Abstract
Nanocrystalline coatings of a Sn-Sb-Ni-CNT composite were applied on titanium mesh using a dip coating and annealing procedure, and investigated as anode material for ozone generation in perchloric acid. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to reveal the electrode composition and morphology, and voltammetry and current efficiency measurements for electrochemical characterization. Addition of CNT resulted in coatings with a higher loading, a higher onset potential for oxygen evolution and in a higher current efficiency for ozone generation. A long term test of the CNT-containing electrodes at a constant current density (53.5 mA/cm2) for 17 h indicated a stable anode potential.
Export citation and abstract BibTeX RIS
In recent years there has been an increasing interest in ozone generation, mainly due to its high oxidation power. Ozone is an environmentally clean reagent as its decomposition into O2 at normal temperature leaves non-toxic products. Among its many applications, ozone is commonly used in water and waste treatment and for wood pulp bleaching.
Electrochemical ozone production (EOP) has advantages over the classic corona method for ozone production, for example high concentrations in the gas and liquid phases, simple system design and low voltage operation. In the electrochemical process, ozone is generated at the anode surface. However, ozone generation severely competes with O2 evolution which is thermodynamically favorable. (see Eqs. 12):
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/159/5/D265/revision1/jes_159_5_D265eqn1.jpg)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/159/5/D265/revision1/jes_159_5_D265eqn2.jpg)
So, to reach high ozone yield and current efficiency (CE) the inhibition of O2 evolution is required. This can be achieved in several ways, such as by suitable choice of electrode material (high oxygen overpotential anodes are favorable to minimize the oxygen evolution), operating temperature and of the electrolyte composition, in particular the use of electrolyte additives that partially inhibit the oxygen evolution reaction (OER) via the blocking of its active sites. See references by Foller et al.1 and by Da Silva et al.2 for reviews on EOP.
Various anode materials have been studied for electrochemical ozone production such as platinum,1 PbO2,3–5 Si/TiOx/Pt/TiOx,6,7, Pt-TaOx,8 boron doped diamond (BDD),9 and glassy carbon.10 Recent reports on mixed oxide electrodes of Sn, Sb and Ni prepared by thermal decomposition on titanium show high current efficiency, depending on operating conditions, at moderate temperatures and anode potentials.11–14 In particular, operation using a flow through cell showed remarkably high current efficiencies of up to 50%.12 Wang et al.11 varied the composition of the coating solution and concluded an optimum molar ratio of Sn:Sb:Ni of 500:8:1for the highest current efficiency of ozone.
Carbon nanotube (CNT) based composites are advanced materials that are attracting much attention because of their unique structures and mechanical properties, also CNTs can conduct electrons, have high adsorption capacities and high surface areas. Doping TiO2 films with CNT has shown to increase the photoelectrolytic catalytic activity, compared to undoped TiO2.15,16, Sn-Sb oxide coatings doped with CNT showed an increased efficiency for electrochemical phenol oxidation compared to undoped oxides.17 In the same study accelerated life time tests indicated that additions of CNT can increase the service life for the Sn-Sb oxide coatings. Studies of composites of metal oxides with CNTs for supercapacitors have shown positive effects of the CNTs, for example an increased electrical conductivity for Co-oxide18 and increased capacitance for metal oxide-CNT composites.18,19,
The goal of the present work is to improve the electrocatalytic activity and efficiency of anodes for electrochemical ozone generation by using CNTs as a dopant in a Sn-Sb-Ni-CNT composite coating on a Ti substrate.
Experimental
A titanium mesh (20 mm × 20mm) was degreased with acetone, rinsed in Millipore water, contacted with boiling oxalic acid for 1 hour and cleaned in an ultrasonic bath in Millipore water for 1 hour. Metal salts, SnCl4 · 5H2O (98%, Sigma Aldrich), SbCl3 (99.5%, Sigma Aldrich), NiCl2 · 6H2O (98%, Merck) were dissolved in 99.5% ethanol, where after concentrated HCl was added dropwise until the solution was clear. In the case of CNT-composite preparation multi walled CNT (MWCNT) with O.D:10-15nm, I.D:2-6nm and length 0.1-10 μm (90%, Sigma Aldrich) were then added, and the solution was sonicated for 2 hours. The titanium mesh was coated by dipping, followed by drying at 110°C for 15 min, and then annealing at 520°C for 30 min. This was repeated 20 times, the last of which the annealing lasted for 1h. All results, except for the UV-vis spectra in Figure 7, refer to the electrodes coated 20 times. The molar ratio of Sn-Sb-Ni-CNT was controlled to be 500/8/1/10 in the coating solution, where the molecular weight for carbon (12 g/mole) was used for the molar ratio of CNT. Four different batches of electrodes were prepared, all containing coatings with as well as without CNT.
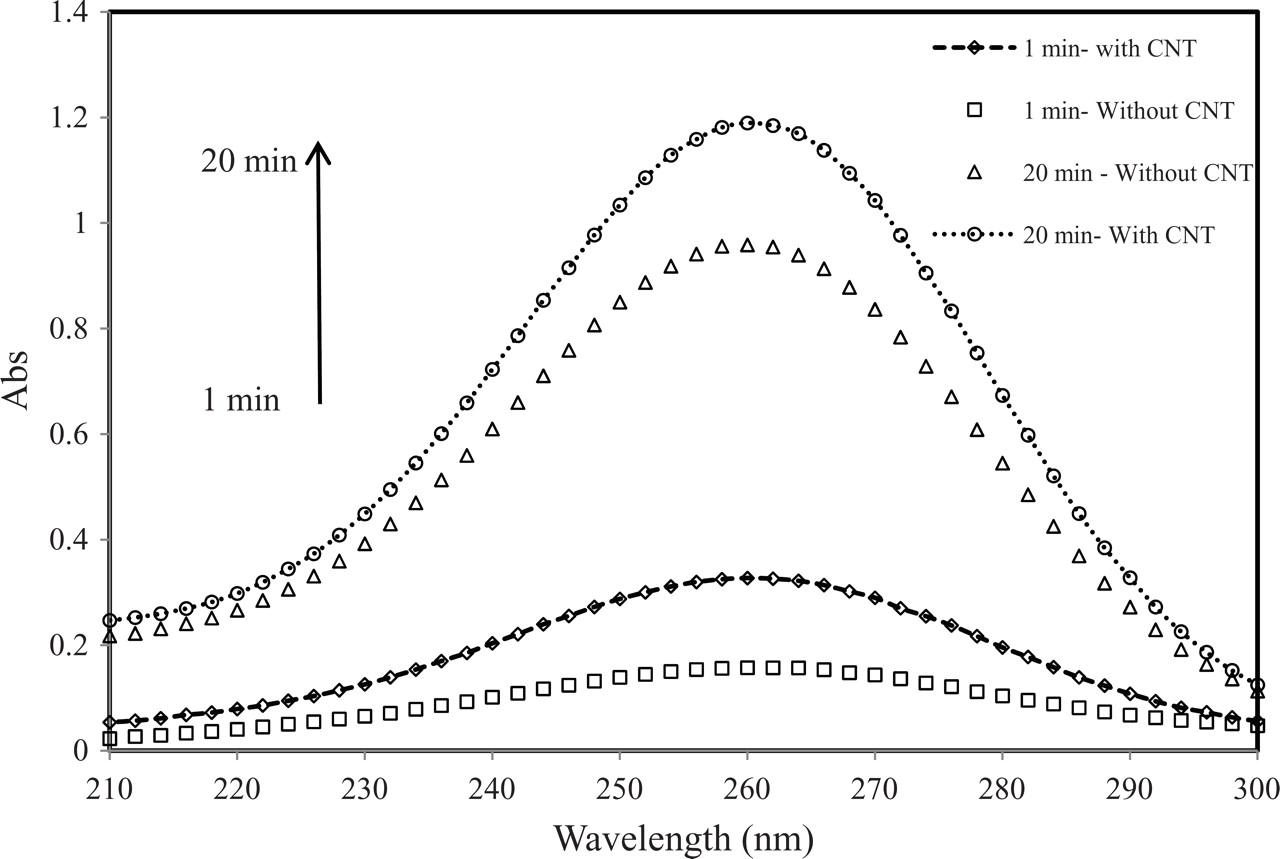
Figure 7. UV-Vis spectra of the electrolyte during electrolysis for coatings with and without CNT.
