Abstract
As an emerging alternative to proton exchange membrane fuel cells (PEMFCs), hydroxide exchange membrane fuel cells (HEMFCs) are more cost-effective due to PGM-free catalysts options, more affordable bipolar plate production, and other potential savings.1 However, unlike PEMFCs, the HEMFC technology is in its early phases and there is a continuous effort to improve the performance and durability through the advancement of materials and optimization of operating conditions.
The polarization curve is one of the most common methods of testing a fuel cell. A typical polarization curve for a fuel cell consists of open circuit potential (crossover losses), low-current behavior (kinetics losses), moderate-current behavior (ohmic losses), and high-current behavior (transport losses). Crossover and ohmic losses are determined by electrolytes, kinetics losses depend on catalysts and transport losses involve gas diffusion and water management. In addition, a triple phase boundary comprised of an electrolyte, an electrode and a gaseous reactant is crucial for the electrochemical reactions. Thus, the optimization of all these aspects is critical for HEMFC performance improvement.
Here, we focus our work on transport phenomena in a fuel cell. The materials we used are hydroxide exchange membranes (HEMs) and ionomers (HEIs) based on poly(aryl piperidinium) (PAP).2 The specific chemical structure (shown in Figure 1) endows PAP HEMs with excellent chemical stability, high conductivity and mechanical robustness, making PAP HEMs/HEIs one of the best choices for HEMFC electrolyte. Furthermore, the use of PAP HEMs/HEIs enables operating HEMFCs at 95 °C, which accelerates reaction rates and decreases heat rejection.
Optimization of fuel cell operating conditions is vital for water management. Water production by hydrogen oxidation reaction (HOR) tends to cause flooding in the anode, while water consumption from oxygen reduction reaction causes cathode to dry-out. In addition, the electro-osmotic drag pulls water from the cathode to the anode (in the reverse direction of PEMFCs), thus worsening the water management problem further. In this work, observable characteristics in the polarization curve for a flooded HEMFC were found, and a model was developed to describe this issue. Then we demonstrate that balanced water management can be obtained by adjusting the anode relative humidity and back pressure.
Limiting current technology is widely employed for PEMFCs to characterize the transport resistance in order to reduce mass-transport losses; however, no one has done this systematically to HEMFCs, although there is a difference between limiting current behavior of PEMFCs and HEMFCs. We have found this technique useful to screen catalysts, optimize catalyst loading and adjust ionomer loading to improve the efficiency of gas diffusion, which is very helpful when switching from oxygen to air on cathode.
Our optimized HEMFC can achieve a peak power density of 2.02 W cm-2 in H2/O2 and 1.33 W cm-2 in H2/air with platinum group metal (PGM) based catalysts (shown in Figure 2). The current density at 0.663 V for cell voltage is about 1.70 A cm-2, which is suitable for hydrogen fuel cell vehicles.
References
B. P. Setzler, Z. Zhuang, J. A. Wittkopf, and Y. Yan, Nat Nanotechnol, 11 (12), 1020-1025 (2016).
J. Wang, Y. Zhao, B. P. Setzler, S. Rojas-Carbonell, C. Ben Yehuda, A. Amel, M. Page, L. Wang, K. Hu, L. Shi, S. Gottesfeld, B. Xu, and Y. Yan, Nature Energy, (2019) doi:10.1038/s41560-019-0372-8.
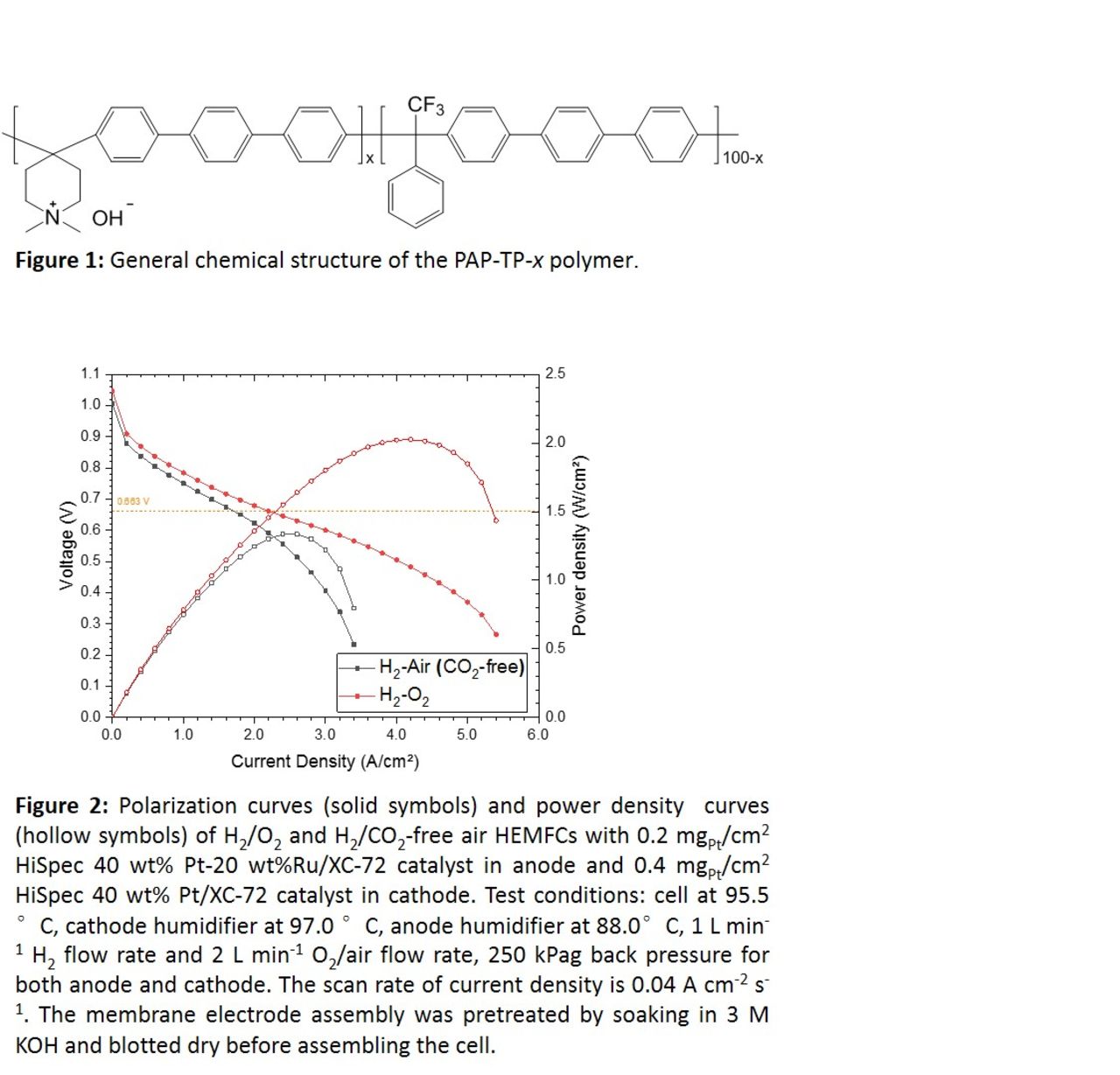
Figure 1
