Abstract
A conjugated aromatic compound comprising multiple fused redox centers, 5,7,12,14-tetraaza-6,13-pentacenequinone (TAPQ), is reported as an electrode of lithium-ion battery for the first time. It not only reaches a high reversible capacity but also obtains an excellent cycle ability. In addition, DFT calculations as well as FTIR, XPS and XRD analysis are performed to demonstrate the electrochemical reaction mechanism. This novel electrode can also be used in sodium and potassium batteries with a similar redox mechanism.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Nowadays, the extensive exploitation of fossil fuels and the rising environmental issues push scientists to pursue innovative techniques for generating and storing energy. The concept of lithium battery (LIB) firstly turned into reality in 1968 and was commercialized by Sony Corporation in 1991. After that, it has gained so much attention as a sustainable energy storage system and largely influenced our daily life. In order to meet the need of applications, considerable development has been made in the past few decades. During this process, the electrode materials, the electrolytes, membranes as well as binders have been greatly improved. In terms of the cathodes or anodes for the battery, the inorganic materials, especially the lithium transitional metal oxides, have been most intensively studied.1 While the first reported organic electrode2 can be traced back to the time almost as same as the birth of lithium batteries, it lost its appeal afterwards. More recently, researches on organic electrodes have been revived in search of high performing materials, yet it is still in the early stage.
There are three major advantages of the organic electrodes. Firstly, they are constituted almost all by light atoms, and multi-electron reactions are produced, thereby easily obtaining high capacities. Secondly, chemical diversity and structural flexibility of the organics permit us to design whatever we want so as to match the specific properties of electrodes. Thirdly, the organic compounds can be synthetized based on natural resources or be retrieved directly from natural biomass, and it is not necessary to exploit transitional metal resources. Thus, it definitely has great potentials to become the new generation of "green electrodes". The redox centers of organic electrodes can be divided into several categories, for instance, the doping/dedoping of ions in conducting polymers,3 the broken and rebuilt of S-S bond in organodisulfides,4 the donating and accepting electrons of radical polymers,5 the oxidation/reduction of carbonyl and imine groups in conjugated compounds,6 etc.
As the organic compounds often have poor electron conductive, even though the conjugated system is of benefit to electron transportation as well as charge delocalization, it gains great concerns compared with other structures. C=O bond and C=N bond are two mostly common-in-use redox centers. The former is a widely-used organic moiety displaying oxidative ability,7 while the latter has been studied as anode active center in Schiff bases because of its low voltage platform.8–10 Several C=N bonds can also get together to form a π-conjugated N-containing heteroaromatic molecule, producing electrochemical reactions.11 Inspired by the above properties, here we report a conjugated aromatic compound, 5,7,12,14-tetraaza-6,13-pentacenequinone (TAPQ), combining the C=O bond with the C=N bond as redox centers, used as a battery cathode for the first time. The compound with multiple fused redox centers showed its good redox activities by mixing with conductive carbon. Then we will do DFT calculations to demonstrate the revolution of electrochemical reaction when cycling, which will be confirmed by the ongoing experimental measurements, including FTIR, XPS and XRD analysis. Our results not only provide a novel example of a multi-electron redox active organic molecule as cathode in a Li battery (Na and K also) with a fairly good cycling performance especially at high current loading, but also clarify the reaction mechanism of multiple fused redox centers.
Experimental
Materials synthesis
The straightforward synthetic approach to TAPQ is depicted in Scheme 1.12 It started from commercially available 1,2-phenylediamine and 2,5-dihydroxy-1,4-benzoquinone which were mixed together and stirred at melting state for 5 hours. Then the produced 5,14-dihydroquinoxalino[2,3-b]phenazine was converted to 5,7,11,14-tetraaza-6,13-pentacenequinone by oxidation with potassium dichromate in sulfuric acid.