An Ag/AgCl (KCl sat.) electrode was used as the reference electrode and platinized titanium as counter electrode. Electrogeneration of ozone was performed galvanostatically in a jacketed undivided cell with volume 250 mL. Temperature was controlled by a Julab VC 12B (Germany) water bath. Electrolysis experiments were carried out with a potentiostat/galvanostat AUTOLAB PGSTAT 302N. The electrolyte was made from HClO4 p.a. grade from Merck and Millipore water. The concentration of dissolve ozone was determined using a UV/Vis spectrophotometer (Ultraspec 3000, Pharmacia Biotech, England). For the spectrophotometer 1 mg/ L dissolved ozone corresponds to an absorbance of 0.098 at the wavelength of 258 nm.11,14, The current efficiency (CE) of the ozone generation was determined from the following equation7 (Eq. 3)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/159/5/D265/revision1/jes_159_5_D265eqn3.jpg)
where nExp and nth (Qth/zF) are the number of moles of O3 obtained experimentally (0.098 × absorption at the wavelength of 258 nm) and calculated theoretically, respectively, z is the number of electrons (equals six in the present case, see Eq. 1), and Qth equals I· t (where I is the current applied for a time period t). The gas leaving the electrochemical cell was not analyzed. Therefore the ozone current efficiencies reported here may be underestimations, as some of the ozone produced may have ended up in the gas phase. Each experiment was repeated at least once.
X-ray diffraction (XRD) patterns of the coating films were recorded on a XRD instrument (Siemens D5000, XRD), with an operating voltage of 40 kV and current of 30 mA and 2θ = 10–90°. Surface morphology was characterized using a Ultra-High Resolution FE-SEM Hitachi S-4800.
Results and Discussion
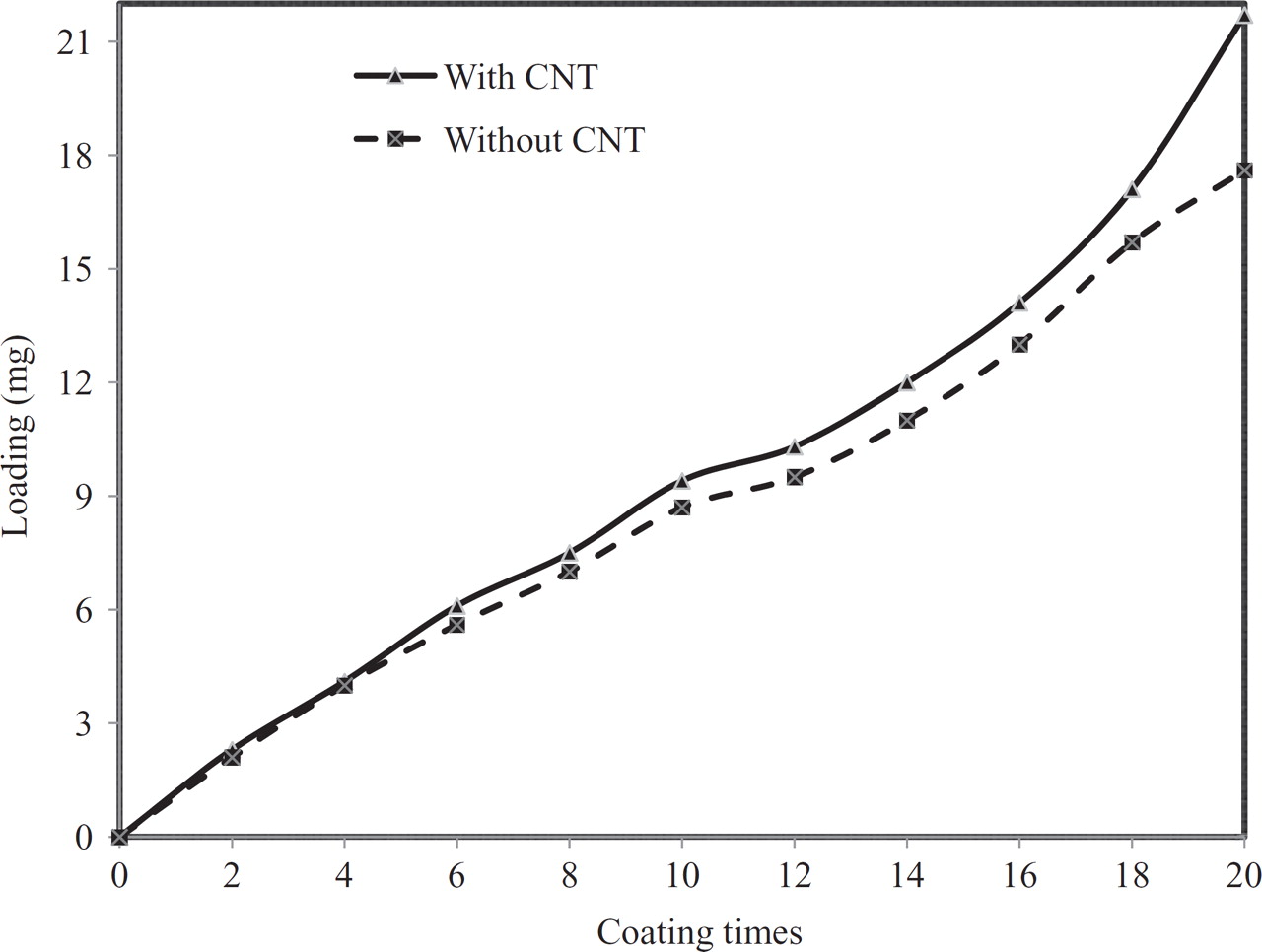
As seen in Figure 1 the loading in the dip coating procedure reached higher values when CNT was present in the coating solution, and the effect became more pronounced with increasing number of layers. All four sets of electrodes showed the same trend. For the coatings shown in Fig. 1 the final loadings, after 20 layers, were 7.75 mg/cm2 for the Sn-Sb-Ni-CNT composite and 6.29 mg/cm2 for the Sn-Sb-Ni coating.
