Abstract
Separators with ultra-high strength, superior heat-resistance and excellent electrolyte wettability are in urgent demand for the development of high safety and high-power density lithium-ion batteries. Herein in this paper, specially designed polyphenylene sulfide separator is prepared via thermally induced phase separation using binary diluent to improve the performance of LIBs for the first time. The effect of binary diluent composition on the polyphenylene sulfide separator performance is investigated by both computational simulation and experimental studies respectively. The as-prepared polyphenylene sulfide separator exhibits tortuous and highly porous structure, which endows the separator superior electrolyte wettability. Furthermore, it also results in higher ionic conductivity (1.69 mS cm−1), lithium-ion transference number (0.57) and interfacial stability of the polyphenylene sulfide separator. In particular, the polyphenylene sulfide separator presents excellent thermal dimensional stability (without shrinkage up to 280°C), self-extinguishing and ultra-high strength (tensile strength of 119 MPa, Young's modulus of 7.55 GPa) benefiting from the high performance polyphenylene sulfide. More importantly, the LiFePO4/Li cell using the polyphenylene sulfide separator shows satisfactory cycling stability (0.064% capacity fading per cycle at 2 C) and superior rate performance. These desirable performances suggest that the polyphenylene sulfide separator is very attractive for high safety and high-power density LIBs.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lithium-ion batteries (LIBs) have gained an unprecedented interest in electric vehicles, hybrid electric vehicles and utility storage after their success in portable electronic devices.1–4 Unfortunately, two main challenges namely safety risk and power density hinder their commercial development.5,6 It is generally recognized that the safety issues of batteries are inseparable from the dimensional thermo-stability and mechanical integrity of the separator.7–9 As a critical component of LIBs, the separator plays the dual roles of preventing direct contact between anode and cathode while providing the lithium-ion transport channels.10,11 In other words, superior mechanical and thermal stability of the separator is essential to avoid short-circuit and even explosion, while high wettability and low ionic resistance of the separator enables the excellent electrochemical performance of LIBs.12–14
Up to now, commercial polyolefin separators have been dominant in LIBs over decades for their advantages of good chemical stability and acceptable mechanical strength.15 However, their low porosity, poor wettability and inferior dimensional thermos-stability limit their further application in high-power batteries.16 Therefore, it is mandatory to develop an alternative separator with ultra-high strength, superior heat-resistance and excellent electrolyte wettability.17 Many approaches have been carried out to circumvent aforementioned shortcomings of the polyolefin separators. For example surface modification (such as surface coating,18 surface grafting19 and blending)20 and constructing available polymer skeletons are both widely employed to improve the electrolyte wettability and thermal stability of the separator.21–23 Unfortunately, the former usually needs complicated manufacturing process and is limited in service time as the weak bonding force between the modified layer and the polymer matrix.24 Consequently, developing advanced materials with ultra-high strength, superior heat-resistance and excellent electrolyte wettability is a systematic engineering.25,26
More recently, high performance polymers have been widely applied owing to their good chemical and electrochemical stability, superior heat and flame resistance as well as robust mechanical strength.27 And these inherent qualities promote the high performance polymers an available choice for LIBs separator, such as polyimide (PI),28,29 polytetrafluoroethylene (PTFE),30 polyphenylene sulfide (PPS)31 and poly (ether ether ketone) (PEEK)32 separator. In generally recognized, these high performance polymers cannot be directly used for LIBs separators as their extreme dissolubility. To overcome this problem, one widely investigated solution is to modify the chemical structure of them, which sacrifices the mechanical strength and thermal stability of the separators to a certain extent.33,34 Quite recently, Wang et al.31 employed PPS-based composite separator for LIBs, which was the only one report on PPS for LIBs. However, the introduction of SiO2 and poly (vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) leads to the separator fabrication process more complex and expensive. Meanwhile, the general non-solvent induced phase separation (NIPS)28,35,36 and electrospinning37,38 method are not available for the formation of PPS separator as its poor solubility in any solvent. On the contrary, thermally induced phase separation (TIPS) is an effective method to prepared interconnect and porous semi-crystalline separators.39,40 And the key point of TIPS is the miscibility of the skeleton polymer with diluent, which influences the performance of the prepared separator.41
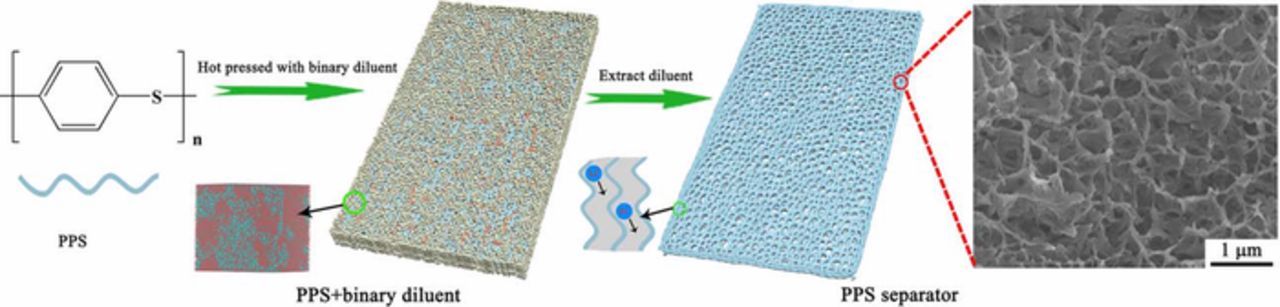
In these connections, in this paper, we prepared the PPS porous separator with unique bicontinuous pore structure via TIPS using binary diluent for the first time (Figure 1). The effect of binary diluent composition on the performance of PPS separator was fully investigated by molecular dynamics (MD) and mesoscopic dissipative particle dynamics (DPD) simulations.42 Comparing with common commercial separators, the PPS separator exhibits definite advantages in porosity, wettability and electrochemical performance when employed for assembling LIBs. Most importantly, the PPS separator shows enhanced mechanical strength, thermal stability and flame retardancy, which is presumably highly suitable for safe LIBs.
Figure 1. Schematic illustration of the procedure of PPS separator. The insets represent the mesoscopic morphology from DPD simulation and channel of lithium-ion migration.
Experimental
Materials
Polyphenylene sulfide (PPS, PL005, Mn = 94,000) was purchased from Chengdu Letian Plastic Co. Ltd. (Chengdu, China). Polyether sulfone (PES, JF1003, Mn = 30,000) was purchased from SABIC Innovative Plastic (America). Diphenyl sulfone (DPS) and diphenyl ketone (DPK) in analytical reagent grade were purchased from Aladdin Co. Ltd. (Shanghai, China). Dimethyl sulfoxide (DMSO analytical reagent grade) was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). The liquid electrolyte was supplied by DoDoChem (Suzhou, China), which consisted of 1 mol L−1 LiPF6 in the mixture of dimethyl carbonate, diethyl carbonate and ethylene carbonate (1:1:1, volume ratio), referred to as "electrolyte" hereinafter unless specified otherwise. The commercial PE separator prepared by wet-method was obtained from Asahi KASEI (Japan) as comparison in this study. All chemicals were purchased commercially and used as received.