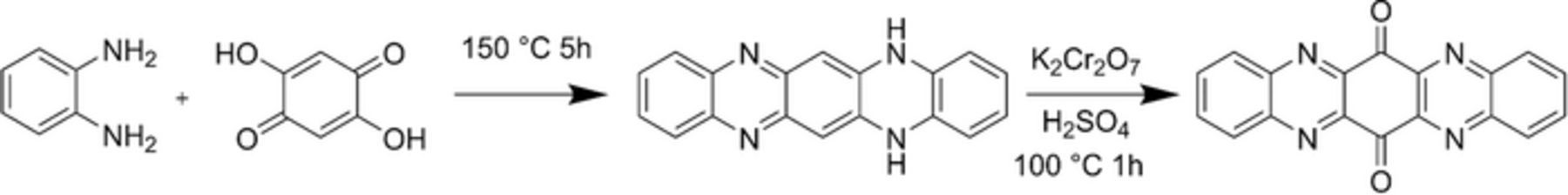
Scheme 1. Synthesis of 5,7,12,14-tetraaza-6,13-pentacenequinone.
Electrochemical characterizations
The electrode was firstly prepared by spreading the slurry of 40 wt% TAPQ, 50 wt% conductive carbon (Super P), and 10 wt% poly-amide imide (PAI) onto the aluminum foil. Then it was dried overnight in the 60°C vacuum oven and punched to circular pieces with a diameter of 14 mm. Electrochemical cells were assembled in an Argon-filled glove box by employing the lithium foil as the counter electrode and the glass fiber as the separator. To inhibiting the dissolution of the reduced organic electrode, a high-salt-concentration electrolyte, that is 4M solution of LiTFSI in tetraethelyneglygol dimethylether (TEGDME), was selected as the electrolyte. Galvanostatic charge-discharge measurement was carried out on a Land CT 2001A battery test system at room temperature. Cyclic voltammetry (CV) tests were performed on an electrochemical workstation CHI660B.
Physical characterizations
The structure of raw material and electrode was characterized by X-ray diffraction (XRD) (BrukerD8 Advance, Germany) using Cu-Kα radiation (λ = 0.1540 nm) at 40 kV, 40 mA. X-ray photoelectron spectroscopy(XPS) measurement was conducted by a PHI 5300C electron spectroscopy for chemical analysis (ESCA) system with a monochromatic Mg-Kα X-ray source. Fourier transform infrared spectra were recorded