Figure 1. Loading as a function of coating times during the dip coating procedure.
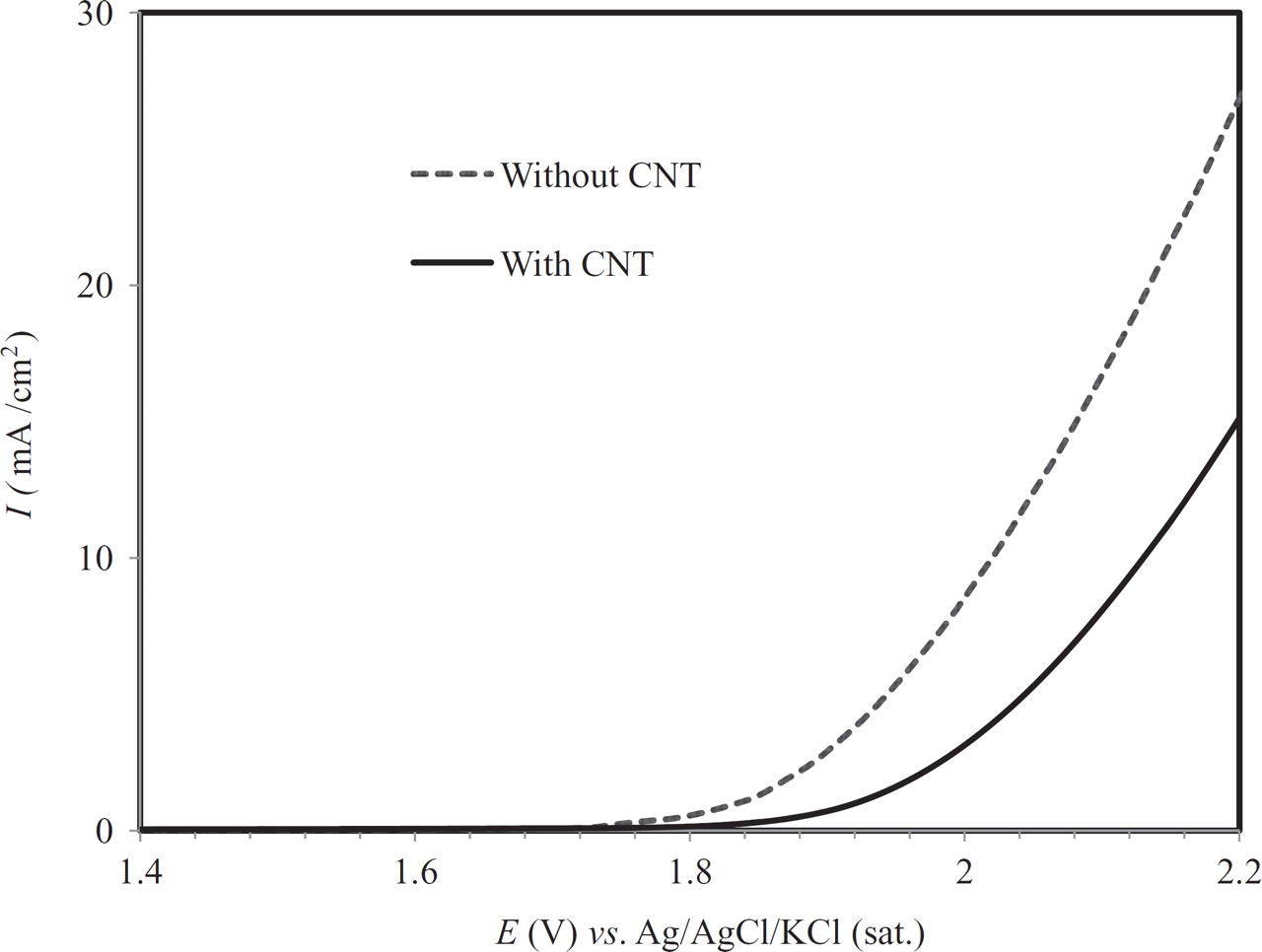
Anodic linear sweeps in perchloric acid in Fig. 2 indicate a difference in the onset potential of the oxygen evolution between the electrode with CNT (∼1.9 V vs. Ag/AgCl) and without CNT (∼ 1.75 V vs. Ag/AgCl), thus the kinetics for oxygen evolution was strongly suppressed on both materials. The rate of oxygen evolution reaction (OER) would be considered when comparing the performance of a set of electrodes toward electrochemical ozone production (EOP), as a low rate of OER is favored. The rate of oxygen evolution on the electrode with CNT was lower than on the other electrode, particularly at high potentials typically used for electrochemical ozone production (EOP).
Figure 2. Anodic linear sweeps at a scan rate of 50 mV/ s in 0.1 M HClO4 at 20°C.
