Abstract
1. Introduction
Pyroprocessing is a promising technology for a fast reactor cycle with a highly concentrated minor actinides (MA: Np, Am and Cm)-bearing metallic fuel. Separation of actinides (An) from fission products such as lanthanide elements (Ln) is one of the difficult challenges to establish practical pyroprocessing. Liquid Cd cathode (LCC) with LiCl-KCl bath has been generally used for An recovery in our process [1], however, An/Ln separation performance using LCC was not high enough to fabricate a highly concentrated MA-bearing fuel from materials having a high ratio of Ln/An. Our recent studies showed that a combination of liquid Ga cathode with LiCl-KCl bath had potential to give a higher An/Ln separation performance, and currently we are developing an innovative pyroprocessing using the liquid Ga electrode. Solubility of U and Pu in liquid Ga is smaller than in liquid Cd. Therefore, the solid precipitates (An-Ga alloy) formation could have a significant influence on designing the process compared to the case of using LCC. However, the influence of the solid precipitates formation on An/Ln separation performance is unclear.
In this study, galvanostatic electrolysis test on the recovery of U, Pu and Am in liquid Ga was carried out to investigate the influence. Precipitation of Pu-Ga alloy was observed by SEM/EDX on the cross section of Ga electrode after the electrolysis, and separation factors (SFs) for each metallic elements (M) based on Ce, which is defined as (M/Ce concentration ratio in liquid Ga)/(M/Ce concentration ratio in the salt phase), were calculated to evaluate An/Ln separation performance.
2. Experiment
All experiments were performed in glove boxes with high-purity Ar atmosphere. Cd-0.1wt%Li alloy anode, liquid Ga cathode and Ag/AgCl reference electrode were used in LiCl-KCl eutectic melt at 773K. Two runs of galvanostatic electrolysis experiments were carried out applying the constant current of 5~10 mA (cathodic current densities: 3.76~7.53 mA/cm2). In the first experiment, An were recovered in the liquid Ga electrode from LiCl-KCl melt containing An chlorides, where concentrations of recovered An in the liquid Ga were almost the solubility. In the second experiment, two times larger amounts of An than the solubility were recovered in liquid Ga from LiCl-KCl melt containing both An and Ln chlorides. After the electrolysis, the Ga and the molten salt were sampled and dissolved into nitric acid. Concentrations of elements in the samples were analyzed by ICP-AES. γ spectrometry was also used to analyze Am concentration in samples. Cross section of solidified Ga electrode was observed by SEM/EDX.
3. Results and discussion
It was confirmed that SFs of An seen in Table 1 were around ten times higher values than those obtained in the case of LCC. Furthermore, the SFs of An in the case of the amount of An recovered in liquid Ga exceeding solubility (second experiment) were comparable to those in the case of the An concentration below solubility. The SEM/EDX observation showed that grain shaped precipitates with 1~10 μm was formed homogeneously inside the Ga and the size of the precipitates increased with the amount of recovered An in liquid Ga. Chemical form of the precipitates was assigned to be Pu-Ga alloy by the EDX. It was also found that the precipitates did not accumulate on the surface of liquid Ga. In the present experimental conditions, the amount of An in the liquid Ga had an influence on the precipitation formation, while the precipitation had no influence on An/Ln separation performance.
Acknowledgement
This work is supported by the Innovative Nuclear Research and Development Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Reference
[1] S. Kitawaki, et al. Nucl. Technol., 162 (2008) 118-123.
[2] T. Murakami et al., J. Nucl. Radiochem. Sci., 16 (2016) 5-10.
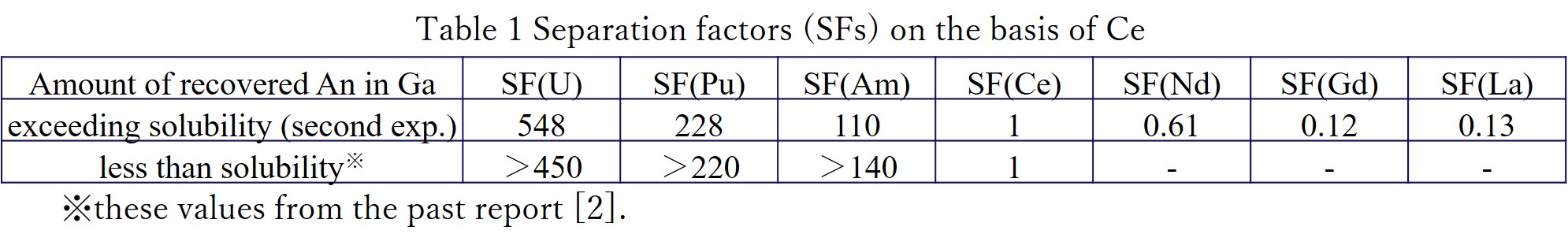
Figure 1