with pellets made of active power scraped from the electrode and KBr on an FTIR (Nicolet iS10). For all the ex situ measurement, the electrodes were obtained by disassembling the coin cells at three states (as-prepared, fully discharged to 1.4V and fully recharged to 3.5V in the first cycle). Then the electrodes were rinsed with dimethylether (DME).
Computational calculations
Geometry optimization and energy calculation of the molecules were performed using Gaussian 09 quantum chemistry package13 based on the Unrestricted B3LYP method14 and the basis set of 6-31+G(d). The polarizable continuum model (PCM) was employed, with static relative permittivity and dynamic relative permittivity set to 7.9 for solvent TEGDME.15
Results and Discussion
Structural characterization of TAPQ
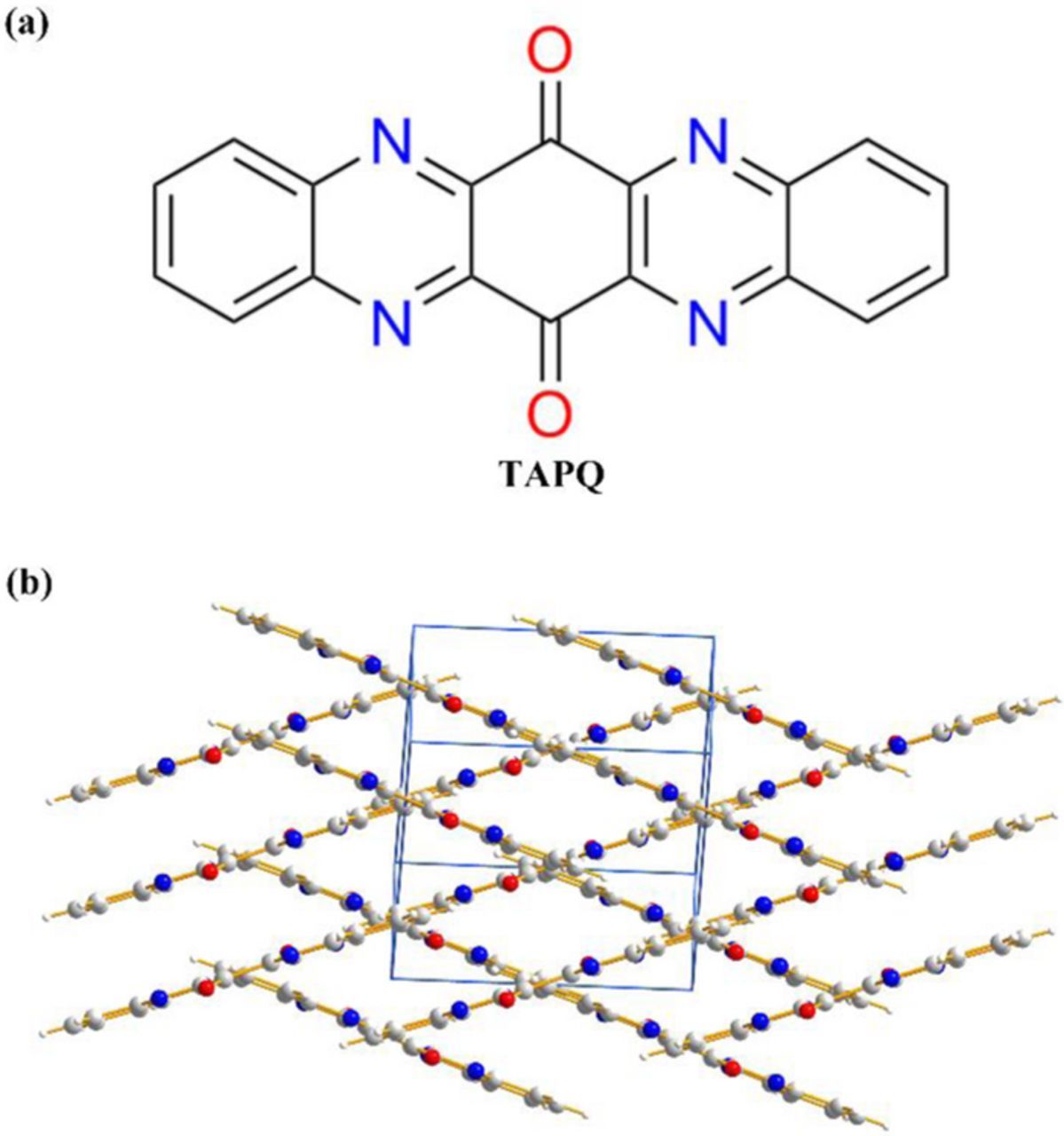
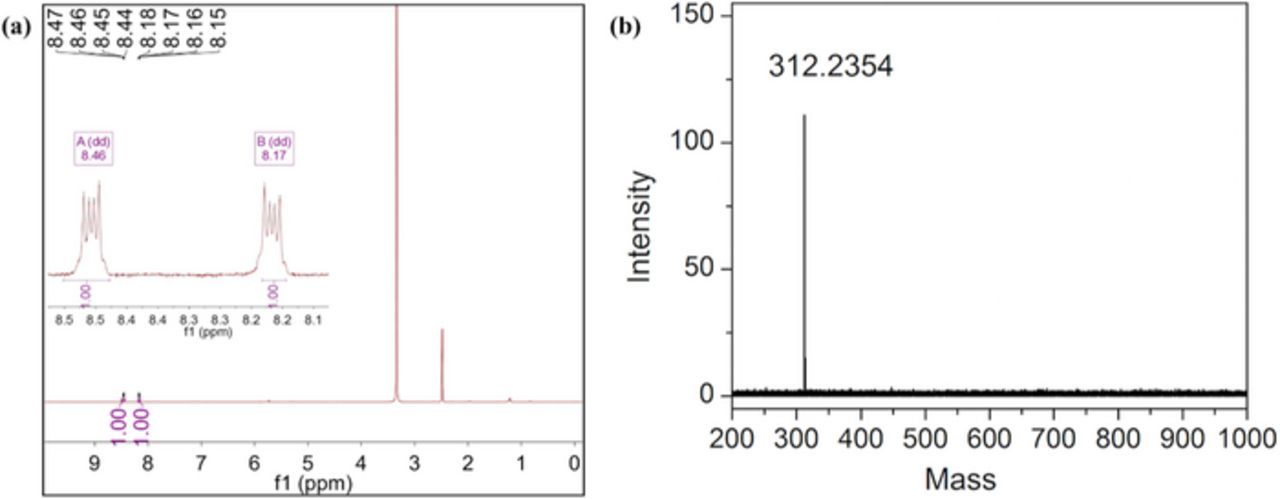
The obtained product was characterized by 1H NMR and MALDI-TOF mass spectroscopy and was found to be consistent with the proposed structure (Fig. 1). The crystal structure of TAPQ was obtained from Cambridge Crystal Structure Database (CCDC: 761663).12 It has a coplanar structure and hence tends to stack due to the strong π-π interactions. The interlayer distance between two neighboring molecules is 3.32 Å, which facilitates the charge transfer among the molecular layers. Moreover, intermolecular hydrogen bonds can be found between C–H and N/O of two parallel molecules, which forms a three-dimensional network and produces a channel for lithium ions (Fig. 2).
