Abstract
Background: Recent studies have shown that endothelial progenitor cells (EPCs) contribute to lung repair after lipopolysaccharide (LPS)-induced lung injury and infusion of LPS decreased early EPCs in human peripheral blood. However, the effects of LPS on endothelial colony-forming cells (ECFCs) remain to be determined. Objective: To investigate possible effects of LPS on the functional activity of ECFCs. Methods: ECFCs were isolated from human umbilical cord blood and characterized. ECFCs at passages 3-5 were treated for 24 h with either LPS or vehicle control. Their viability, migration and in vitro vasculogenesis activity were assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, modified Boyden chamber and in vitro angiogenesis assays, respectively. ECFC adhesion was assessed by replating cells on fibronectin-coated dishes and subsequent counting of adherent cells. Results: Incubation with LPS dose-dependently inhibited the viable, migratory, adhesive and in vitro vasculogenesis capacity of ECFCs. Conclusion: LPS impaired the functional activity of ECFCs.
Introduction
Acute lung injury (ALI) is a critical illness syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates that are not attributed to left atrial hypertension. The crude incidence of ALI was estimated at 78.9/100,000 person-years in the USA [1]. In humans, the most common cause of ALI is sepsis, therefore administration of the Gram-negative bacterial endotoxin lipopolysaccharide (LPS) has been used as a model of sepsis-related lung injury. Although LPS is often used as a stimulus for lung injury in several species [2,3,4], the pathophysiology of LPS-induced lung injury is not thoroughly well known. Recent studies have demonstrated that destruction of pulmonary endothelium is one of the main histological changes in the lung tissue in ALI during the acute exudative phase [5]. Activation and damage of pulmonary endothelium is the hallmark of ALI, so endothelial repair after vascular injury is important for the prevention and treatment of ALI.
Endothelial progenitor cells (EPCs), first isolated by Asahara et al. [6], are mainly derived from the bone marrow, and can be mobilized to the peripheral circulation where they contribute to the repair of injured endothelium and to the formation of new blood vessels [7]. Increasing evidence suggests that there are two different types of EPCs from circulating mononuclear cells (MNCs): early EPCs and endothelial colony-forming cells (ECFCs), which are also called late or blood outgrowth endothelial cells, or late EPCs [8,9,10]. Although both EPCs are derived from MNCs, they have different morphologies, growth patterns and function in vitro [8,9]. Recently, associations between early EPCs and chronic obstructive pulmonary disease [11], ALI [12] and bacterial pneumonia [13] have been reported. More recently, relationships between stem cells, progenitor cells and lung diseases have been widely discussed [14,15,16]. Furthermore, infusion of LPS led to a significant decrease in peripheral early EPCs in humans [17]. Unlike early EPCs, ECFCs have much less been studied. The effect of LPS on the functional activity of ECFCs remains to be determined. Here we investigated the effect of LPS on ECFCs.
Materials and Methods
Isolation, Culture and Characterization of ECFCs
The study was approved by the Ethics Committee of the Shantou University Medical College. It was mainly carried out from December 2010 to July 2012. ECFCs were isolated, cultured and characterized according to previously described techniques [18,19]. Briefly, total MNCs were isolated from umbilical cord blood by Ficoll density gradient centrifugation and cultured on 6-well plates coated with human fibronectin (Chemicon, Billerica, Mass., USA) in endothelial cell basal medium-2 (EBM-2; Lonza, Walkersville, Md., USA) supplemented with EGM-2 MV single aliquots (Lonza) consisting of 5% fetal bovine serum, vascular endothelial growth factor, basic fibroblast growth factor-2, epidermal growth factor, insulin-like growth factor-1, ascorbic acid, gentamicin sulfate, amphotericin-B and hydrocortisone. After 24 h of culture, nonadherent cells and debris were removed by washing with EGM-2 medium, and new medium was applied. The medium was exchanged daily for 7 days and then every other day until the first passage. ECFCs were characterized as adherent cells double positive for 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (DiI-acLDL; Invitrogen, Grand Island, N.Y., USA) uptake and fluorescein-isothiocyanate-conjugated Ulex europaeus agglutinin lectin (UEA-1; Sigma-Aldrich, St. Louis, Mo., USA) binding using an inverted fluorescent microscope. They were further documented by demonstrating the expression of von Willebrand factor (vWF), kinase insert domain receptor (KDR), CD105, CD146 and CD144 (vascular endothelial-cadherin, VE-cadherin) but not CD11b, CD14 and CD45 (polyclonal rabbit anti-vWF antibody, anti-KDR antibody, anti-CD105 antibody, anti-CD146 antibody, anti-CD144 antibody, anti-CD11b antibody, anti-CD14 antibody and anti-CD45 antibody; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif., USA).
