Summary
In 2008 Dr. Michael D. Iseman of National Jewish Health said, “In roughly 55 years we have squandered our precious legacy of chemotherapy for…tuberculosis [TB].” William R. Bishai, MD, PhD, Director of the The KwaZulu-Natal Research Institute for Tuberculosis and HIV, Durban, South Africa, echoed this idea, saying that “HIV and multidrug- resistant [MDR] strains of TB threaten to reverse a half century of partial control of TB.” He was referring to the TB and HIV coepidemics in sub-Saharan Africa.
- HIV & AIDS
- Bacterial Infections
In 2008 Dr. Michael D. Iseman of National Jewish Health said, “In roughly 55 years we have squandered our precious legacy of chemotherapy for…tuberculosis [TB].” William R. Bishai, MD, PhD, Director of the The KwaZulu-Natal Research Institute for Tuberculosis and HIV, Durban, South Africa, echoed this idea, saying that “HIV and multidrug-resistant [MDR] strains of TB threaten to reverse a half century of partial control of TB.” He was referring to the TB and HIV coepidemics in sub-Saharan Africa.
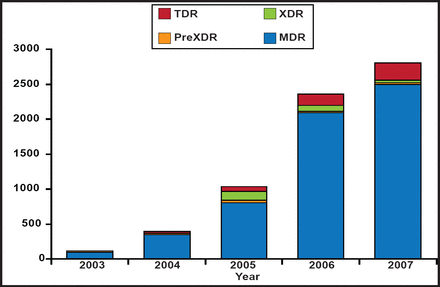
In South Africa the TB rate is 971/100,000 and 18% of all adults aged 15 to 49 years are infected with HIV. In the South African province of KwaZulu-Natal, the TB rate is among the highest globally (1200/100,000) with 3000 MDR- and 300 extremely drug-resistant (XDR)-TB cases/year and rising (Figure 1). Dr. Bishai outlined a series of MDR treatment principles that are being used in South Africa to combat this epidemic. In the intensive or injectable phase, clinicians are using 5 drugs (4 oral and 1 injectable) for a minimum of 6 months, followed by a continuation phase during which at least 4 oral drugs are used for at least 18 months from the date of sputum culture conversion. The GeneXpert® System (an automated, cartridge-based nucleic amplification assay for the simultaneous detection of TB and rifampicin resistance directly from sputum) has made a difference and confirms drug susceptibility about 68% of the time, but 15% of time there are discordant findings. Patient management is complicated and includes counseling, baseline and serial audiography, and an aggressive blood work plan and side effect monitoring program. Social workers are used where possible and a contact investigation is attempted, although it is difficult to accomplish. Response to therapy is monitored by cultures and weights, and serial X-rays at 6, 12, and 24 months. Confirmed culture conversion, defined as 2 consecutive negative smears and cultures at least 30 days apart, is required before the patient can return to public workplaces.
“Totally” Drug Resistant TB in KwaZulu-Natal.
Reproduced with permission from WR Bishai, MD, PhD.
There is reduced chance of cure for patients with XDR-TB and a very high risk of premature death. These patients should be hospitalized. In terms of treatment, resistance is assumed if a drug is used for more than a month with no change in smears or cultures.
There are a variety of problems in managing MDR- and XDR-TB patients. Co-conditions (pediatrics, pregnancy, diabetes mellitus, renal insufficiency acute or chronic liver disease, thyroid disease, HIV, immune reconstitution inflammatory syndrome [IRIS], and infection control) need to be considered in addition to treatment regimens. Psychiatric complications are a particular problem. In South Africa, there are not enough beds and there is a waiting list, which has led clinicians to consider outpatient programs as the only rational option. Another option that is being considered is repurposing drugs such as vitamin D and verapamil.
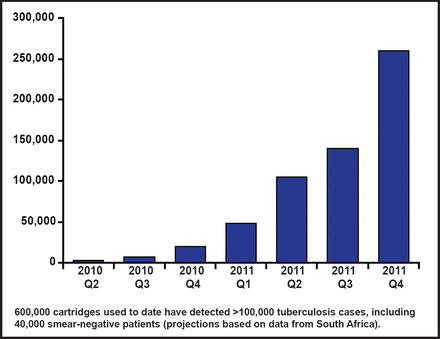
Mark Perkins, MD, Foundation for Innovative New Diagnostics, Geneva, Switzerland, discussed some of the new diagnostic methods available for MDR-TB. The World Health Organization has endorsed several methods for drug susceptibility testing in TB endemic countries: indirect phenotypic (solid and liquid culture, nitrate reductase, and colorimetric redox), direct phenotypic (microscopic observation drug susceptibility), and direct genotypic (line probe assay, and the Xpert Mycobacterium tuberculosis and resistance to rifampicin [Xpert MTB/RIF] assay). The Xpert MTB/RIF system has specificity for RIF resistance detection of 99.1%. The use of the Xpert MTB/RIF cartridge in the public sector of developing countries has increased dramatically since the WHO endorsement (Figure 2).