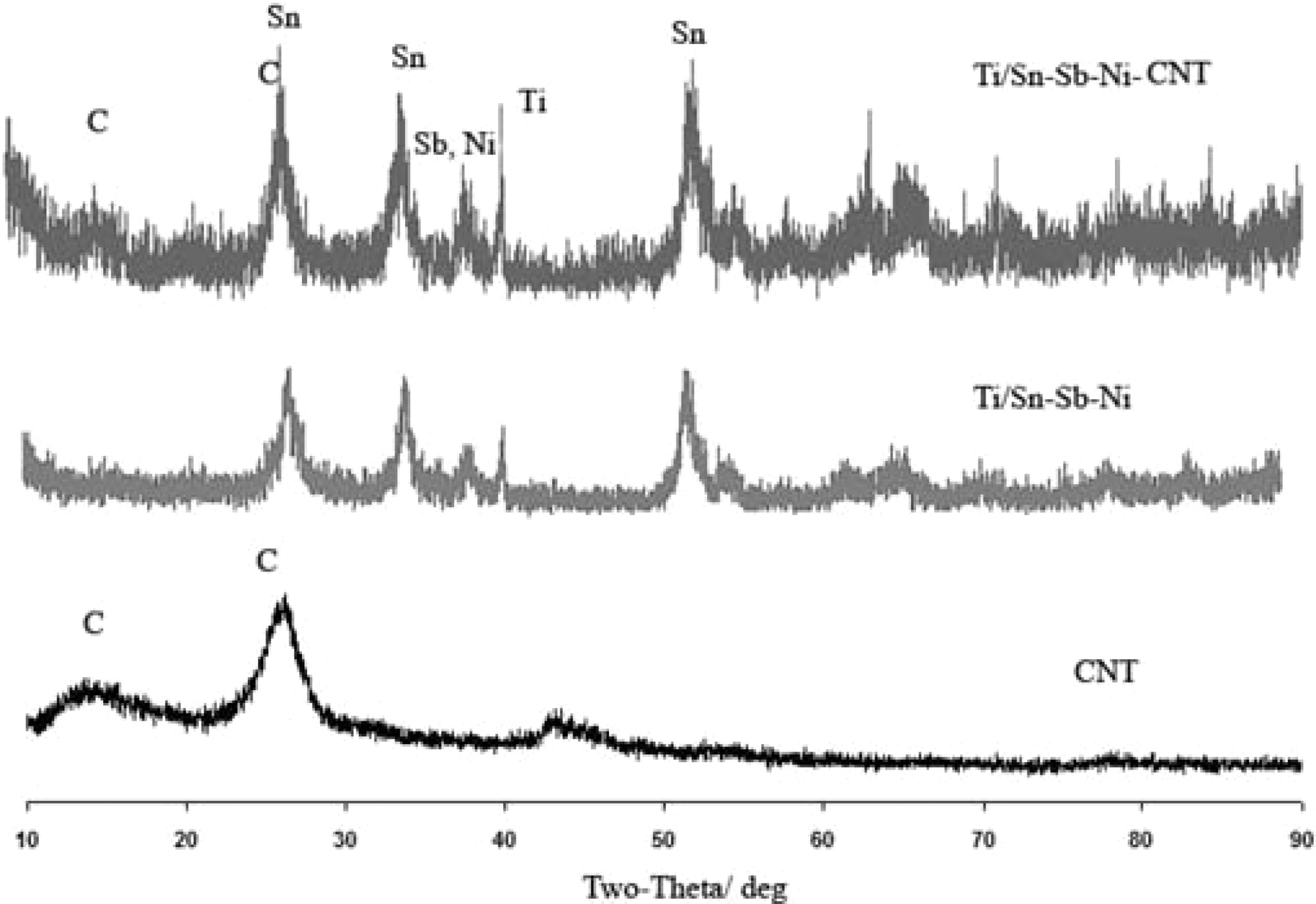
If reversing the sweeps in Fig. 2, cyclic voltammetry (CV), no peaks or shoulders were found. There was no cathodic current on the reverse scan, and consecutive CV scans were reproducible. In Figure 3 XRD spectra for CNT and the two types of coatings produced are given. The crystal size and structure of the composite are important factors for the efficiency of the anode, especially when the crystal size is decreased to the nanoscale.17,20, The average grain sizes were calculated by Scherrer equation:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/159/5/D265/revision1/jes_159_5_D265eqn4.jpg)
Figure 3. XRD spectra of the Ti/Sn-Sb-Ni with and without CNT as well as for CNT.
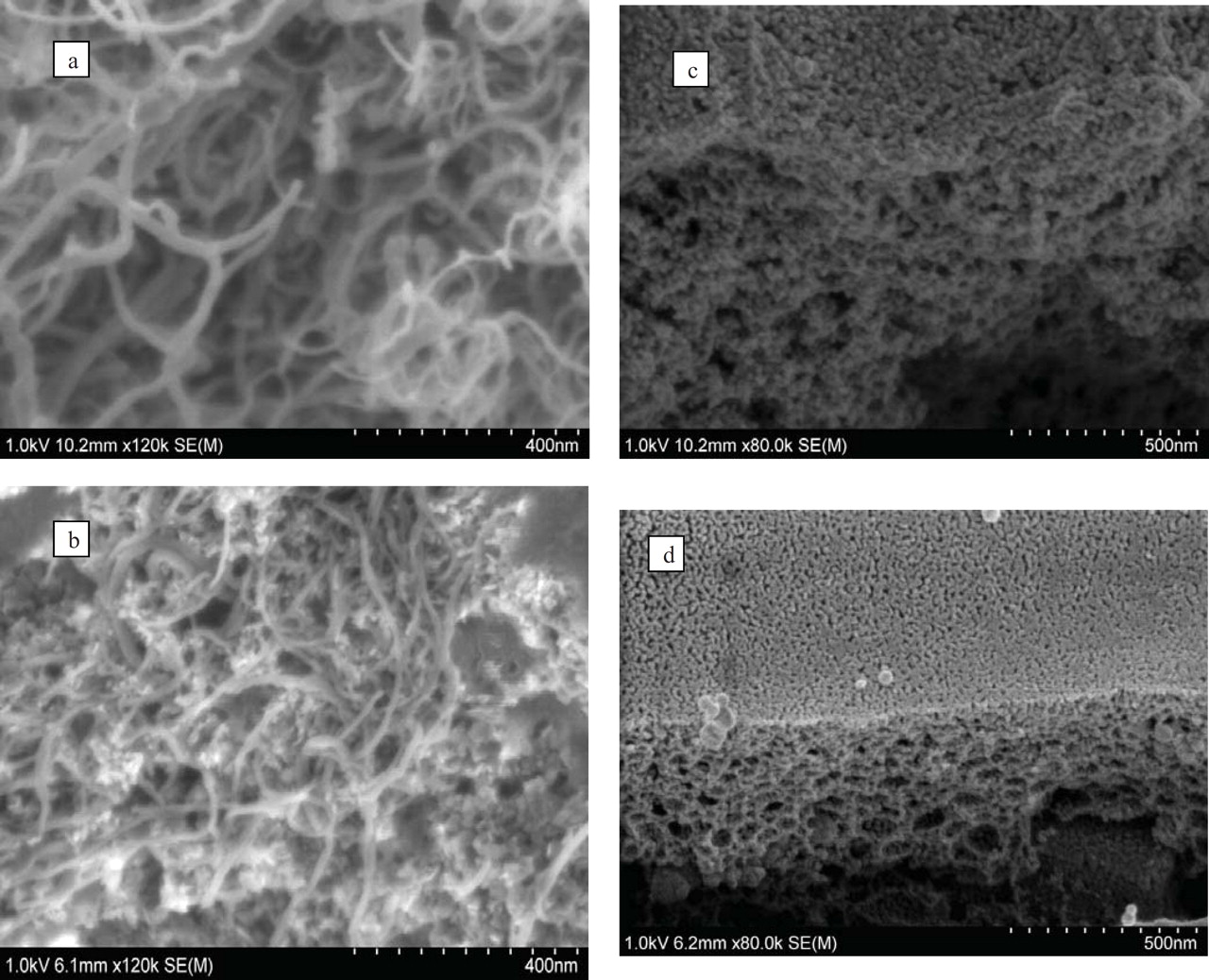
In Eq. 4 L is average grain size (nm), k is Scherrer constant, λ is the wavelength of X-ray, fwhm is obtained from the diffraction peak width at half maximum, θ is the diffraction angle (rad). The average particle size of the composite, L, was under 10 nm and it was confirmed in SEM images, see Figure 4.
