Abstract
Lithium-oxygen (Li-O2) batteries have a theoretical energy density of 3,500 Wh/kg, which is ten times greater than that of typical lithium-ion batteries [1]. However, the practical performance of Li-O2 batteries is hindered by the limited cycle life and low round-trip efficiency. Among the attributed reasons are parasitic side-reactions, including electrolyte decomposition and carbon corrosion leading to cathode degradation [2]. Thus, novel materials design is essential for combating the aforementioned side-reactions. Promising strategies toward this goal include the following: (1) introduction of redox mediators, whose primary purpose is to decrease the charge over-potential [3]; (2) use of highly crystalline carbon cathodes, which reduces the carbon corrosion over many cycles [2]. However, each of these strategies on its own cannot fully address the limitations of Li-O2 batteries. Their performance must be examined at the system level with the understanding of the reaction mechanisms in order to develop the viable Li-O2 battery technology.
In this work, we show that the combined effect of lithium iodide (LiI) and graphite yields a stable Li-O2 battery over many cycles. LiI acts as the redox mediator, while graphite provides a highly crystalline carbon cathode. Figure 1 shows the impact of LiI and graphite on the electrochemical performances of Li-O2 batteries under the restricted capacity of ~0.3 mAh/cm2 (i.e., 3 hours for charge and discharge) at an intermediate cycling rate. Our findings are based on the characterization of Li-O2 battery using the dimethyl sulfoxide (DMSO) based electrolyte. We compare the Li-O2 battery with the addition of LiI to the DMSO electrolyte and graphite as cathode (referred to as "LiI/graphite battery") to the battery with DMSO electrolyte and activated carbon cathode (referred to as "baseline battery"). For the electrochemical performance, the LiI/graphite battery has a lower charge over-potential (3.44V) and a significantly improved cyclability compared to the baseline battery (Figure 1C). To understand the electrochemistry responsible for these performance differences, we examined the morphology and the phases of discharge products formed in LiI/graphite batteries and baseline batteries using scanning electron microscopy (SEM) and X-ray diffraction (XRD). The role of graphite in the reduction of carbon corrosion was studied using X-ray photoelectron spectroscopy (XPS) by tracking the C1s spectral peak, and XRD by tracking the (002) diffraction peak shift. With regards to morphology after discharge, SEM shows that the cathode surface is coated with dense flower-like carpets for the LiI/graphite battery, which is not observed in the baseline battery. The XRD examination of the cathode after discharge in both LiI/graphite and baseline battery show reflections from both lithium peroxide (Li2O2) and lithium hydroxide (LiOH). For the baseline battery, whose overpotential increases cycle-to-cycle, SEM and XRD reveal a passivated cathode surface without crystalline diffraction peaks. In contrast, for the LiI/graphite battery, the SEM and XRD shows dense carpets with LiOH as the dominant phase. Thus, we find that the formation of flower-like LiOH carpets during discharge is essential to the DMSO-based Li-O2 battery cyclability at intermediate cycling rates. While LiOH has been reported as a side product in the previous works, our results suggest that it plays an essential role in viable Li-O2 battery technology [4]. Together, our results demonstrate a promising materials strategy based on reducing the overpotential build-up with redox mediator and improving the carbon cathode stability.
Acknowledgements: This work is supported by the Energy & Biosciences Institute through the EBI-Shell program. All characterizations were carried out at the Materials Research Laboratory Central Research Facilities, University of Illinois. JQ and SP contributed equally to this work. The authors thank Dr. Richard Haasch for XPS characterization.
References:
[1] W. J. Kwak et al., "Lithium-Oxygen Batteries and Related Systems: Potential, Status, and Future," ACS Applied Materials and Interfaces. 2020.
[2] Y. Bae et al., "Tuning the carbon crystallinity for highly stable Li-O2 batteries," Chem. Mater., 2016.
[3] T. Liu, J. P. Vivek, E. W. Zhao, J. Lei, N. Garcia-Araez, and C. P. Grey, "Current Challenges and Routes Forward for Nonaqueous Lithium-Air Batteries," Chemical Reviews., 2020.
[4] T. Liu et al., "Cycling Li-O2 batteries via LiOH formation and decomposition," Science., 2015.
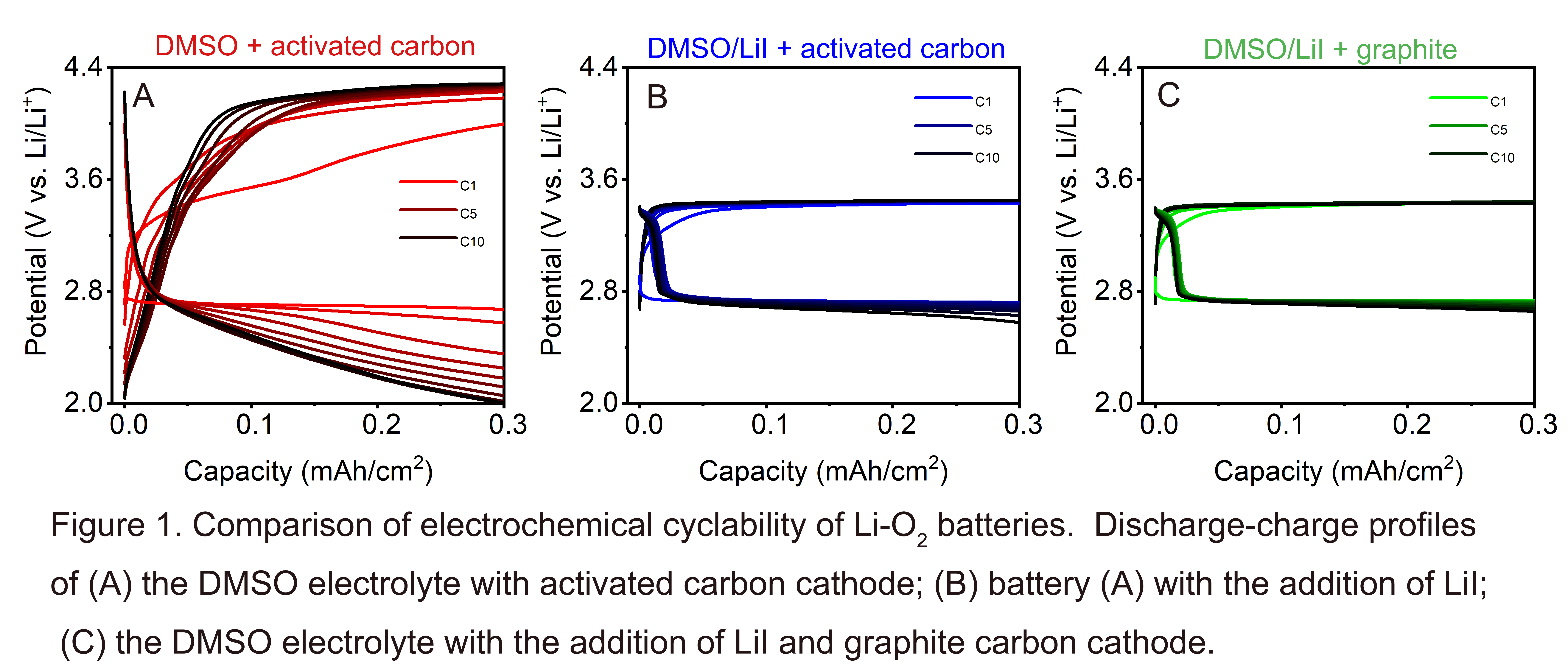
Figure 1