Figure 1. (a), (b) Chemical structure and crystal packing of TAPQ.
Figure 2. (a), (b) 1H NMR spectrum and MALDI-TOF Mass spectrum of 5,7,12,14-tetraaza-6,13-pentacenequinone in deuterated DMSO.
Electrochemical characterization
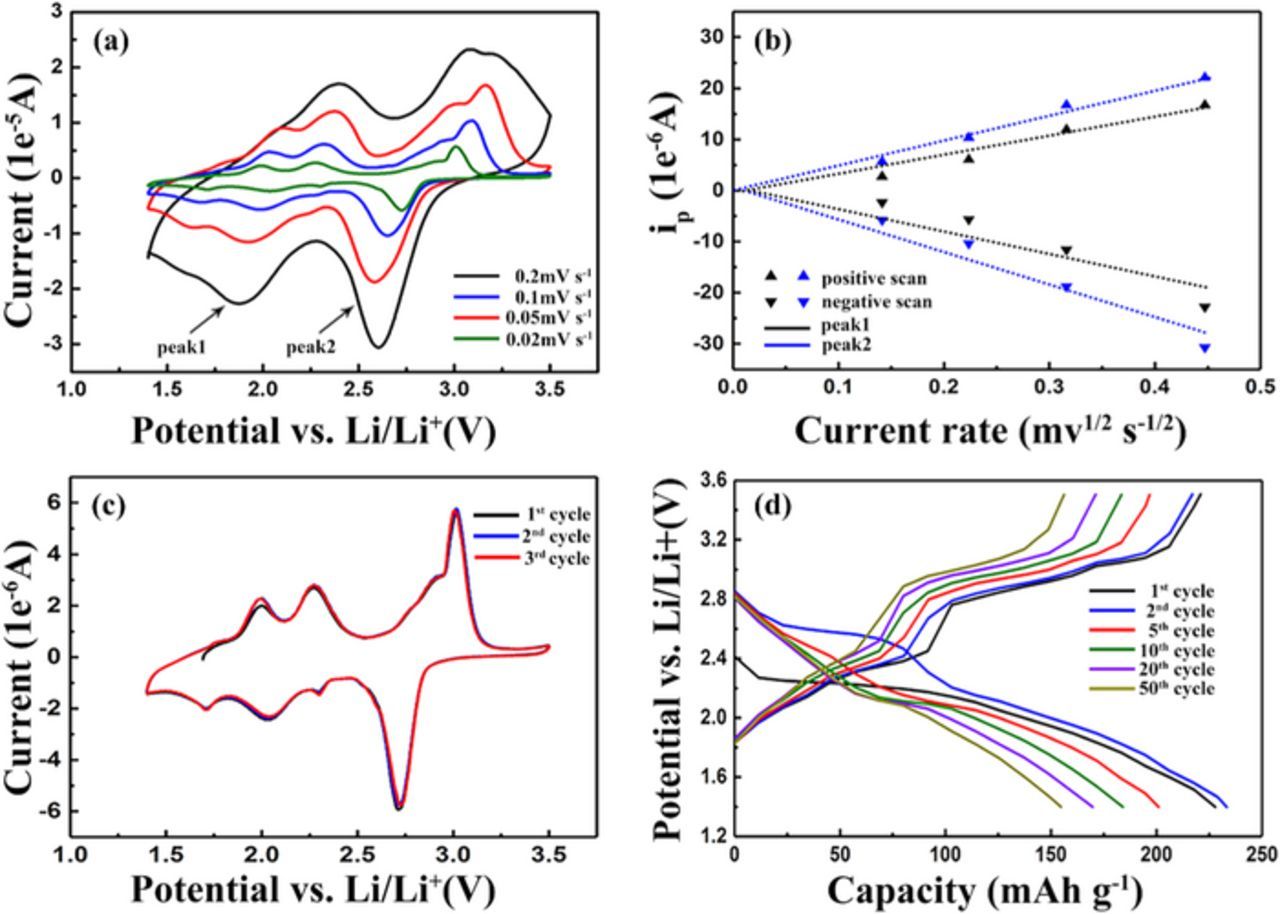
The cyclic voltammetry (CV) properties were initially evaluated between 1.4 and 3.5V vs. Li/Li+ at four different scan speeds and the results (Fig. 3a) clearly point to the multiple redox reactions. Four pairs of oxidation/reduction peaks display at 3.0/2.7V, 2.9/2.3V, 2.3/2.0V, 2.0/1.7V, corresponding to the four lithiated processes respectively. For various scanning rates, the peak currents constantly rise along with the increased scan speeds. What's more, there is a linear relation between the peak currents and the square root of sweep rates for both the positive and negative scans, indicating that the redox process of TAPQ is diffusion-controlled (Fig. 3b). For each specific scan rate (Fig. 3c), the curves in three subsequent cycles overlapped quite well without any polarization, correlated with the high reversibility of the cell.
