Abstract
Polymer electrolyte fuel cell (PEFC) has tremendous interests due to its attractive properties such as low-temperature operation and high power density. For PEFC, Pt nanoparticles (NPs) loaded on carbon support (Pt/C) is widely used as the cathode, in which large amount of costly Pt is required to accelerate the oxygen reduction reaction (ORR) kinetics of PEFC [1]. However, NPs catalysts have some drawbacks such as low durability, and low catalytic activity due to aggregation. Moreover, high cost and limited availability of this precious metal hinder the large-scale commercialization. To overcome these challenges, development of low Pt cathode catalyst with high catalytic activity and durability for ORR in PEFC is highly desirable. Herein, Pt-alloy nanowires (NWs) catalyst was prepared with Co. Addition of a second metal into Pt to form Pt-metal alloy NWs catalyst can improve the electronic and geometric parameters of Pt metal, such as Pt-Pt interatomic distance and surface structure, and consequently enhances the ORR activity and stability of the catalyst [2].
To prepare the Pt-Co NWs/C catalyst, a simple and efficient method was used. For synthesis of Pt-Co NWs, 19.6 mg Pt(acac)2, 25.7 mg Co(acac)2, 135.0 mg glucose, 60.0 mg PVP (mw. 40000) and 1.8 mg W(CO)6 were mixed with 2 mL 1-octadecene (ODE) and 3 mL oleylamine (OAm). After sonication for 60 min, the mixed solution was further heated up to 140 ºC for 6 hours. Next, the Pt-Co NWs was obtained by centrifugation (9,000 rpm for 10 min), and subsequently a facile selective precipitation process was employed to purify NWs. For preparation of Pt-Co NWs/C catalysts, the ethanol reduction method was used. Pt-Co NWs/C catalyst was obtained by centrifugation, then washed with ethanol, and dried overnight. Subsequently, annealing treatment was performed under a mixing atmosphere of hydrogen and argon with an annealing temperature of 450 ºC for 12 h. After that, XRD, TEM, and EDX measurements were performed to examine the structures, morphology, and composition of the catalysts. Then, tree-electrode cell was used to evaluate the electrochemical active surface area (ECSA) estimated from hydrogen adsorption/desorption peak using cyclic voltammetry (CV). Current-voltage (I-V) measurements of the single cell (Pt loading of 0.1 mg-Pt cm−2-MEA) were performed at cell temperature of 80 ºC, a scan rate of 50 mV s−1, and a voltage range of 0.2-1.2 V. The accelerated durability test (ADT) was performed.
Fig. 1 shows the XRD patterns of Pt-Co NWs/C catalyst, and compared with commercial Pt-Co/C and Pt/C catalysts. For Pt-Co NWs/C catalyst, some diffraction peaks were observed at ca. 2θ = 39.7º, 46.1º, and 67.4º assigned to (111), (200), and (220) reflections of Pt. The peak of Pt-Co NWs was slightly shifting to higher values as compared with the peak of standard Pt (JCPDS#88-2343). The shift in the peak position can be attributed to the formation of alloy crystal. Fig. 2 shows the TEM image of Pt-Co NWs. The nanocrystal exhibits a uniform morphology with a diameter of ca. 1.5 nm and a length of 64 nm. The chemical composition of the as-prepared catalyst is examined by EDX, which is consistent with the reported literatures. The area-specific activity of Pt-Co NWs/C estimated from the mass activity (0.80 V) divided by ECSA is ca. 0.70 mA cm−2, and the corresponding value of Pt-Co/C is ca. 0.13 mA cm−2, as shown in Fig. 3. Comparison of I-V polarization and power density curves obtained with Pt-Co NWs/C and commercial Pt-Co/C catalysts are shown in Fig. 4. Pt-Co NWs/C exhibits low cell voltage (0.37 V) than that of the commercial Pt-Co/C catalyst (0.46 V) at 1.0 A cm–2. Maximum power densities (Pmax) of Pt-Co NWs/C and Pt-Co/C are 0.39 W cm–2 and 0.59 W cm–2, respectively. However, the reduction of Pmax after ADT is suppressed for Pt-Co NWs/C catalyst. The Pmax losses for the Pt-Co NWs/C and Pt-Co/C are 3.4% and 20.9%, respectively.
Therefore, it can be concluded that the lifespan of a PEFC prepared with Pt-Co NWs/C catalyst under steady-state operation can be very long. The Pmax could be achieved by enhancing ECSA, since the area-specific activity of Pt-Co NWs/C is higher than that of commercial catalysts. Increasing activity of Pt-Co NWs/C would be a future task.