Figure 4. SEM images of (a) neat CNT (b) CNT and metal salts in dried coating solution (c) Ti/Sn-Sb-Ni coating (d) Ti/Sn-Sb-Ni-CNT coating.
The SEM morphologies of the samples with and without CNT are presented in Fig. 4. Image a shows neat CNT and image b shows CNT after drying a sample of the coating solution, thus containing Sn-, Sb- and Ni-compounds. Images c and d show the electrode coating morphology without and with CNT, respectively. In a smaller magnification (not shown) both coatings had mud cracked appearances with homogeneously distributed cracks on their surfaces. As seen in the cracks of Fig. 4c, coatings with CNT showed a higher porosity. Pores with diameters of approximately 50–200 nm were more frequent in the CNT-containing coatings than in the coatings without CNT.
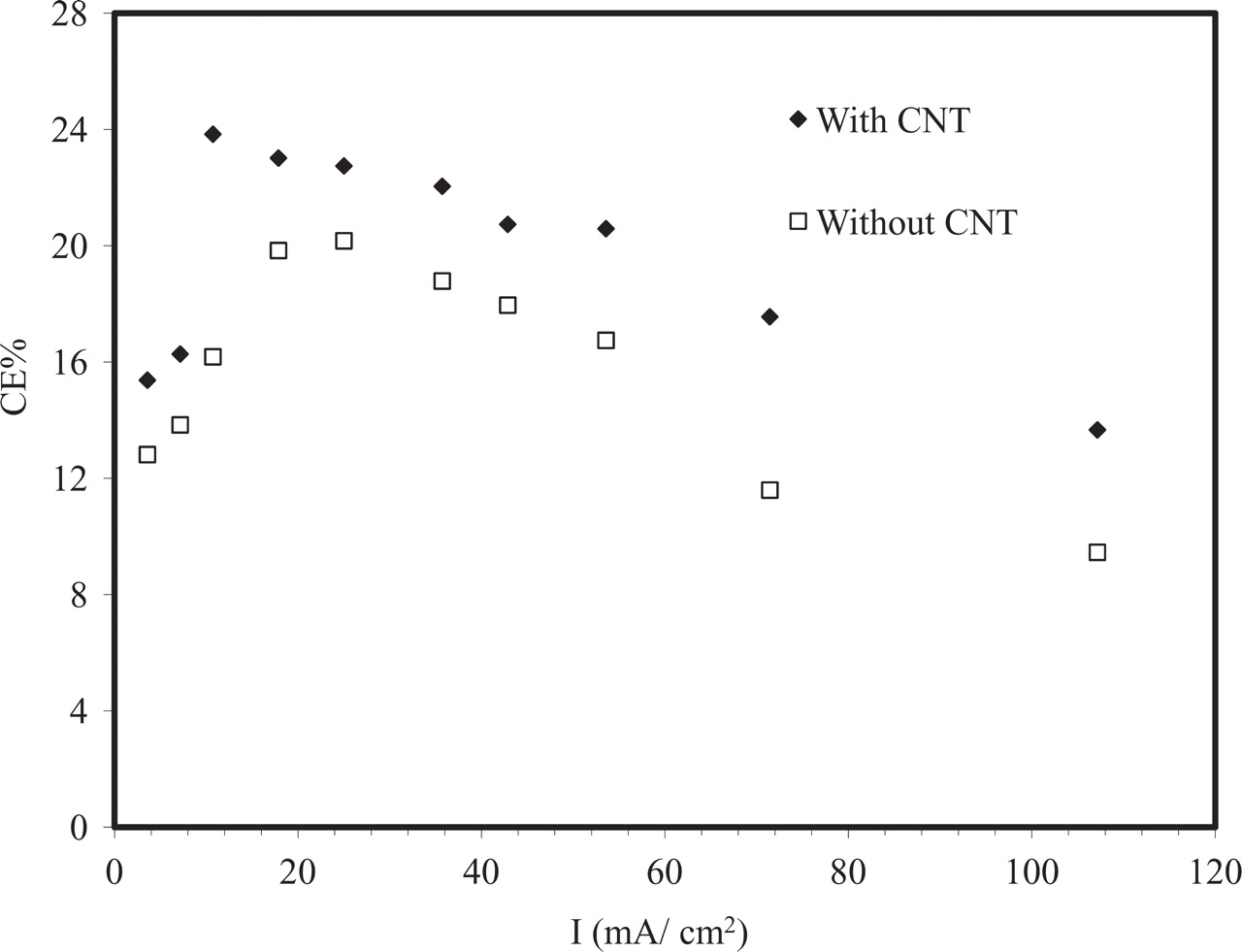
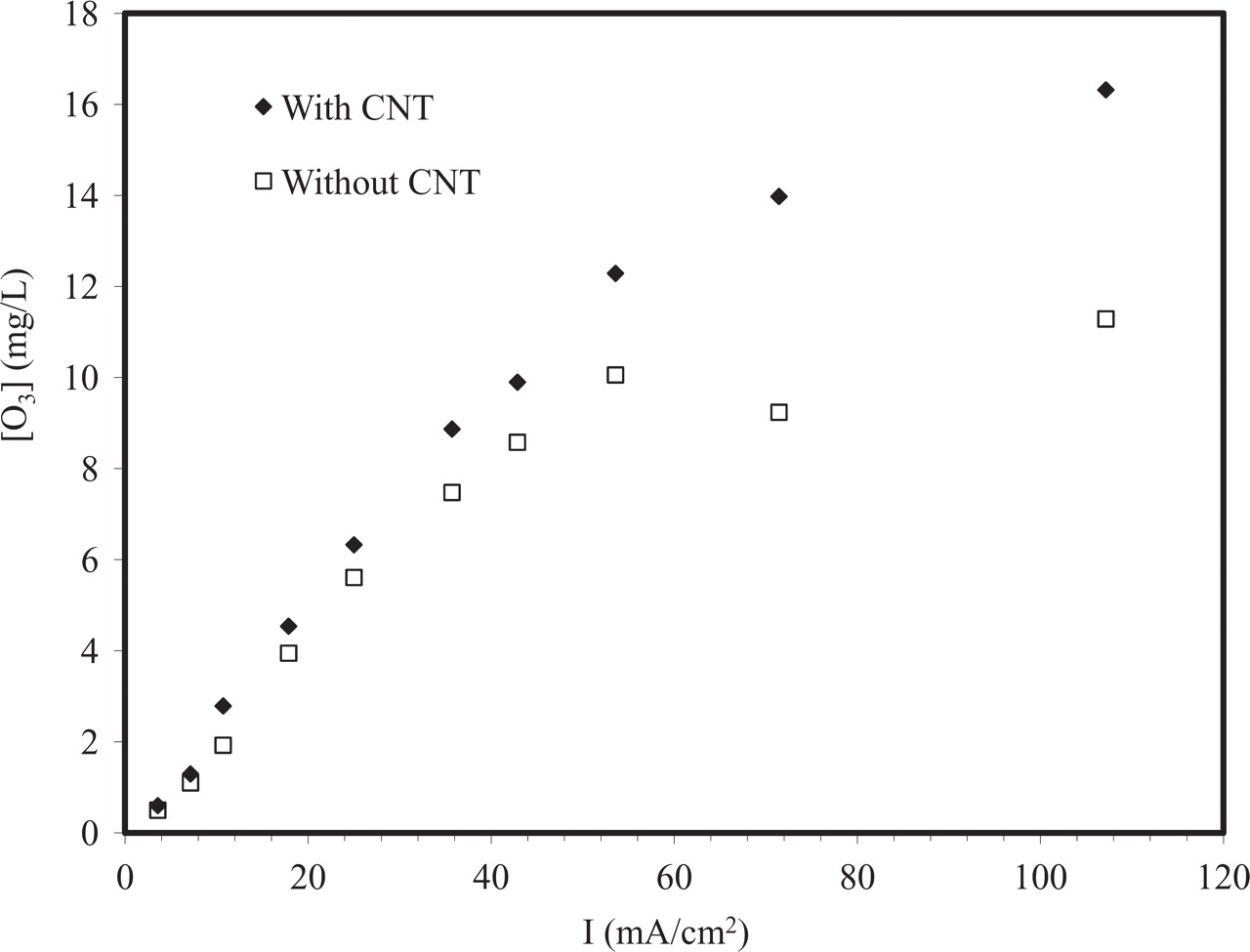
The current efficiency (CE%) and concentration of ozone generated as a function of the current density are shown in Figures 5 and 6, respectively. The results show that when using Ti/Sn-Sb-Ni-CNT electrode as anode, the current efficiency reached 24% and the ozone concentration reached over 17 mg/L after 20 min electrolysis time. At the same conditions, the Ti/Sn-Sb-Ni anode reached 16% CE and an ozone concentration of 10 mg/ L. At this stage the reason for the beneficial effect of CNT is not clear and some possible explanations are briefly discussed follow. The slightly higher loading for the CNT containing coatings may have an impact, as in an earlier study14 the CE% for ozone generation increased with increasing coating