Western Blot
The expression of CD144 protein was investigated. Proteins (25 μg) were denatured, loaded onto a 8% polyacrylamide gel, resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred to a PVDF membrane. Membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.05% Tween-20 and incubated with rabbit polyclonal anti-CD144 at a dilution of 1:200 overnight at 4°C, followed by a 1-hour incubation with a secondary goat polyclonal anti-rabbit antibody at a dilution of 1:1,000. The protein bands were visualized with an ECL kit (Thermo Scientific, Waltham, Mass., USA).
Protocols
ECFCs were used at passages 3-5. Cells were serum depleted for 24 h before experiments and incubated with 0.1, 1 and 10 μg/ml LPS for 24 h.
ECFC Viability Assay
ECFC viability was determined with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. ECFCs were digested with 0.25% trypsin, cultured in a 96-well culture plate (200 µl/well) and then treated with either LPS (0.1-1 and 10 μg/ml; Sigma-Aldrich) or vehicle control. After being cultured for 24 h, ECFCs were supplemented with 10 µl MTT (5 g/l, Sigma-Aldrich) and incubated for another 4 h. Then the supernatant was discarded by aspiration and the ECFC preparation was shaken in 150 µl dimethyl sulfoxide for 10 min before optical density was measured at 490 nm.
Migration Assay
The contribution of EPCs to reendothelialization after endothelial injury represents a multistep that includes recruiting, rolling, adhesion, migration and differentiation. To evaluate the effect of LPS on ECFC migration, a modified Boyden chamber assay was used. In brief, ECFCs were detached with 0.25% trypsin and then 2 × 104 ECFCs in 200 µl EBM-2 were seeded in the upper chamber of a Transwell cell culture insert (8-µm pore size; BD Biosciences, Bedford, Mass., USA). Vascular endothelial growth factor (50 ng/ml, PeproTech, Rocky Hill, N.J., USA) in EBM-2 was placed in the lower chamber. After incubation for 24 h at 37°C, the upper side of the membrane was wiped gently with a cotton ball. Then the membranes were washed with phosphate-buffered saline (PBS) and fixed with methanol. For quantification, cells were stained with 4',6-diamidino-2-phenylindole solution. Cells migrating into the lower chamber were counted manually in 5 random high-power (×100) microscopic fields (HPF), and the average number of cells/HPF was determined.
Cell Adhesion Assay
ECFCs were washed with PBS and gently detached with 0.25% trypsin. After centrifugation and resuspension in EGM-2, identical cell numbers were replated onto fibronectin-coated culture dishes and incubated for 30 min at 37°C. Nonadherent cells were removed by washing with PBS and adherent cells were counted by independent blinded investigators.
In vitro Vasculogenesis Assay
Analysis of capillary formation in Matrigel (BD Biosciences) was performed according to the manufacturer's protocol. Matrigel (30 µl) was aliquoted into a 96-well plate and incubated at 37°C for 60 min. After trypsinization, 1 × 104 ECFCs were suspended with 100 µl culture medium and plated onto the pre-incubated Matrigel. After incubation for 3-9 h, tube formation in the Matrigel was observed under a microscope and the number of closed units in a random field of each well was quantitated.
Statistical Analysis
All data are presented as means ± SD. Differences between group means were assessed by an unpaired Student's t test for single comparisons or by ANOVA for multiple comparisons using SPSS 16.0. A value of p < 0.05 was considered significant.
