Abstract
The demand for lithium-ion batteries continues to grow as the need for renewable energy increases. Over the past few decades, lithium-ion batteries have seen widespread adoption in portable electronics, and they are increasingly being used in electric vehicles. To satisfy the energy density needed for these next-generation applications, new anode materials with high-energy densities are required. Traditional graphite is a very stable intercalation-based anode, but its low capacity has driven many researchers to identify alternatives. Silicon (Si) has one of the highest gravimetric energy densities known when alloyed with lithium. Unlike graphite which is limited to 1 lithiumion per 6 carbon atoms (LiC6), the lithium-silicon alloy Li15Si4 has nearly 4 lithium ions per silicon atom. This gives silicon a theoretical capacity of 3579 mAh/g, compared to the 372 mAh/g for graphite. However, the challenge with silicon (and most alloy anodes) is its poor stability— as the alloying of lithium and silicon is accompanied with a 300% volume expansion. On a microscopic scale, this causes material/electrode swelling and pulverization, which in turn causes severe capacity fade in a few cycles.
The binder is known to play an important role in the cycle stability of silicon-based anodes for lithium-ion batteries. Polyvinylidene fluoride (PVDF) has traditionally been the binder used in the preparation of both graphite anodes and lithium metal oxide cathodes. It is electrochemically stable; that is, it is not reduced at the low potential of the anode (~5 mV vs Li) and is not oxidized at the high potential of the cathode (5 V vs Li). However, PVDF has three main disadvantages. First, PVDF cannot accommodate the large volumetric expansion of silicon lithiation. As a result, batteries using PVDF as a binder with silicon will experience a very rapid drop in capacity. Second, PVDF is not water soluble and requires the use of organic solvents like N-Methyl-2-pyrrolidone (NMP) during electrode processing. Finally, the cost of PVDF is approximately 20 USD/kg, which is several times greater than competing binders like sodium carboxymethylcellulose (NaCMC) at 6 USD/kg.
Next-generation binders should be inexpensive, able to accommodate the expansion of silicon, and use environmentally friendly processing. Natural biopolymers (such as NaCMC and xanthan gum XG) are a promising class of binders that offer several advantages over traditional polyvinylidene fluoride (PVDF). Biopolymers contain many carboxylate and hydroxyl functional groups which aid in the formation of noncovalent bonding with the oxide surface of silicon. In particular, xanthan gum is a high molecular weight polysaccharide produced extracellularly by the bacteria Xanthomonas campestris. It is water soluble and thus eliminates the need for organic solvents. In addition, its high molecular weight is known to provide better capacity retention, owing to the long molecular chains that can accommodate silicon expansion. While existing studies have explored the fundamental properties of these biopolymer binders and their interaction with silicon, there has been little research on the use of these binders under realistic processing conditions (≤ 10 wt% binder). Herein, we optimize the electrochemical performance of both NaCMC and XG-based silicon electrodes with a low binder content. In addition, we report results for both a high-silicon (≥80 wt%) and a practical low-silicon (20 wt%) composite, all while using nano silicon prepared by industrial-scale synthesis. Figure 1 below shows the capacity retention of different electrode preparations. A higher initial capacity is expected with 80% silicon. However, the overall stability is superior for the low silicon-high graphite (G) composites.
Keywords: high-capacity anodes; binders; biopolymers; silicon; lithium-ion batteries
Figure 1. Capacity vs cycle number for 1st Generation Si-based electrodes. Cycled at C/10.
References
H. Yim, S. Niketic, N. Salem, O. Naboka, and Y. Abu-Lebdeh, J. Electrochem. Soc., 164, A6294–A6302 (2017).
Zhao, S. Niketic, C. H. Yim, J. Zhou, J. Wang, and Y. Abu-Lebdeh, ACS Omega, 3, 11684–11690 (2018).
M. Courtel and Y. Abu-Lebdeh, "Use of Xanthan Gum as an Anode Binder," Patent 10,483,546 B2, 2019.
Li, Z. G. Wu, Y. M. Liu, Z. W. Yang, G. K. Wang, Y. X. Liu, Y. J. Zhong, Y. Song, B. H. Zhong, and X. D. Guo, Ionics (Kiel)., 27, 1829–1836 (2021).