thickness for Ni-Sb-SnO2 coatings produced by dip coating. Also, the higher porosity observed for the CNT-containing coatings may lead to more efficient transport of reactants and products, in particular ozone, in the electrode structure. Suggested reaction mechanisms for electrochemical ozone generation involve several adsorbed intermediates,5 and incorporated CNT may affect the adsorption properties of the coatings.
Figure 5. Effect of current density on the current efficiency after 20 min polarization in 0.1 M HClO4 at 20°C.
Figure 6. Effect of current density on the ozone concentration after 20 min polarization in 0.1 M HClO4 at 20°C.
The decrease of CE% with increasing the electrolysis current density is usually observed for EOP and is generally explained by an increase in local heating at the electrode, see discussion in section 3.3 about temperature effects on CE. Another possible reason is related to the competition of side reactions. Increasing the electrolysis current, leading to a higher anode potential, may increase the number of active sites for OER on the expense of EOP and thus lowering the relative rate of EOP compared to the rate for OER. This would lead to decreased efficiency for ozone generation with increased current density in the high current region.6
Figure 7 shows UV spectra of the ozone containing electrolyte, where the first spectrum was collected after 1 minute of electrolysis. The peak absorbance increased with electrolysis time and was consistently higher for electrolysis with the CNT-containing electrodes compared to when electrodes without CNT were used.