Fabrication of PPS separators
The PPS separators were fabricated via TIPS using binary diluent, where DPS or DPK was used as the first diluent and PES as the second diluent. The concentration of PPS in the mixture was kept at 25%, and the weight ratios of the first diluent (DPS or DPK) with the second diluent (PES) in the binary diluent were 1/0, 1/1, 2/1, 3/1, 5/1, 8/1, 10/1 and 0/1 in turn. Firstly, PPS and binary diluent were mixed at 250°C with vigorous stirring for half hour under nitrogen protection to obtain a homogeneous solution. Subsequently, the homogenous solution was quenched in liquid nitrogen to solidify for avoiding phase separation. Then the solidified sample was chopped into powder and hot calendered in a mold with vulcanizing press at 250°C, 4 MPa to form a uniform film. Finally, the binary diluent in the film was extracted by DMSO for 12 h at 120°C and the porous PPS separator was obtained after volatilization of DMSO in oven at 50°C for 12 h. According to the composition of the binary diluent, PPS separators prepared with DPS or DPK as the first diluent were denoted as PPS1 and PPS2, respectively. Besides, the detail codes of PPS separators and composition of the binary diluent were listed in Table S2 and Table S3.
Physical characterization of PPS separators
The morphologies of the separators were characterized by a filed-emission scanning electron microscope (SEM, FEI Quanta 400, Netherlands) operating at 20 kV. Pore size distributions of the separators were measured by mercury intrusion porosimetry (Micromeritics AutoPore IV 9500, America). Air permeability of the separators was determined by Schopper air permeability (XS-TQD10, China). Contact angle measurements were conducted on a contact angle goniometer (SL200B, China) using the electrolyte as the operating fluid. Mechanical properties of the separators (70 mm × 4 mm) were measured by a universal tensile tester (QJ2118, China) with a strain rate of 2 mm min−1 at room temperature. Thermal stability of the PPS separators were investigated with thermogravimetric analyzer (PerkinElmer Pyris Diamond TG/DTA, America) from 20 to 720°C and different scanning calorimetry (DSC, DSC 200 PC, Netzsch, Germany) from 30 to 350°C at a heating rate of 10°C min−1 under N2 atmosphere.
Porosity of the separator was examined by measuring the weight of the dry separator and the separator with encapsulated n-butanol in pore. Afterword, the porosity of the separator was calculated according to Equation 1:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/166/8/A1644/revision1/d0001.gif)
where md and mw are the weight of the separator before (dry) and after soaking (wet) in n-butanol for 4 h, respectively, while ρb is the density of n-butanol (0.809 g cm−3) and V is the volume of the dry separator.
Electrolyte uptake of the separator was measured by immersing the separator in electrolyte for full soaking, which was calculated according to Equation 2:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/166/8/A1644/revision1/d0002.gif)
where w0 and w1 are the weight of the separator before and after soaking in the electrolyte for 4 h, respectively.
Thermal shrinkage of the separator was determined by measuring the dimensional change before and after exposing the separator at different temperature for 1 h and the shrinkage was calculated according to Equation 3:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/166/8/A1644/revision1/d0003.gif)
where the s0 and s1 are the surface area of the separator before and after heat-treatment under different temperature.
Electrochemical measurements
Electrochemical measurements of the separators were tested by assembled coin-type cells in an argon-filled glove box. Ionic conductivities (σ) of the separator was determined by measuring electrochemical impedance spectroscopy (EIS, Solartron 1287, England) within the frequency ranging from 100 kHz to 0.1 Hz under an amplitude of 5 mV. The tested 2025 cell was assembled by sandwiching an electrolyte-soaked separator between two stainless steels (SS/separator-electrolyte/SS). And then the ionic conductivity (σ) was calculated from Equation 4:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/166/8/A1644/revision1/d0004.gif)
where Rb is the bulk resistance, d is the thickness of the separator and S is the area of the stainless steel, respectively.
Electrochemical stability of the separator was performed by linear sweep voltammetry (LSV) with a sweep rate of 5 mV s−1 and a potential range of 0–6 V (vs Li+/Li). The tested 2025 cell was assembled by sandwiching the electrolyte-soaked separator between stainless steel with lithium metal (SS/separator-electrolyte/Li). To analyze the interfacial resistance between the separator and lithium electrode, lithium/separator-electrolyte/lithium cell (Li/separator-electrolyte/Li) was assembled and measured by EIS in the frequency range from 100 kHz to 0.1 Hz under voltage amplitude of 10 mV.
Lithium-ion transference number ((tLi+) of the separator was investigated by the method base on the combination of chronoamperometry (CA) and EIS. The cell which assembled with Li/separator-electrolyte/Li was measured before and after polarization (10 mV), and the lithium-ion transference number (tLi+) was defined by Equation 5.
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/166/8/A1644/revision1/d0005.gif)
where Io and Is are the initial and steady-state current, respectively, the ΔV corresponds to the polarization potential (10 mV). Ro and Rs are the interfacial resistances before and after polarization, respectively.
Interface stability between separator and lithium electrode was conducted by lithium periodic stripping/plating test with Li/separator-electrolyte/Li cell. The cell was measured under 1 mA cm−2 current density and charge/discharge lasted for 1 h at each step. To fabricate the cathode for half-cell testing, the LiFePO4 power was mixed with Super P and polyvinylidene fluoride at a weight ratio of 8:1:1 with N-methy-2-pyrrolidone as the solvent (the loading of LiFePO4 is around 2.0 mg cm−2). The battery performance of the LiFePO4/separator-electrolyte/Li half-cell was tested on LAND-CT2001A (Wuhan, China) multichannel battery tester. And the galvanostatic charge/discharge test was conducted within a potential ranging from 2.5 to 4.2 V at various C-rates.
Unless otherwise noted, all experiments were performance at room temperature (around 25°C). And all the results can be reproducible.
