Abstract
As a clean energy conversion device, the fuel cell has attracted much attention over the past decade. Among the different types of fuel cells, the polymer electrolyte fuel cell (PEFC) shows promise for automotive, backup power, and portable device applications, due to its low operating temperature, high power density, quick dynamic response, cold start capacity and other advantageous features 1, 2. Various numerical and experimental studies of PEFCs have been conducted 3, 4. Experimental equipment has developed substantially; however, it remains limited with respect to conducting in-situ measurements for PEFCs. As an alternative, numerical approaches offer useful tools for obtaining local internal data. The utility of the latter is increasing in the light of the increasing capacity of supercomputers.
Commercial computational fluid dynamics (CFD) software has been widely used, and provides relatively straightforward solvers for fuel cell applications 5-7. However, there are several drawbacks concerning model development and application, e.g., non-transparency, licensing limitations, parallelization, etc. Open-source libraries are much more flexible with respect to the transparency of source code, model development, free utilization between multiple institutes and large-scale parallelization.
A model, which is capable of reproducing all major physico-chemical hydrodynamic processes within a PEFC, is developed and implemented within the open-source CFD source library, OpenFOAM. All major transport phenomena, including two-phase fluid flow, inter-phase heat and mass transfer, and ionic and electronic transfer, are taken into account. An Eulerian-Eulerian formulation is applied for the two-phase flow in the gas channels, a Leverett J-function is utilized for liquid water transfer in the porous regions, and a Butler-Volmer relationship employed to describe the electrochemical reaction. A two-potential model is used to describe proton and electron transfer. A multiple region technique is applied for domain decomposition in the various components, including air/fuel passages, membranes, and electrodes.
Figure 1. Numerical and experimental results. (a)-(d). in-house Designed. 8; (e). Design of Schneider et al. 9.
The model is applied to an in-house-designed PEFC prototype,8 which features three serpentine flow channels, as is shown in Figure 1 (a). The comparison of polarization curves is shown in Figure 1 (b). The present model predicts overall performance with good agreement. Figure 1 (c) and (d) display the current density in the membrane and liquid water saturation in the cathodic catalyst electrode, respectively. In general, the current density decreases from the inlet to the outlet, whereas the liquid water saturation increases. However, both current density and liquid water saturation show higher values under the lands. Liquid water is prone to accumulate in these regions, which increases the local electric conductivity, and therefore the local current densities.
Validation of the model is also facilitated by means of a comparison of the local current density with experimental measurements,9 as shown in Figure 1 (e). It can be seen that these values increase from under-land to under-channel regions, a feature which is captured both by the model and the experimental data. This contrasts with those shown in Figure 1 (a)-(d), where the gases are 90% humidified.
Further investigations will focus on the comparison of polarization curves under a wide variety of operating conditions, varying such parameters as temperature, relative humidity, etc. The local current density distribution measured with the S++ device will also be used to provide additional validation for the present model.
M. Andersson, S. B. Beale, M. Espinoza, Z. Wu and W. Lehnert, Applied Energy, 2016, 180, 757 - 778.
G. Zhang and K. Jiao, Journal of Power Sources, 2018, 391, 120 - 133.
A. Z. Weber, R. L. Borup, R. M. Darling, P. K. Das, T. J. Dursch, W. Gu, D. Harvey, A. Kusoglu, S. Litster, M. M. Mench, R. Mukundan, J. P. Owejan, J. G. Pharoah, M. Secanell and I. V. Zenyuk, Journal of The Electrochemical Society, 2014, 161, F1254-F1299.
H.-W. Wu, Applied Energy, 2016, 165, 81 - 106.
S. Chen, Z. Xia, X. Zhang and Y. Wu, Energy Procedia, 2019, 158, 1678 - 1684.
G. Zhang and K. Jiao, Energy Conversion and Management, 2018, 176, 409 - 421.
L. Fan, G. Zhang and K. Jiao, Energy Conversion and Management, 2017, 150, 763 - 774.
Y. Shi, H. Janßen and W. Lehnert, Energies, 2019, 12.
I. A. Schneider, S. von Dahlen, A. Wokaun and G. G. Scherer, Journal of The Electrochemical Society, 2010, 157, B338-B341.
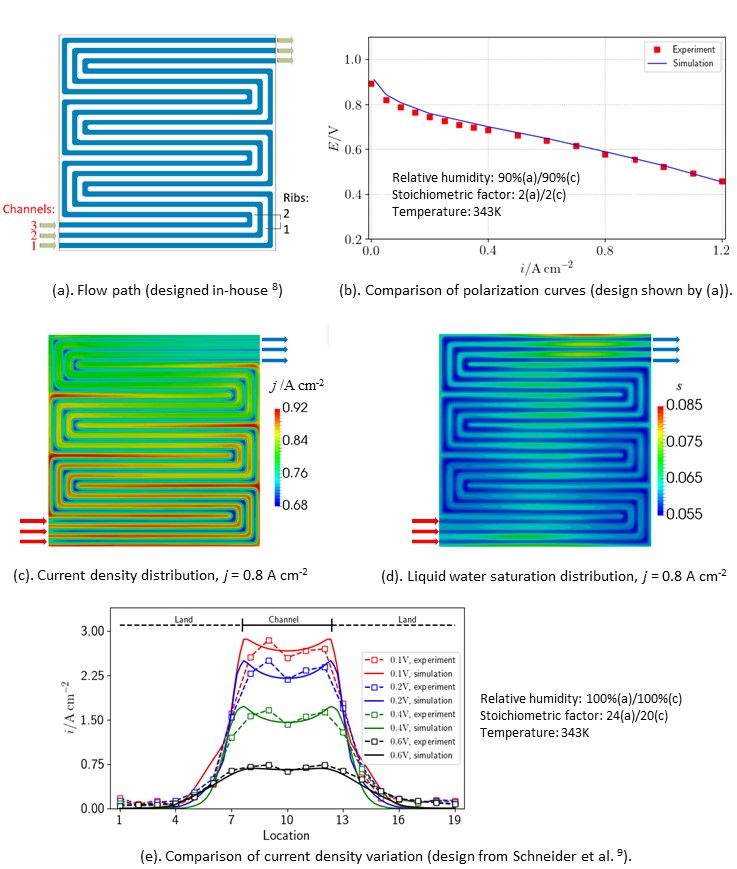
Figure 1