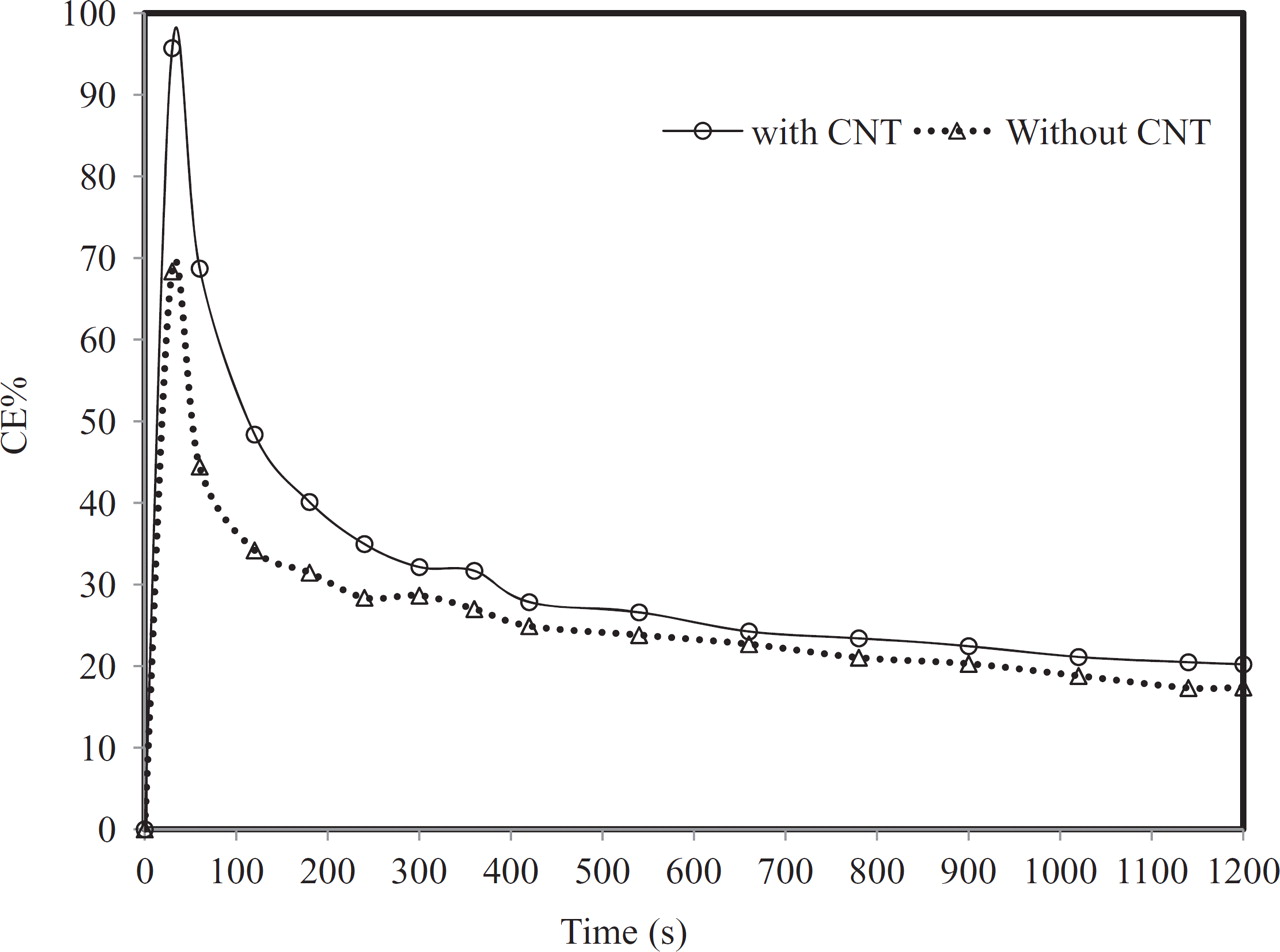
The change of CE% with increasing electrolysis time is shown in Fig. 8. The CE reached over 95% after 60 sec of electrolysis, and then quickly declined to below 30% after 1200 sec. The reason for this peak in CE% may be related to the buildup of reaction intermediate concentrations until steady state conditions. Similar peaks were found in a previous study.14
Figure 8. Influence of CNT doping on the CE% in 0.1 M HClO4 using a constant current of 53.5 mA/ cm2 for 20 min at 20°C.
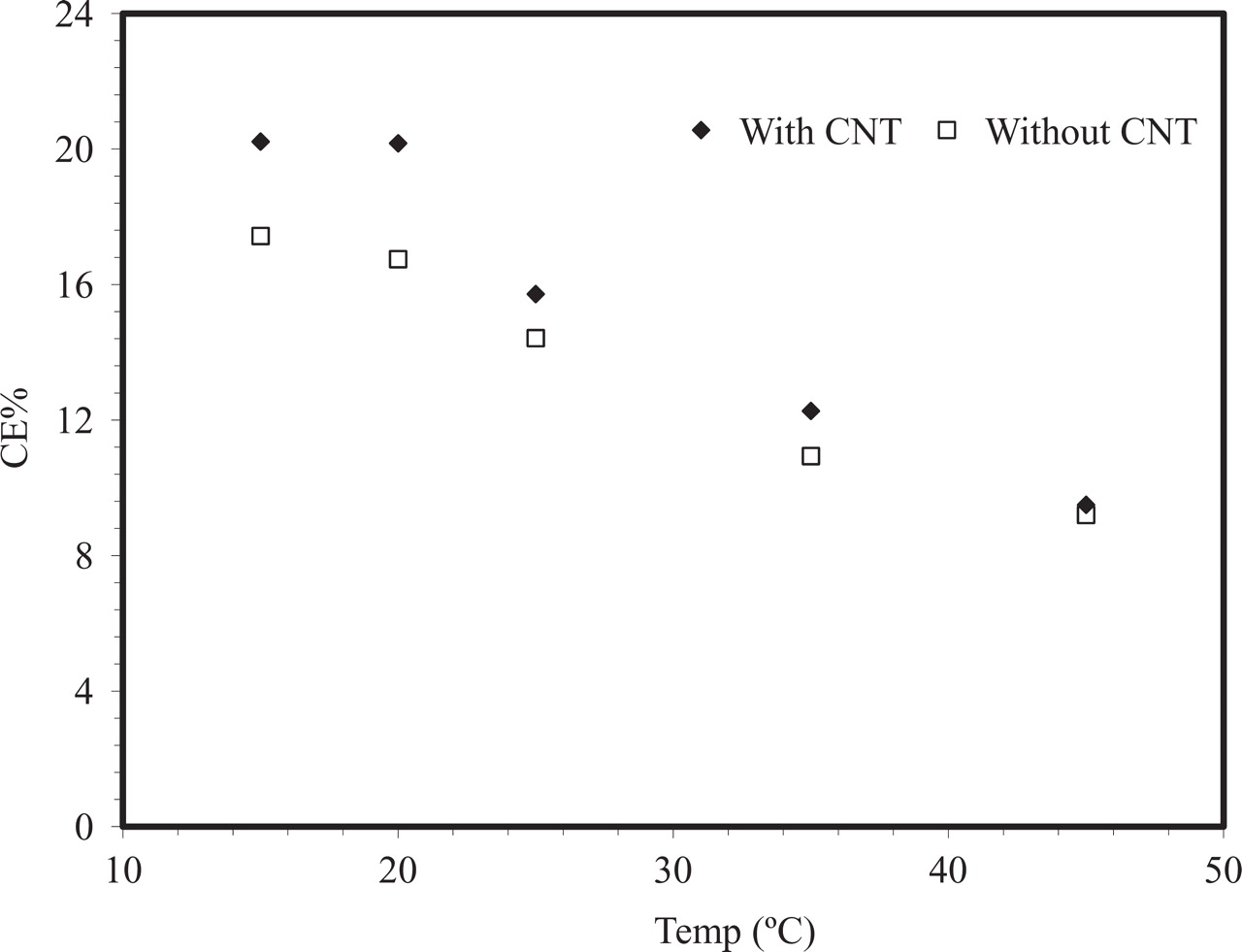
The influence of the temperature on EOP depends on electrode material and on the nature of the electrolyte. Temperature vs CE% curves between 15°C and 45°C given in Fig. 8 show that CE% values decreased with increasing temperature. This phenomenon is usually found in studies of EOP, and has been attributed to a lower O3 decomposition rate as the temperature is decreased.5 The reason may also be lower rate of decomposition of a reaction intermediate of EOP at lower temperature. Electrogeneration of other highly oxidized species, such as perchlorate21 and peroxodisulfate22 in aqueous solutions with competing oxygen evolution, have also shown increased current efficiencies when lowering the temperature. As seen in Fig. 9 the positive effect of CNT on CE% is more pronounced at low temperature.