Results and Discussion
Computer simulation and experimental study of binary diluent
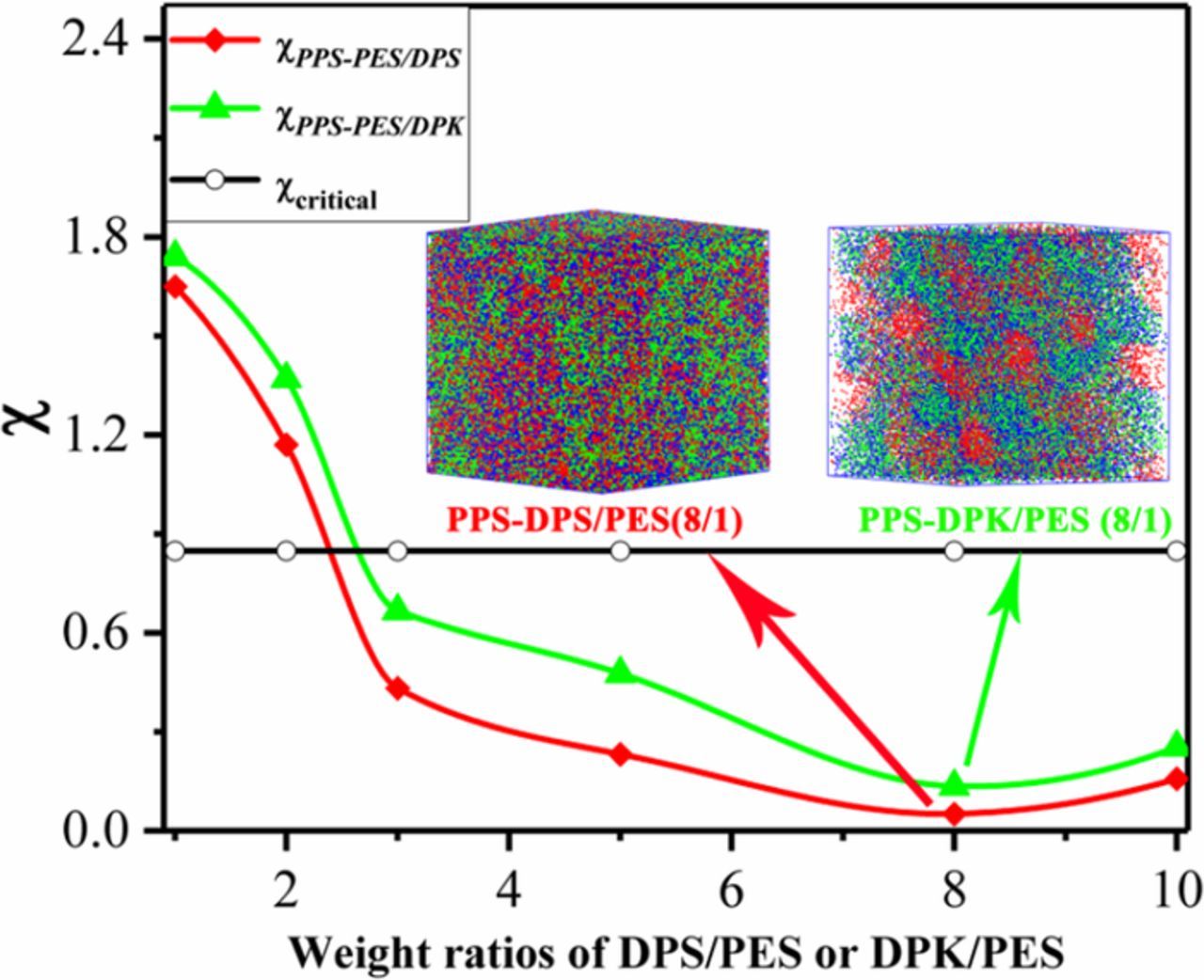
The unique microstructure PPS separator was prepared via an extraordinary thermally induced phase separation (TIPS) process with binary diluent (Figure 1). Diphenyl sulfone (DPS) or diphenyl ketone (DPK) was selected as the first diluent due to its high boiling point and strong interaction with PPS,43 while polyether sulfone (PES) was used as the second diluent because of its good compatibility with DPS or DPK but poor compatibility with PPS.44 As can be seen clearly from Figure S1, the PPS-PES film was destroyed when immersed in the extracting agent due to the agglomeration of PES, while the PPS-binary diluent film can remain stable. Meanwhile, the PPS separator prepared using DPS or DPK as unary diluent also show nonporous structure (Figure S2) due to the good fluidity of DPS or DPK above melting point. According to the aforementioned, we can conclude that the porous PPS separator cannot be obtained by unary diluent. A suitable diluent composition should be the one having good compatibility with PPS, which makes a vital function in the microstructure of PPS separator after extracting diluent. For further illustration, molecular dynamics (MD) and mesoscopic dissipative particle dynamics (DPD) simulations were carried out to study the compatibility of the binary diluent composition with PPS.45,46 The compatibility was evaluated by Flory-Huggins interaction parameter (χ) which calculated at ambient temperature (300K) and further confirmed by the mesoscopic morphology from DPD simulation, as shown in Figure 2. Moreover, the details of the simulations and calculations are described in the supporting information, as shown in Table S1, Table S2 and Table S3 in turn. From these results, it is found that the χ values of PPS and unary diluent systems are larger than critical value, which manifests PPS and unary diluent systems are incompatible.47 On the contrary, when the weight ratios of first diluent (DPS or DPK) and second diluent (PES) in the binary diluent are greater than 3 (including 3), the PPS and binary diluent systems indicate compatible (Figure 2). Besides, the mesoscopic morphologies of PPS and binary diluent systems demonstrate no obvious phase separation when the weight ratio was 8/1, and the compatibility of the system is better with DPS as first diluent.
Figure 2. Flory-Huggins interaction parameter (χ) versus the weight ratios of DPS/PES or DPK/PES. The inset images are the mesoscopic morphologies from DPD simulation of PPS1-81 and PPS2-81 systems.
Morphology and pore structure
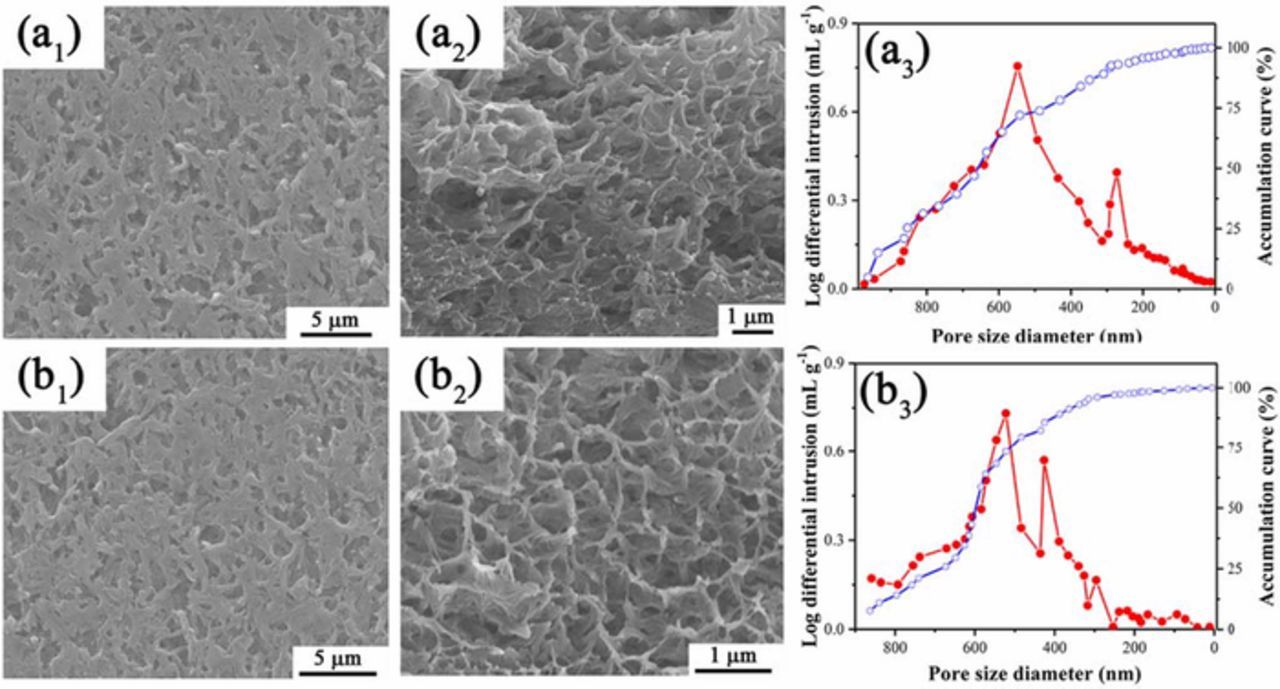
The SEM images (Figure 3) of PPS1-81 and PPS2-81 separators both show a unique uniform microstructure with 3D interconnected pore structure. The pore size distributions of both PPS1-81 and PPS2-81 separators measured by mercury intrusion porosimetry are also shown in Figure 3 (a3) and Figure 3 (b3). It can be clear seen that the PPS separator exhibits a narrow pore size distribution with an average pore size of around 500 nm. Such a tortuous, sub-micro range and uniform distribution pores structure is generally considered to effective to retard lithium dendrite growth and achieve stable lithium-ion deposition at high rates, thus playing a significant role in mitigating self-discharge and avoiding internal short-circuit.48,49 Besides, the morphologies of PPS separators prepared with other composition of binary diluent are shown in Figure S3 and Figure S4. It should be noted that a uniform porous PPS separator can be obtained when the weight ratio of first diluent to second diluent is higher than 3 (including 3), which is consistent with previous simulation results. Moreover, the pore structure of the PPS separators are slightly affected by the difference of the weight ratios of first diluent and second diluent.