Acknowledgement
The authors would like to thank the New Energy and Industrial Technology Development Organization (NEDO) for financial support of this work.
References:
F. Godínez-Salomon, R. Mendoza-Cruz, M. J. Arellano-Jimenez, M. Jose-Yacaman, C. P. Rhodes, ACS Appl. Mater. Interfaces, 9, 18660-18674 (2017).
V. Č olić, A. S. Bandarenka, ACS Catal. 6, 5378-5385 (2016).
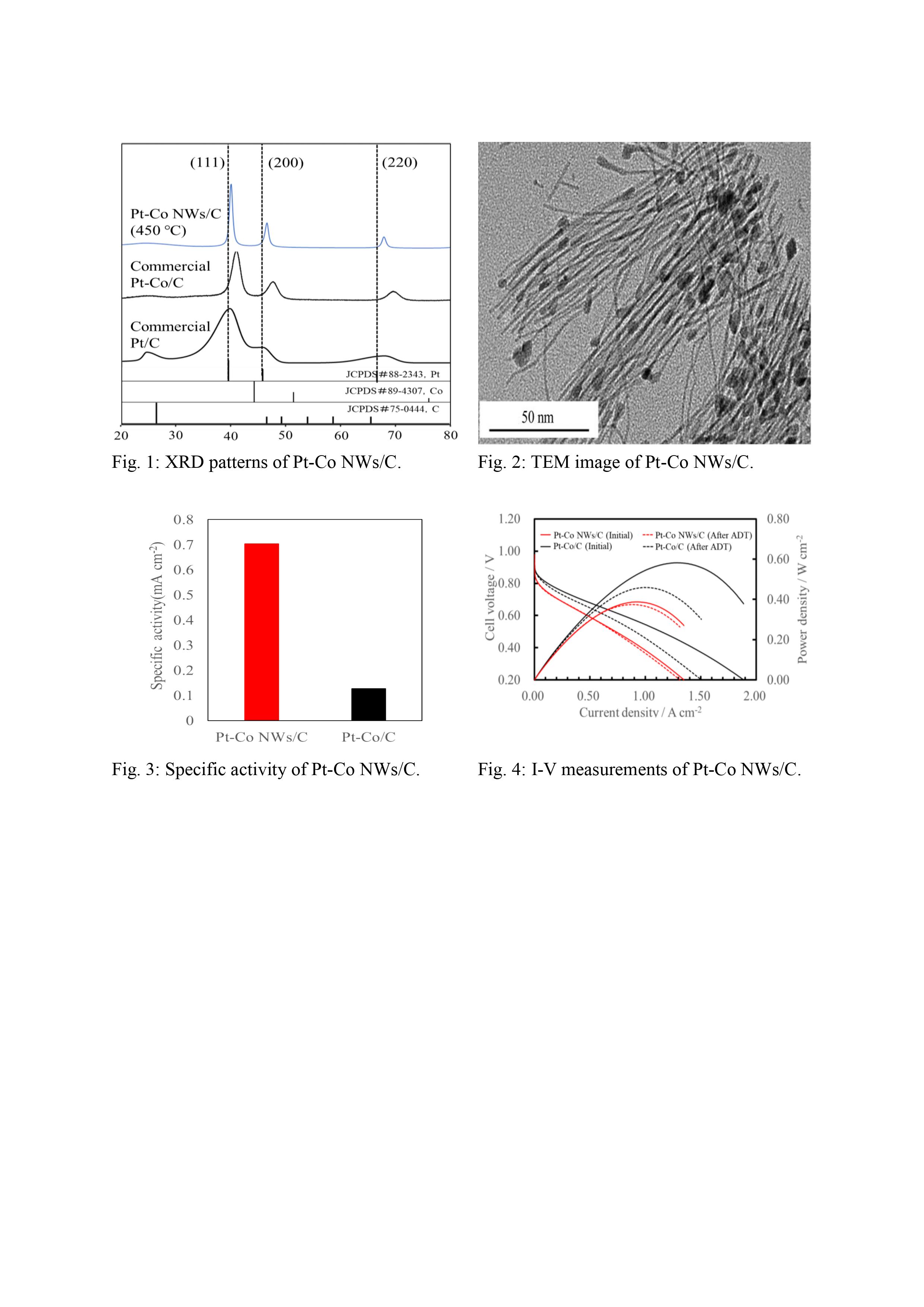
Figure 1