Figure 9. Influence of the temperature on the CE% in 0.1 M HClO4 measured after 20 min polarization at 53.5 mA/ cm2.
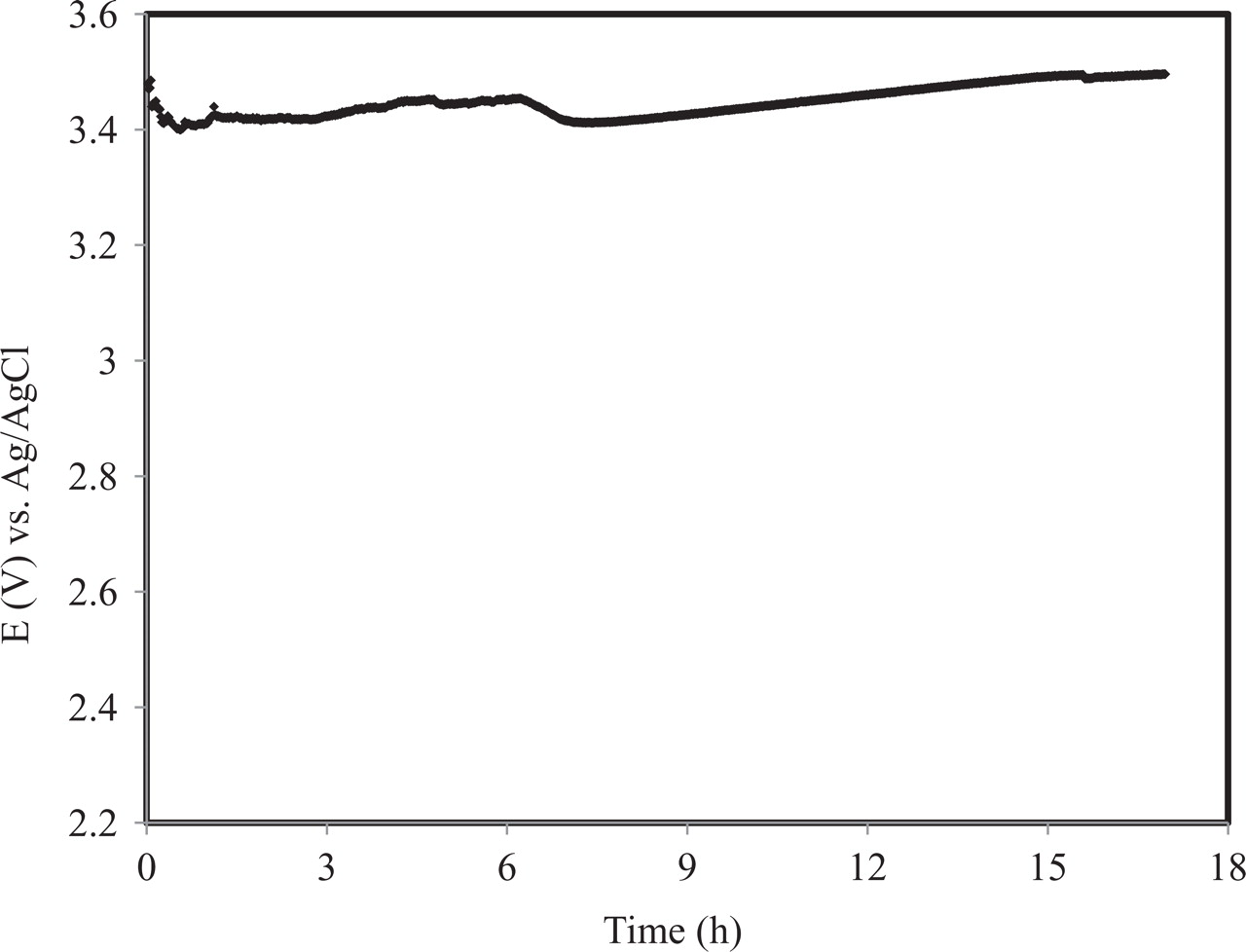
One of the important criterions is electrode stability especially when it is used in acidic media and high anodic potential. The stability of the electrode was indicated by measuring the change in the electrode potential with time upon applying a constant current density of 53.5 mA/cm2 in 0.1 M HClO4. The electrode potential decreased at the beginning and then remained fairly constant for a 4.5 h, see Fig. 10. The initial anodic potential which started at around 3.48 V (vs. Ag/AgCl) slowly decreased down to 3.44V at 4.5 h and then slowly increased up to 3.49 V after 17 h.
Figure 10. Chronopotentiometry, Ti/Sn-Sb-Ni-CNT electrode in 0.1M HClO4 at 53.5 mA/ cm2.
Conclusions
A dip coating and annealing procedure was used to synthesize nanocrystalline Ti/Sn-Sb-Ni coatings and Ti/Sn-Sb-Ni-CNT composite coatings for electrochemical ozone production. The electrodes were characterized by SEM, XRD and linear sweeps and in current efficiency measurements at varying temperature and current density. The addition of CNT resulted in coatings with a higher loading, a higher onset potential for oxygen evolution and in a higher current efficiency for ozone generation. The largest effect of CNT-addition on current efficiency was seen at low temperature and at high current density. Current efficiencies well over 20% were obtained for the CNT-containing coatings also which also indicated a relatively stable anode potential for at least 17 h at 53.5 mA/cm2.
Acknowledgments
Permascand AB is greatly acknowledged for providing titanium mesh used in the study and also the authors wish to express their thanks to Ministry of Science, Research and Technology of the Islamic Republic of Iran for financial support.