Figure 3. SEM images (a1, a2, b1, b2) and pore size distribution (a3, b3) of (1) PPS1-81 and (2) PPS2-81 separators: (a1, b1) surface morphologies, (a2, b2) cross-section morphologies.
Thickness, porosity and air permeability value of the PE and PPS separator are the basic physical properties to reflect their pore structure. As shown in Table I, the thickness of the as-prepared PPS separator is around 29 μm, which is suitable for high-power density LIB.50 Meanwhile, all as-prepared PPS separators exhibit higher porosity than commercial PE separator, which mainly attributes to their bicontinuous 3D pore structure formed by TIPS with binary diluent. The air permeability, which is calculated by the volume of gas passing through a certain area for a fixed time under pressure, can be applied to quantitatively evaluate the connectivity of pore structure and further reflected lithium-ion migration.51 It can be seen that the PPS separators have higher air permeability over PE separator due to their highly interconnected and tortuous structure. In this sense, we can conclude that the porosity and air permeability of the PPS separators increase with increasing the weight ratios of first diluent to second diluent, which is agreement with SEM observation. What's more, the PPS1-81 and PPS2-81 separators possess the highest porosity and air permeability among all PPS separators, facilitating to enhance the migration of lithium-ion.
Table I. Thickness, porosity, air permeability value and electrolyte uptake of PE and PPS separators.
| Sample | Thickness (μm) | Porosity (%) | Air permeability value (μm Pa−1·s−1) | Electrolyte uptake (%) |
|---|---|---|---|---|
| PE | 25 | 45.0 | 0.697 | 115 |
| PPS1-31 | 29 | 59.8 | 0.780 | 280 |
| PPS1-51 | 29 | 67.2 | 1.25 | 357 |
| PPS1-81 | 29 | 73.5 | 1.72 | 409 |
| PPS1-101 | 30 | 73.5 | 1.60 | 392 |
| PPS2-31 | 29 | 56.0 | 0.703 | 271 |
| PPS2-51 | 29 | 61.0 | 1.10 | 349 |
| PPS2-81 | 28 | 69.7 | 1.59 | 384 |
| PPS2-101 | 29 | 71.0 | 1.59 | 376 |
Wettability and electrolyte uptake
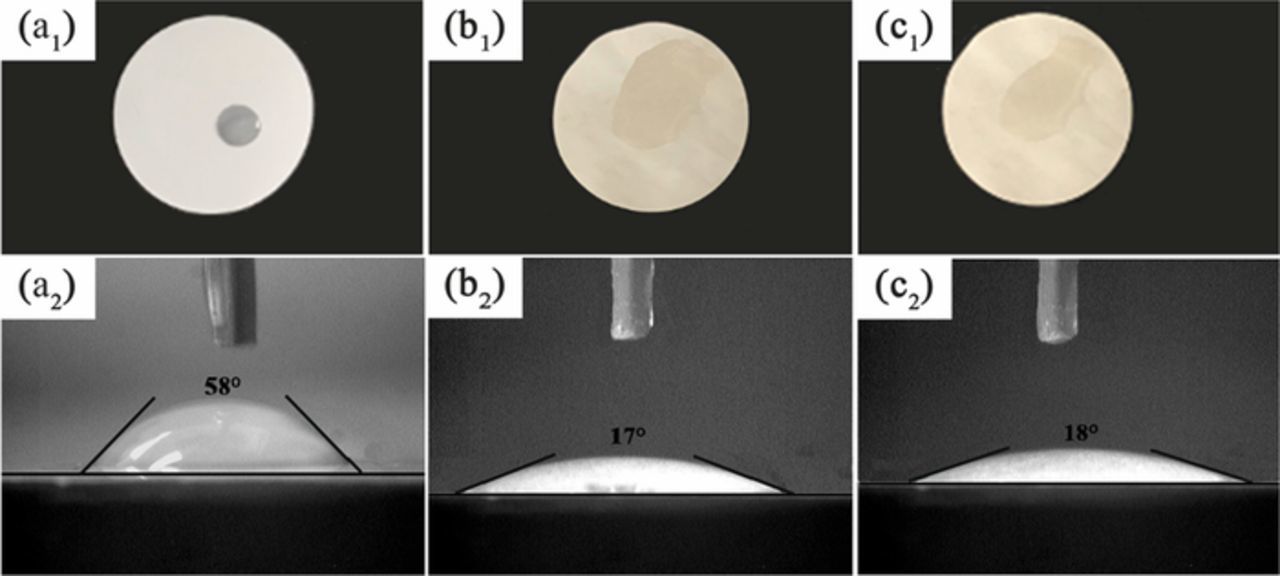
Fast and well-distributed wetting of the electrolyte of whole separator is beneficial to the rapid and uniform transport of lithium-ion.52 Therefore, electrolyte spreading test and contact angle measurement were conducted to analyze the wettability of a separator. As shown in Figure 4, the liquid electrolyte droplet was quickly and easily absorbed into PPS separator in a few seconds, whereas the electrolyte formed a bead on the PE separator after a long time. In addition, the contact angle measurement with electrolyte was further carried out to verify the wettability of the separator. After the same time interface contact (10 s) of separator toward electrolyte, the PPS separator shows a contact angle of around 17°, which is much lower than that of PE separator (58°). The poor wettability of PE separator can be explained as the hydrophobicity of polyethylene and the low affinity between the PE separator and electrolyte.21 On the contrary, the strong interaction existed between PPS chains and carbonate electrolyte due to the presence of polar groups, which further leads to the superior wettability of the PPS separator.