Results
Characterization of ECFCs
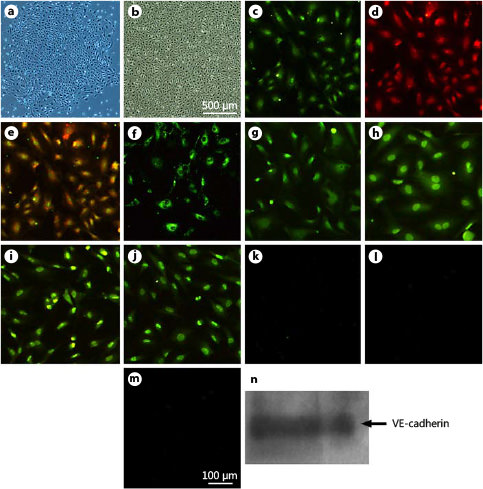
ECFCs appeared after 1-2 weeks as small colonies (fig. 1a) in cultures of MNCs from the human umbilical cord blood and developed cobblestone-like cell morphology over time. During passaging, cells retained an endothelial-like morphology and formed cobblestone-like monolayers (fig. 1b). Fluorescence microscopy showed that ECFCs took up DiI-acLDL and bound UEA-1 (fig. 1c-e). Immunophenotyping revealed that ECFCs expressed the following endothelial cell surface antigens vWF (fig. 1f), KDR (fig. 1g), CD105 (fig. 1h), CD146 (fig. 1i) and CD144 (fig. 1j), but did not express CD11b (fig. 1k), CD14 (fig. 1l) and CD45 (fig. 1m). Furthermore, CD144 expression was verified by Western blot (fig. 1n).
Morphology and characterization of ECFC. Small colonies appeared after 1-2 weeks in cultures of MNCs (a). ECFC with cobblestone-like morphology were selected, passaged and grown to confluence (b), shown to endocytose DiI-acLDL (c) and bind UEA-1 (d). Merged image of both stains (e). ECFC expressed vWF (f), KDR (g), CD105 (h), CD146 (i) and CD144 (j) but did not express CD11b (k), CD14 (l) and CD45 (m). The expression of CD144 (VE-cadherin) was also verified by Western blot (n).
Morphology and characterization of ECFC. Small colonies appeared after 1-2 weeks in cultures of MNCs (a). ECFC with cobblestone-like morphology were selected, passaged and grown to confluence (b), shown to endocytose DiI-acLDL (c) and bind UEA-1 (d). Merged image of both stains (e). ECFC expressed vWF (f), KDR (g), CD105 (h), CD146 (i) and CD144 (j) but did not express CD11b (k), CD14 (l) and CD45 (m). The expression of CD144 (VE-cadherin) was also verified by Western blot (n).
Effect of LPS on ECFC Viability
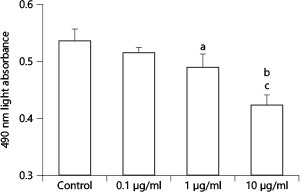
The effect of LPS on ECFC viability was determined using an MTT assay (fig. 2). LPS dose-dependently decreased ECFC viability at concentrations of 0.1-10 μg/ml, reaching a maximum at 10 μg/ml LPS (10 μg/ml LPS vs. control: 0.424 ± 0.017 vs. 0.536 ± 0.021, 490 nm light absorbance, p < 0.01). Furthermore, ECFC viable activity was significantly lower at 10 than at 1 μg/ml LPS (10 vs. 1 μg/ml LPS: 0.424 ± 0.017 vs. 0.490 ± 0.023, p < 0.05).
Effect of LPS on ECFC viability. LPS dose-dependently decreased ECFC viability with a nadir at 10 μg/ml LPS. Means ± SD. n = 4. a p < 0.05, b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS group.
Effect of LPS on ECFC viability. LPS dose-dependently decreased ECFC viability with a nadir at 10 μg/ml LPS. Means ± SD. n = 4. a p < 0.05, b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS group.