Xpert MTB/RIF Cartridge Use in the Public Sector of Developing Countries, by Quarter.
Reproduced with permission from M. Perkins, MD.
The main obstacles to the development and use of improved TB diagnostic tools are lack of laboratory infrastructure (92% of testing capacity is in 5 countries), lack of reference standard, and limited commercial opportunity. Hopefully some of the emerging technologies can offset these obstacles.
There are number of predictors of success and treatment failure in patients with drug resistant TB. One of the parameters for success is the ability to selectively apply surgery. John Mitchell, MD, University of Colorado School of Medicine, Denver, Colorado, USA, discussed the surgical procedures available to patients with MDR- and XDR-TB.
Factors favoring surgery include a pattern of drug resistance so extensive that it compromises the likelihood of a medical cure, localized lung damage that might be a focus of persistent disease and/or further acquired resistance, allergies or intolerance to essential medications, and lack of access to curative chemotherapy. Among the benefits of surgery are rapid bacteriologic conversion, removal of bronchiectasis/fibrotic lung, and increased chance of cure in some patients. However, no procedure is without risk; for this type of surgery the risks are morbidity and mortality related to surgery, potential long-term functional deficits, and transmission in the health facility.
Anatomic resections are the usual procedure and they are now most often performed using a minimally invasive technique with video-assisted thoracoscopic surgery lobectomy being the most common method. Mortality of 0% to 5.0% and morbidity rates of 11.5% to 34.7% are reasonable and can be expected. Cure rates are about 80% to 98%. From a compilation of studies Dr. Mitchell derived an overall treatment success, defined as repeated negative cultures over 6 months, of 84% (95% CI, 78 to 89; p<0.001) following surgery.
Mel Spigelman, MD, Global Alliance for TB Drug Development, New York, New York, USA, discussed the status of several new TB drugs in clinical development but also highlighted the fact that the pipeline is still inadequate, the time for clinical development is still too long, and not enough resources are being allocated to TB research and development.
Drugs in Phase 3 that show promise include delamanid, gatifloxacin, moxifloxacin, and rifapentine. Dr. Spigelman presented data (Trial 204) showing that treatment with delamanid results in a significant (p=0.008) 53% increase in sputum culture conversion at 2 months versus placebo. In another study in patients with latent M. tuberculosis infection, weekly therapy with rifapentine plus isoniazid for 3 months was as effective as 9 months of isoniazid alone in preventing TB and had a higher treatment-completion rate [Sterling TR et al. N Engl J Med 2011]. Phase 3 noninferiority trials with gatifloxacin and moxifloxcin are in process in Africa and other third-world countries.
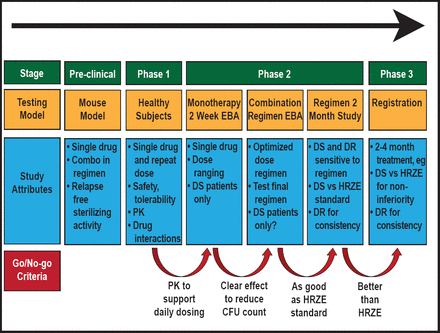
Unified Drug Sensitive/Drug Resistant Development Pathway for Novel TB Drug Regimens is a new pathway being used for the development of TB treatment regimens to improve clinical development time (Figure 3). The first example of the use of algorithm is the Phase 2 combination early bacterial activity study [New Combination 1; NC001], which compared treatment with various combinations of moxifloxacin, pyrazinamide, PA-824, and TMC207 to the standard therapy, Rifafour, to reduce the log colony-forming units over time. Treatment results were consistent with those seen in earlier preclinical mouse model studies, indicating the usefulness of the mouse model. This method was also used to detect early bactericidal activity using BID or QD dosing of sutezolid, a new oxazolidinone with activity against M. tuberculosis [Wallis RS et al. IAC 2012 Abstract THLBB02].
Unified Drug Sensitive/Drug Resistant Regimen Development Path.
EBA=early bacterial activity; DS=drug sensitive; DR=drug resistant; PK=pharmacokinetics; HRZE=isoniazid/rifampicin/pyrazinamide/ethambutol
Reproduced with permission from the Global Alliance for TB Drug Development.
- © 2012 MD Conference Express®