Figure 4. Electrolyte spreading tests (a1, b1, c1) and contact angles (a2, b2, c2) of (a) PE, (b) PPS1-81 and (c) PPS2-81 separators.
Moreover, the electrolyte uptake of the separator was investigated and displayed in Table I. It can be clearly seen that the electrolyte uptake of the PPS separators increase with increasing the weight ratios of first diluent to second diluent, which is consistent with the relationship between porosity and air permeability. Similarly, the PPS1-81 and PPS2-81 separators show the highest electrolyte uptake (409% and 384%), which are significantly higher than that of the PE separator (115%). The excellent electrolyte uptake of PPS separator is due to the high porosity and strong affinity with electrolyte. In summary, the PPS separator exhibits enormous advantage in absorbing and storing liquid electrolyte, leading to higher ionic conductivity, larger lithium-ion transference number and better interfacial stability.
Mechanical properties
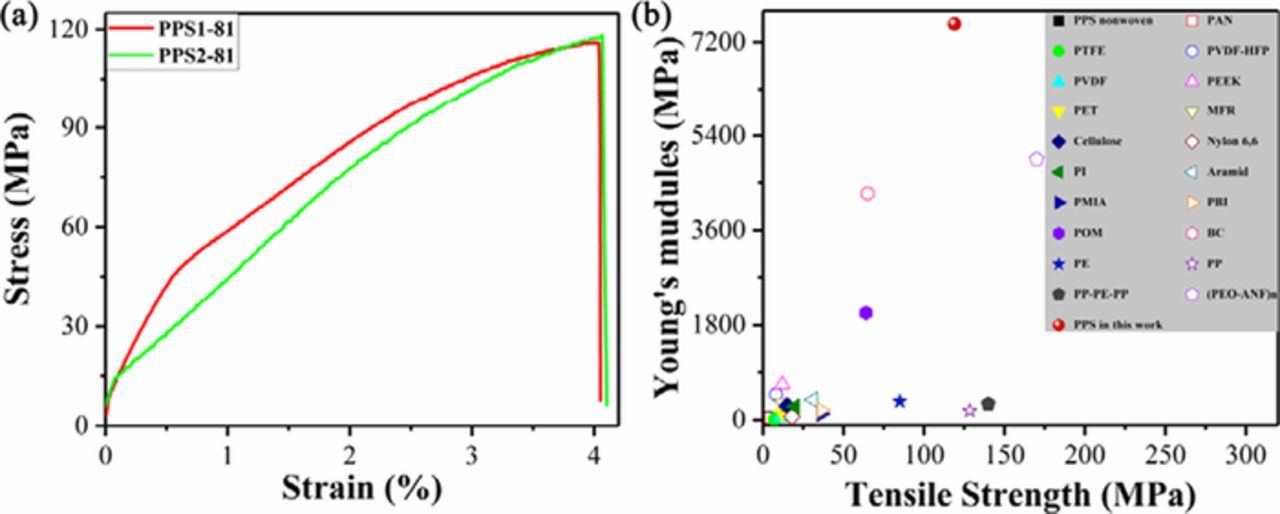
A qualified separator should not only be robust enough to withstand high tension during assembling, but also capably address additional safety concerns caused by external mechanical abuse, brusque change of temperatures, or accidental high current density.53 In other words, the separator with sufficient mechanical strength to ensure integrity is of great significance for the safety of LIBs. As presented in Figure 5a, the PPS separators show ultra-strong mechanical properties with tensile strength of 119 MPa and Young's modules of 7.55 GPa, which is much higher than the current polymer separators (such as PE: 85 MPa and 0.35 GPa, Figure 5b).24,30–32,54–68 According to the predication that the separator with high Young's modulus (i.e., G' > 6 GPa, which is 1.3 times of metallic lithium) can prevent lithium dendrite proliferation,69 the as-prepared PPS separator should be sufficient to suppress the lithium dendrite extension. Moreover, the uniform and tortuous pore structure with appropriate size of the PPS separator can also provide for stable lithium-ion deposition, which is beneficial for preventing the formation of dendrite.70 Such ultra-high strength of the PPS separator not only stems from high intrinsic strength in high performance polymer PPS, but also results from the unique pore structure of the as-prepared separator. Therefore, it is believed that the PPS separator with such ultra-strong mechanical property is capable of addressing the safety problem of the LIBs caused by mechanical destruction of separator.
Figure 5. (a) Stress-strain curves of PPS1-81 and PPS2-81 separators; (b) Comparative evaluation of tensile strength and Young's module of the as-prepared PPS and other current polymer separators (in dry state). The corresponding separators are: PPS nonwoven, polyphenylene sulfide nonwoven; PAN, polyacrylonitrile; PTFE, polytetrafluoroethylene; PVDF-HFP, poly(vinylidene fluoride-co-hexafluoropropylene); PVDF, poly(vinylidene fluoride); PEEK, poly(ether ether ketone); PET, polyethylene terephthalate; MFR, melamine formaldehyde resin; Cellulose; Nylon 6,6; PI, polyimide; Aramid; PMIA, poly(m-phenylene isophthalamide); PBI, polybenzimidazole; POM, polyformaldehyde; BC, bacterial cellulose; PE, wet-method polyethylene; PP, Celgard 2500 polypropylene; (PEO-ANF)n poly(ethylene oxide)-aramid nanofibers.