Figure 3. (a) CV profiles of TAPQ electrode at scan rates of 0.02, 0.05, 0.1, 0.2 mV/s between 1.4 and 3.5 V. (b) The linear relationship between the currents of two clearly distinguishable peaks and the square root of current rates. (c) CV profiles at the scan rate of 0.02 mV/s in three subsequent cycles. (d) The galvanostatic discharge/charge curves of various cycles at 2C. (1C = 515.5mAh/g).
The voltage plateaus observed in the galvanostatic charge/discharge profile (Fig. 3d) completely correspond with the CV results. With regard to the TAPQ/Li battery at 2C, it delivered a reversible capacity of 274.6mAh/g in the first cycle and still rendered 164.5 mAh/g after 50 cycles, yielding both a great initial capacity and a high capacity retention of 60%. The dramatic capacity decay was only observed in the first five cycles and the subsequent stabilized capacity approximately corresponds to two electrons reaction. We speculate that although a C=O bond is shared with two adjacent C=N bonds to chelate with lithium cations, one carbonyl group can only stably accommodate one lithium ion because of the steric hindrance effect, just like the phenomenon demonstrated in other quinone-based organic electrode batteries.16,17 For this reason, only half of its maximum capacity is highly reversible. Much less capacity loss was detected afterwards, and it is mainly due to the dissolution of the organic active material in the electrolyte. Although the electrolyte with ultrahigh salt concentration was selected to decrease the solubility of reduced product, it could not avoid the dissolution completely.
