Abstract
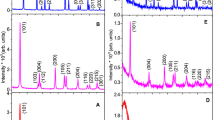
Titania-silica mesoporous materials have been synthesized by a modified sol-gel technique using a cationic surfactant. The synthesis process was studied using statistical design of experiments to achieve the best conditions for titania-silica preparation. The synthesized materials were characterized by XRD, FT-IR, SEM and surface area measurements. The XRD and SEM results showed an amorphous structure of titania-silica. The surface area measurements using nitrogen adsorption showed type-IV isotherms which indicate the formation of mesoporous structure. A high surface area can be obtained (685 m2/g). The crystal size of titania-silica calculated using Scherrer’s equation was found to be in the range 8–15 nm.
Similar content being viewed by others
References
C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature 359, 710 (1992).
A. Sayari and P. Liu, Microporous Mater. 12, 149 (1997).
F. Schüth, Chem. Mater. 13, 3184 (2001).
X. He and D. Antonelli, Angew. Chem. Int. Edn. Engl. 41, 214 (2002).
D. M. Antonelli and J. Y. Ying, Angew. Chem. Int. Edn. Engl. 34, 2014 (1995).
R. L. Putnam, N. Nakagawa, K. M. McGrath, N. Yao, I. A. Aksay, S. M. Grunner and A. Navrotsky, Chem. Mater. 9, 2690 (1997).
V. F. Stone and R. J. Davis, Chem. Mater. 10, 1468 (1998).
D. M. Antonelli, Microporous Mesoporous Mater. 30, 315 (1999).
H. Yoshitake, T. Sugihara and T. Tatsumi, Chem. Mater. 14, 1023 (2002).
D. T. On, Langmuir 15, 8561 (1999).
D. Khushalani, G. A. Ozin and A. Kuperman, J. Mater. Chem. 9, 1491 (1999).
S. Cabrera, J. E. Haskouri, A. Beltrán-Porter, D. Beltrán-Porter, D. D. Marcos and P. Amorós, Solid State Sci. 2, 513 (2000).
P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature 395, 583 (1998).
P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Chem. Mater. 11, 2813 (1999).
M. Muhammed Yusuf, H. Imai and H. Hirashima, J. Non-Cryst. Solids 285, 90 (2001).
H. Hirashima, H. Imai and V. Balek, J. Non-Cryst. Solids 285, 96 (2001).
M. Muhammed Yusuf, H. Imai and H. Hirashima, J. Sol-Gel Sci. Technol. 25, 65 (2002).
M. Muhammed Yusuf, H. Imai and H. Hirashima, J. Sol-Gel Sci. Technol. 28, 97 (2003).
M. Muhammed Yusuf, Y. Chimoto, H. Imai and H. Hirashima, J. Sol-Gel Sci. Technol. 26, 635 (2002).
Y. Xu, W. Zheng and W. Liu, J. Photochem. Photobiol. A 122, 57 (1999).
E. Gallagher, C. M. O’Brien, A. G. M. Scannell and E. K. Arendt, J. Food Eng. 56, 269 (2003).
S. L. Shyu and L. S. Hwang, Food Res. Int. 34, 133 (2001).
W. G. Cochran and G. M. Cox, in: Experimental Designs, 2nd edn, p. 335. Wiley, New York, NY (1957).
R. Myers and D. C. Montgomery, Response Surface Methodology. Wiley, New York, NY (2002).
C. Liu, Y. Liu, W. Liao, Z. Wen and S. Chen, Biotechnol. Lett. 25, 877 (2003).
A. Vohra and T. Satyanarayana, Crit. Rev. Biotechnol. 23, 29 (2003).
Y. R. Abdel-Fattah and Z. A. Olama, Process. Biochem. 38, 115 (2002).
J. Zhang, I. Boyd, B. J. O’Sullivan, P. K. Hurley, P. V. Kelly and J. P. Senateur, J. Non-Cryst. Solids 303, 134 (2002).
X. T. Yoko, K. Kamiya and K. Tanaka, J. Mater. Sci. 25, 3922 (1990).
C. P. Jaroniec, M. Kruk, M. Jaroniec and A. Sayari, J. Phys. Chem. B 102, 5503 (1998).
M. Kruk, M. Jaroniec and A. Sayari, Langmuir 13, 6267 (1997).
K. Katsunori and S. S. Puyam, J. Phys. Chem. B 103, 3563 (1999).
X. Zhang, F. Zhang and K.-Y. Chan, Appl. Catal. A: Gen. 284, 193 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, R.M., El-Midany, A.A. & Othman, I. Effect of synthesis conditions on preparation of mesoporous titania-silica by a modified sol-gel technique using a cationic surfactant. Res. Chem. Intermed. 34, 629–639 (2008). https://doi.org/10.1163/156856708784795671
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856708784795671