Thermal stability, shutdown function and flame retardancy
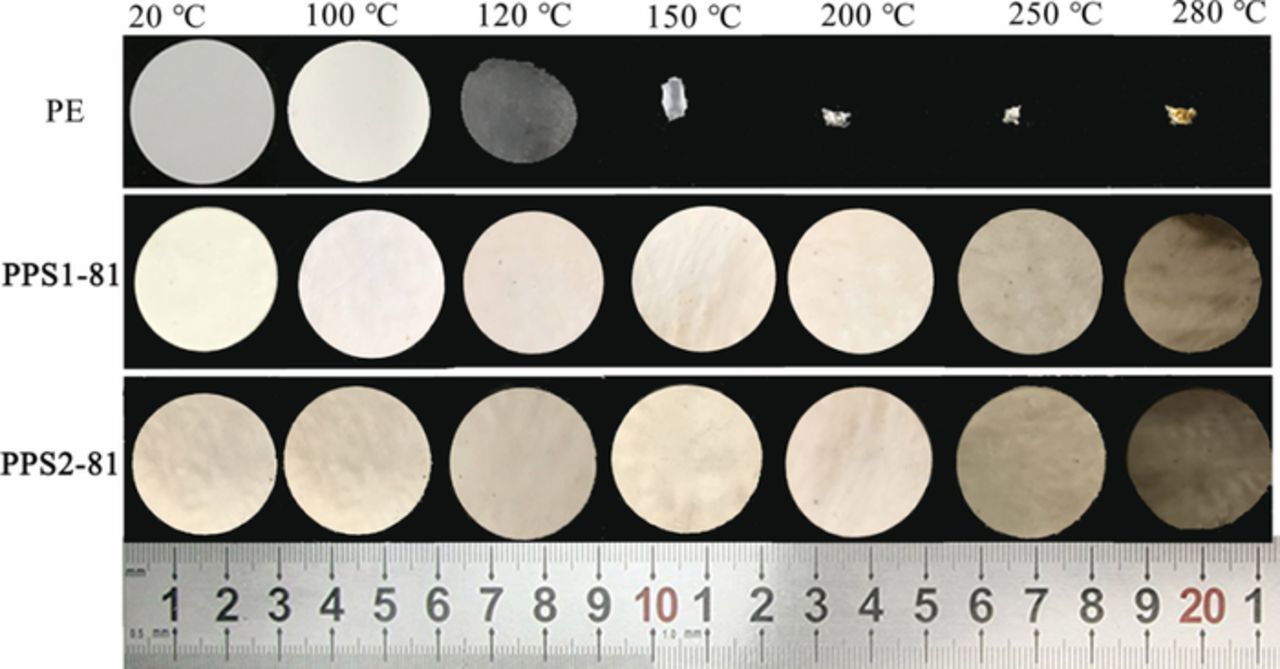
As well known, over-heating, overcharging, internal and external short-circuit can trigger the battery to failure or thermal runaway with fire or even explosion.12,71 It means that the thermal dimensional stability of the separator is a vital factor associated with the safety of LIBs. Figure 6 shows the thermostability of the separators treated at different temperature of 100, 120, 150, 200, 250 and 280°C for 1 h, and then the shrinkage of dimensional change before and after heat-treatment is presented in Figure S5 (a). It is found that the PE separator suffers thermal shrinkage at 120°C and shrinks drastically upon 150°C with a dimensional shrinkage of more than 80%. In sharp contrast, the PPS separator can well maintain the original mechanical integrity and show negligible shrinkage (< 2%) even up to 280°C, exhibiting perfect thermal stability for highly safe LIBs. Moreover, differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements were also conducted to evaluate the thermal stability of the separator. As shown in Figures S5 (b) and (c), the PE separator shows an endothermic peak at 135°C for melting, while the PPS separator exhibits the only endothermic peak at 280°C, and the weight of PPS separator remains unchanged until 480°C.
Figure 6. Digital pictures of PE, PPS1-81 and PPS2-81 separators under heat-treatment at different temperature: 20, 100, 120, 150, 200, 250 and 280°C.
In addition, the morphology changes of the PPS separator before and after heat-treatment under 250°C and 280°C also were investigated for evaluating the shutdown function. As shown in Figure S6, it can still observe porous structure on PPS separator even at 250°C, while the pores in PPS separator collapse after heat-treatment under 280°C for 1 h. These results indicate that the PPS separator possesses an obvious shutdown function, which is crucial for preventing thermal runway of LIBs.72 In summary, the PPS separator with excellent thermal tolerance and shutdown feature can effectively prevent internal short-circuit and thermal runaway at evaluated temperature.
Furthermore, the flame retardancy of the separator is another vital aspect to ensure the safety of LIBs. As shown in Figure S7, the commercial PE separator shrinks immediately once being exposed on fire and then burns out completely, whilst the PPS separator exhibits only slightly shrinkage and the fire quickly self-extinguish after the ignition source is removed. The excellent flame-retardant of the PPS separator is attributed to the high refractory of the PPS resin (UL940 grade and the limit oxygen index of the PPS is around 35).73 Furthermore, the PPS separator with super nonflammable property can play a significant role on terminating fire or other accidents under abuse conditions, which further enhance the safety of LIBs.
Electrochemical performance
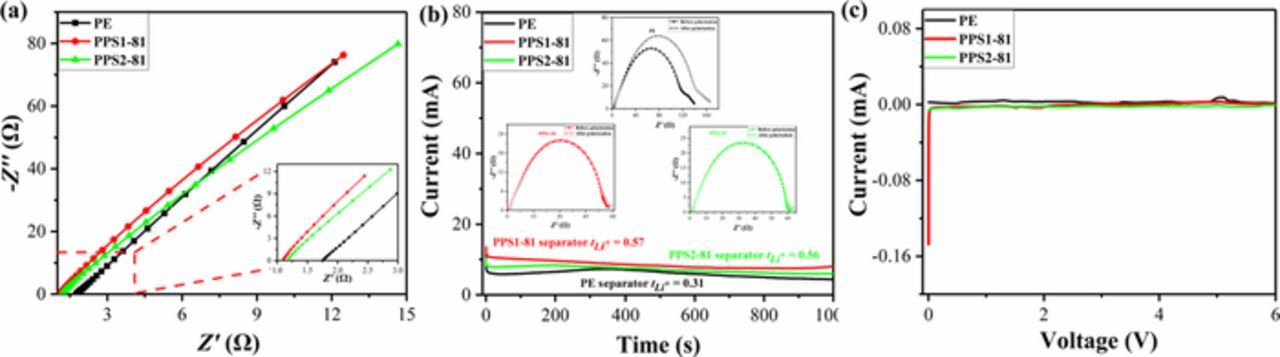
The ionic conductivity of the separator is measured by electrochemical impedance spectroscopy (EIS) accurately, as shown in Figure 7a. The bulk resistance (Rb) of the electrolyte-saturated separator can be determined by the intersection of the Nyquist plots on Z' axis, and it can be found that the Rb of the PE, PPS1-81 and PPS2-81 are 1.75, 1.09 and 1.17 Ω, in turn. The ionic conductivities of the PPS separators are calculated to be 1.69 and 1.61 mS cm−1 according to Eq. 4, which is much higher than that of PE separator (0.76 mS cm−1). Meanwhile, the lithium-ion transference number (tLi+) of the separator is also measured for further investigating the ion transport ability and the effect of concentration polarization.74 As shown in Figure 7b, the lithium-ion transference number of the PE separator is 0.31, while the value of PPS separator is around 0.57. The higher ionic conductivity and lithium-ion transference number of the PPS can be attributed to the following factors: (i) the as-prepared PPS separator possesses high porosity, 3D interconnected and uniform pore structure, which provides abundant channels to promote ion transport; (ii) the superior wettability and electrolyte uptake of the PPS separator may guarantee the number of lithium-ions when the separator soaked in electrolyte; (iii) the polar sulfur atoms of the PPS separator can accelerate the migration of lithium-ion due to the great affinity. Commonly, the PPS separator with higher ionic conductivity and lithium-ion transference number is conductive to lead to better LIBs performance.
Figure 7. (a) Nyquist plots of the SS/separator-electrolyte/SS cells, (b) chronoamperometry (the insets are EIS) of the Li/separator-electrolyte/Li cells and (c) linear sweep voltammetry (LSV) curves of the SS/separator-electrolyte/Li cells with PE, PPS1-81 and PPS2-81 separators.
As an indispensable part in LIB, the separator should be electrochemically stable in the operational voltage of LIB. Liner sweep voltammetry (LSV) was measured to evaluate the oxidation potential of the separator, as shown in Figure 7c. The electrolyte-soaked PPS separator exhibits good oxide stability up to 6.0 V (vs. Li/Li+), which implies the PPS separator is high stable under LIB operating condition, whereas the electrolyte-soaked PE separator exhibits an anodic oxidized at the potentials above 4.8 V. Therefore, the PPS separator is available for LIBs due to the highly electrochemical stability.