Effect of LPS on ECFC Migration
The effect of LPS on ECFC migration was analyzed in a modified Boyden chamber assay (fig. 3). LPS profoundly impaired cell migration at concentrations of 0.1-10 μg/ml: 0.1 μg/ml LPS decreased cell migration slightly (0.1 μg/ml LPS vs. control: 162.5 ± 7.7 vs. 171.0 ± 10.5 cells/HPF, p < 0.05), and 1 μg/ml LPS markedly decreased cell migration of ECFCs (1 μg/ml LPS vs. control: 152.5 ± 10.5 vs. 171.0 ± 10.5 cells/HPF, p < 0.05). The effect reached its maximum at 10 μg/ml LPS (10 μg/ml LPS vs. control: 132.8 ± 7.5 vs. 171 ± 10.5 cells/HPF, p < 0.05).
Effect of LPS on ECFC migration. LPS decreased ECFC migration activity in a concentration-dependent manner. Means ± SD. n = 4. a p < 0.05, b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS.
Effect of LPS on ECFC migration. LPS decreased ECFC migration activity in a concentration-dependent manner. Means ± SD. n = 4. a p < 0.05, b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS.
Effect of LPS on ECFC Adhesiveness
To study the possibility that LPS alters the adhesiveness of cultured human ECFCs, ECFCs were incubated with LPS for 24 h. After being replated on fibronectin-coated dishes, ECFCs preexposed to LPS exhibited a significant decrease in the number of adhesive cells at 30 min at concentrations of 0.1-10 μg/ml (fig. 4), with a maximal effect achieved at 10 μg/ml (10 μg/ml LPS vs. control: 317.8 ± 8.5 vs. 514.0 ± 15.1 cells/96-well plate, p < 0.01).
Effect of LPS on ECFC adhesive activity. LPS decreased ECFC adhesive activity at concentrations of 0.1-10 μg/ml, with a nadir at 10 μg/ml. Means ± SD. n = 4. b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS.
Effect of LPS on ECFC adhesive activity. LPS decreased ECFC adhesive activity at concentrations of 0.1-10 μg/ml, with a nadir at 10 μg/ml. Means ± SD. n = 4. b p < 0.01 vs. control; c p < 0.05 vs. 1 μg/ml LPS.
Effects of LPS on ECFC Vasculogenesis
The effect of LPS on ECFC vasculogenesis was evaluated by an in vitro vasculogenesis assay. After seeding the cells on basement membrane matrix (Matrigel; BD Biosciences), ECFCs manifested tube formation (fig. 5a). The response of the ECFCs to LPS is depicted in figure 5b-d. The number of closed units decreased with increasing LPS concentration (0.1-10 μg/ml) in cells incubated for 24 h (fig. 5e), with peak production at 10 μg/ml LPS (10 μg/ml LPS vs. control: 29.5 ± 1.9 vs. 49.8 ± 4.1 closed units/96-well plate, 40 × field, p < 0.01).
Effect of LPS on ECFC angiogenesis in vitro. Seeding of on Matrigel resulted in capillary network formation (a). LPS inhibited network formation at 0.1 (b), 1 (c) and 10 μg/ml (d). Tube formation was determined by measuring the number of closed units (e). Means ± SD. n = 4. b p < 0.01 vs. control; c p < 0.05 vs. 0.1 μg/ml LPS; d p < 0.05 vs. 1 μg/ml LPS.
Effect of LPS on ECFC angiogenesis in vitro. Seeding of on Matrigel resulted in capillary network formation (a). LPS inhibited network formation at 0.1 (b), 1 (c) and 10 μg/ml (d). Tube formation was determined by measuring the number of closed units (e). Means ± SD. n = 4. b p < 0.01 vs. control; c p < 0.05 vs. 0.1 μg/ml LPS; d p < 0.05 vs. 1 μg/ml LPS.
Discussion
In the present study, we investigated the effect of LPS on the functional activity of ECFCs. The result shows that LPS dose-dependently inhibited the viable, migratory, adhesive and in vitro vasculogenesis capacity of ECFCs.