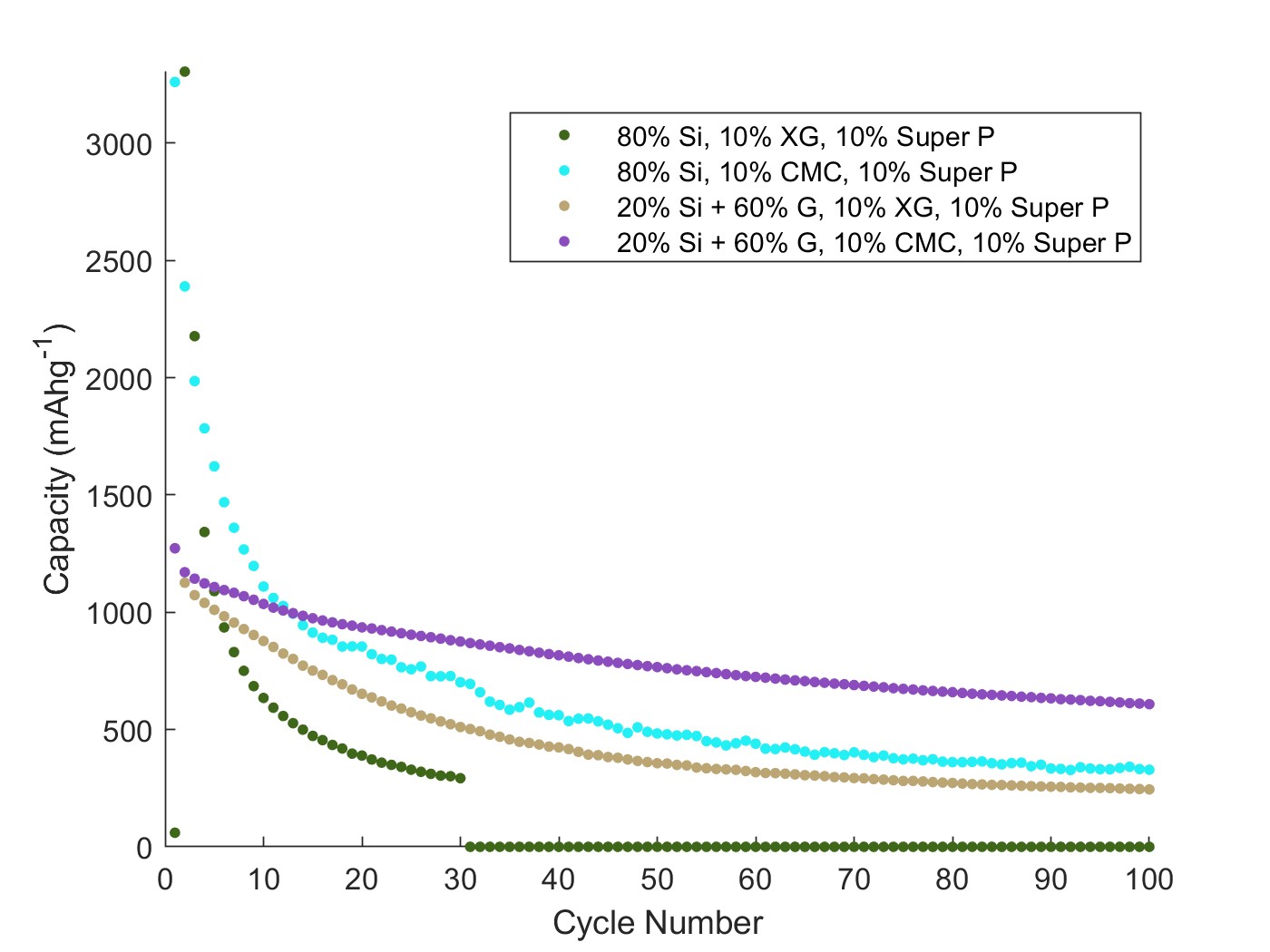
Figure 1