The interfacial compatibility of the electrolyte-soaked separator with lithium electrode is significant for avoiding polarization and further affects the cycle stability and C-rate performance of LIBs. The interfacial resistance variation of the symmetric Li/separator-electrolyte/Li cells over different storage time was characterized by EIS. As shown in Figure 8, the ohmic resistance (Rs) and interfacial resistance (Rct) of the cell with PPS separator (1.77 or 1.85 Ω, 57.6 or 61.2 Ω, as shown in Table II) are much smaller than that of the cell with PE separator (3.14 Ω, 140.6 Ω) after setup. It is considered to be the result of the enhanced intimate contact and compatibility between the PPS separator and lithium electrode. And the interfacial resistance of the cell increases with the prolongation of storage time, which reflects the growth of the solid electrolyte film (SEI). As shown in Table II, the interfacial resistance of the cell with PPS separator increases slowly and exhibits a stable SEI (PPS1-81: 57.6 Ω at setup to 155.1 Ω after 15 days; PPS2-81: 61.2 Ω at setup to 166.3 Ω after 15 days), while that of the cell with PE separator increases rapidly from 140.6 Ω to 390.7 Ω. The improved compatibility of the PPS separator with lithium electrode can be attributed to its excellent wettability and electrolyte retention, which is beneficial to guarantee the number of lithium-ion and expedite its migration. Generally, the stable interface between the separator and lithium electrode means smaller polarization of the cell during cycling.75 The polarization of the lithium symmetric cells with PPS or PE separator was investigated by the galvanostatic lithium-ion string/plating cycling measurement at a current density of 1 mA cm−2, as shown in Figure S8. It can be observed that the cell with PPS separator exhibits extremely stable over potential (∼25 mV at 500 h and ∼30 mV at 2000 h, respectively), while the cell with PE separator shows the increasing polarization (375 mV for 500 h and 60 mV for 2000 h, respectively) and suffer soft short-circuit. The flat, stable plating/strapping cycling and weakened over potential of the cell with PPS separator is due to its rapid, uniform transfer of lithium-ion. The results further indicate that the PPS separator in the cell is beneficial to restrain the growth of lithium dendrites during cycling and enhance the interfacial stability between the separators with lithium electrode, which is of great significance for improving cycle and C-rate performance of the LIBs.
Figure 8. Interfacial impedance spectra of the Li/separator-electrolyte/Li cells with (a) PE, (b) PPS1-81 and (c) PPS2-81 separators after storing different days.
Table II. Fitted impedance parameters of Figure 8.
| PE | PPS1-81 | PPS2-81 | ||||
|---|---|---|---|---|---|---|
| Rs (Ω) | Rct(Ω) | Rs(Ω) | Rct(Ω) | Rs (Ω) | Rct(Ω) | |
| Setup | 3.14 | 140.6 | 1.77 | 57.6 | 1.85 | 61.2 |
| 5 days | 4.70 | 232.2 | 1.91 | 88.2 | 2.04 | 92.3 |
| 10 days | 5.87 | 333.2 | 2.25 | 122.5 | 2.49 | 113.3 |
| 15 days | 7.26 | 390.7 | 2.66 | 155.1 | 2.75 | 166.3 |
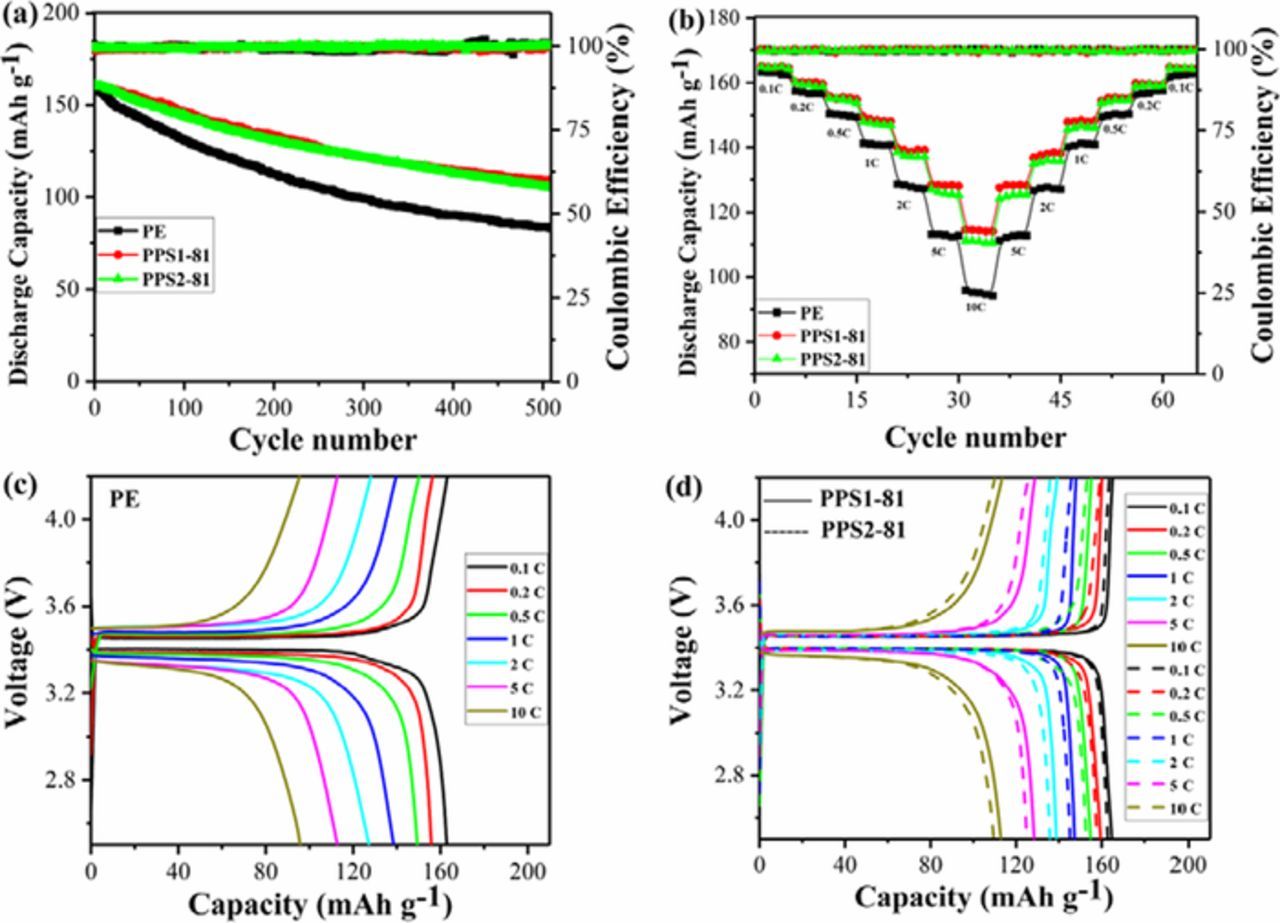
To further investigate the effectiveness of the as-prepared separator on enhancing battery performance, the half-cell assembled with LiFePO4/separator-electrolyte/Li was fabricated to explore the advantages of the PPS separator. The cycling stability of the cells with PE and PPS separators at 2 C from 2.5 to 4.2 V is presented in Figure 8a. The initial discharge capacities of the half-cells with PE, PPS1-81 and PPS2-81 separators are 160.7, 161.3 and 161.2 mAh g−1, and the discharge capacities of these cells fall into 83.7, 109.0 and 105.7 mAh g−1after 500 cycles, in turns. This demonstrates that the cells employing PPS separator (67.6% or 65.6% capacity retention, 0.064% or 0.069% capacity fading per cycle) exhibit a better long-term cycle stability when comparing to the cell with PE separator (52.1%, 0.096% capacity retention, capacity fading per cycle). The superior cycle performance of PPS separator is believed to result from the unique microstructure, high electrolyte uptake, high ionic conductivity, lithium-ion transfer number and future higher interfacial stability, whereas for PE separator, the relatively worse cyclic performance is originated from its poor wettability and serious polarization during cycling.