Since being described by Asahara et al. [6] in 1997, the ‘EPC' has received a vast amount of research interest, especially in relation to cardiovascular diseases. Almost all cardiovascular risk factors as well as various cardiovascular diseases are associated with EPC impairment, both in number and function [20]. Moreover, reduced EPC levels seem to be correlated with endothelial dysfunction [21] and with an increased risk of cardiovascular events [22].
Recently, associations between early EPCs and lung diseases have been investigated. Yamada et al. [13] reported that the number of circulating EPCs significantly increased in patients with pneumonia. In septic patients, the number of EPCs was not only significantly higher than in nonseptic intensive care unit patients and healthy controls but also correlated with survival in septic patients [23]. Burnham et al. [12] reported the number of circulating EPCs in patients with ALI is approximately twofold higher than in healthy control subjects. More importantly, an increased number of circulating EPCs was associated with improved survival [12]. In an animal model of ALI, Yamada et al. [24] have shown that administration of LPS to murine lungs induced rapid release of EPCs into the circulation, which contributed to lung repair after LPS-induced lung injury in mice. Furthermore, transplantation of circulating EPCs or mesenchymal stem cells attenuated ALI induced by oleic acid, hyperoxia and Escherichia coli or pancreatitis-associated lung injury [25,26,27,28,29,30], and improved survival in rats with LPS-induced lung injury [31]. These studies suggest that EPCs are important for lung repair in lung injury. However, in the above studies, EPCs seem to belong to early EPC. Early EPCs appeared after 3-5 days and gradually disappeared after 4 weeks in cultures of MNCs [8]. They have low proliferative capacity and fail to form vessels [9], promote angiogenesis through cytokine secretion [9], and migration and proliferation of mature endothelial cells [32], while the ECFCs in both bone marrow [18] and tissue-resident niches [33] appeared 1-4 weeks after plating, have a high proliferation rate and form blood vessels [8,9], which were regarded as true progenitors of mature endothelial cells. Therefore, from the standpoint of vessel-forming abilities, ECFCs are superior to early EPCs in restoring damaged endothelium and vascular injury. Given the well-established role of EPCs in lung repair, our results might suggest a novel mechanism of action of LPS-induced lung injury: LPS decreases the viable, migratory, adhesive and in vitro vasculogenesis activity of ECFCs in the circulation and tissue, and thus inhibits lung repair processes, which contributes to lung injury.
The mechanisms by which LPS impaired the functional activity of ECFCs remain to be determined. It has been reported that LPS infusion caused a roughly 200-fold increase in TNF levels [17], which inhibited EPC generation [34]. Moreover, TNFα induced apoptosis of EPCs [35], while anti-TNFα treatment had a positive effect on the number and functional properties of EPCs [36]. Xaus et al. [37] have shown that LPS induced apoptosis of macrophages mostly through the autocrine production of TNF-α, which involved Toll-like receptor (TLR4) activation by LPS [38]. Keeping these findings in mind, we speculated that LPS impaired ECFCs partly through activating TLR4 and increasing the level of TNF. However, further studies are needed to confirm our speculation.
A few limitations were apparent in the present study. First, it was a primary study, the mechanisms of action of LPS impairing ECFCs remain to be answered. Secondly, the LPS concentration used in the study is much higher [39] than that in septic patients, in which the concentration is <1 ng/ml [40,41]. The higher concentration of LPS needed may be associated with the resistance of ECFCs to oxidative stress compared to mature endothelial cells [42]. Lastly, the effects of LPS on ECFCs in vivo may be different from those in vitro.
In summary, we found that LPS impaired the functional activity of ECFCs and thus had a detrimental effect on lung repair. Our findings provide additional insight into the mechanism by which LPS induces lung injury.
Acknowledgments
This work was supported in part by the Natural Science Foundation of Guangdong Province (8151503102000017), Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20114404110006) and a basic and clinical research fund of the Shantou University Medical College.
Financial Disclosure and Conflicts of Interest
The authors report no conflicts of interest.