For high-power density application, the LIBs with as-prepared separator should meet the requirement of good C-rate capability.76 The rate performance of the cells with PE and PPS separators were evaluated by measuring the charge-discharge capabilities over a series of current densities from 0.1 to 10 C and back to 0.1 C for 5 cycles each steps, as shown in Figure 9b. It can be observed that the LIB with PPS separator exhibits higher C-rate performance than that with PE separator, especially at high current density. The differences of discharge capacities between the PPS separator-based LIB and PE separator-based LIB become lager when current density increases from 0.1 to 10 C, and it can recover to discharge capacities at corresponding rate during the reverse cycle. Particularly, the discharge capacities of the cell with PPS separator are 1.1, 1.9, 3.7, 5.8, 11.1, 13.5, and 19.3% higher than those of PE separator at the rate of 0.1, 0.2, 0.5, 1, 2, 5 and 10 C, in turns. More detailed rate performance data about the cycle charge-discharge curves of PPS and PE separator based LIBs are shown in Figures 9c and 9d, where the over potential and charge voltage of the PPS separator based-LIB is significantly lower than those in PE separator based LIB, particularly at high current density. For example, the over potential of PPS separator-based LIB is only 100 mV, which is much lower than 300 mV of PE separator-based LIB at the rate of 10 C. In other words, the polarization of the PPS separator-based LIB shows less tremendous than that of PE separator-based LIB, implying lower charge voltage and higher discharge potential. In summary, the LIB with PPS separator is proved to show better cycle stability and C-rate performance that that with PE separator, which can be attributed to the following reasons. Firstly, the as-prepared PPS separator can promote faster, more abundant and uniform lithium-ion transmission due to its unique porous structure and higher electrolyte uptake. Secondly, the excellent compatibility between PPS separator and electrodes can lead to lower interfacial resistance and polarization. Besides, the ultra-high strength of PPS separator is beneficial to restrain the growth of lithium dendrite, which is very important for the safety of LIBs.
Figure 9. Electrochemical properties of the LiFePO4/separator-electrolyte/Li cells with PE, PPS1-81 and PPS2-81 separators: (a) cycling performance of the cells with PE, PPS1-81 and PPS2-81 separators at 2C from 2.5 to 4.2 V; (b) discharge rate capabilities of the cells with PE, PPS1-81 and PPS2-81 separators from 0.1 to 10 C; charge/discharge curves of the cells with PE (c), PPS1-81 and PPS2-81 (d) separators at different rates.
Furthermore, the morphology of the cycled lithium-metal anode was investigated to investigate the effect of separator on the electrochemical and safety performance of LIB as shown in Figure S9. The lithium metal anode disassembled from the cell with PE separator after 500 cycles shows a rough and non-uniform surface and with the formation of needle-like dendrites, which may probably pierce the separator and lead to short-circuit.77 On the contrary, the lithium metal anode disassembled from the cell with PPS separator after 500 cycles displayed a pretty smooth microstructure and suppresses lithium dendrite effectively. The suppressed lithium dendrite formation and growth of the cell with PPS separator not only results from the ultra-high strength of the PPS separator, but also dues to the fact that the PPS separator can provide larger amount, more uniform lithium-ion deposition and lower polarization. Because of the lithium dendrite suppression, the PPS separator will not be impaled and the safety of the PPS separator-based LIBs can be enhanced dramatically. To further evaluate the electrochemical performance of the PPS separator at high temperature, the cycle performance of half-cell with PPS separator, which was treated at 250°C for 1 h, was measured at 0.5 C. As can be seen from Figure S10, the initial discharge capacity of the half-cell is 162.0 or 161.7 mAh g−1 (PPS1-81 or PPS2-81 separator), while the discharge capacity of the cell is 128.3 or 124.6 mAh g−1 after 500 cycles. The result indicates that the electrochemical performance of the PPS separator-based cell was not affected by the process of heat-treatment, which can be attributed to the intact porous microstructure of the PPS separator after heat-treatment at 250°C. Combining the ultra-high mechanical strength, superior thermal stability, excellent cyclic stability as well as C-rate performance, the as prepared PPS separator shows highly promising as the separator for high safety and high power density LIBs.
Conclusions
In summary, a bicontinuous PPS porous separator is firstly prepared via TIPS methodology using binary diluent. The optimal composition of binary diluent is not only experimentally investigated, but also by computing simulation. The as-prepared PPS separator displays a high porosity (73.5%), excellent wettability (contact angel 17°) and electrolyte uptake (409%), consequently resulting in high ionic conductivity (1.69 mS cm−1), high lithium-ion transference number (0.57) and excellent compatibility with electrode. More importantly, the PPS separator exhibits highly thermal stability (without dimensional shrinkage up to 280°C) and flame retardancy, shutdown function, ultra-high mechanical strength (tensile strength of 119 MPa and Young's modules of 7.55 GPa), which endows its prospect in the application of high safety LIBs. Furthermore, the LiFePO4/Li half-cell with PPS separator presents satisfactory cycle stability (0.064% capacity fading per cycle after 500 cycles at 2C) and enhanced C-rate performance, owing to the lower ion transfer resistance and interfacial resistance, lower polarization and stable interface between PPS separator with electrode. In these senses, this work provides a brand new approach for the fabrication of PPS separator for high safety and high-power density LIBs or electrochemical energy storage systems such as lithium-sulfur batteries, sodium batteries and supercapacitors.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (U1601211, 51573215, and 21506260), Guangdong Province Sci & Tech Bureau (2017B090901003, 2016B010114004, 2016A050503001), Natural Science Foundation of Guangdong Province (2015A030310355, 2016A030313354), the Special Project on the Integration of Industry, Education and Research of Guangdong Province (2015B09090100), Guangzhou Scientific and Technological Planning Project (201607010042, 201707010424 and 201804020025), the Fundamental Research Funds for the Central Universities (171gjc37, 18lgpy32).
ORCID
Yuezhong Meng 0000-0003-2997-9841