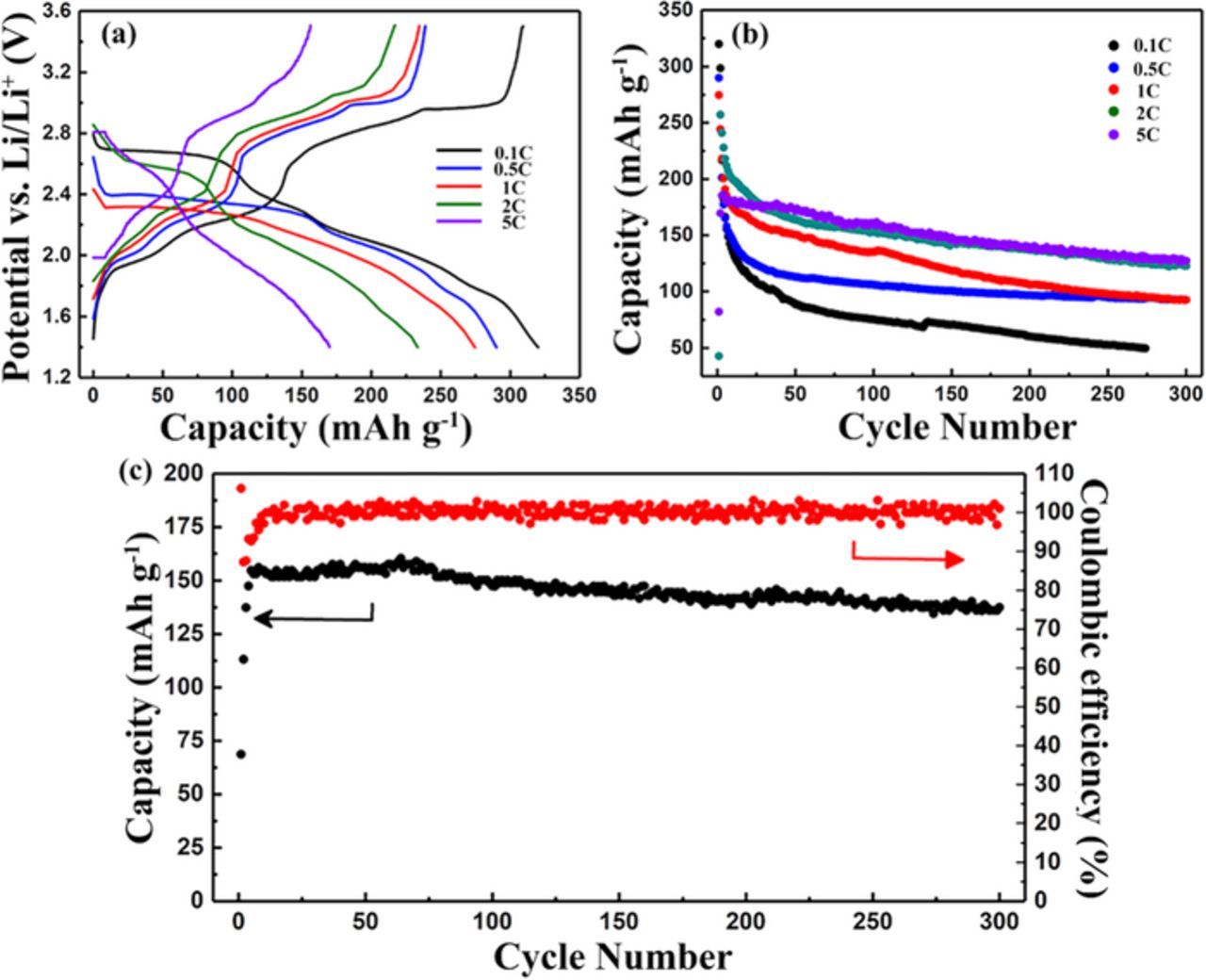
The rate capacities are also investigated (Figs. 4a, 4b) and the battery shows the maximum capacities of 320.0 mAh/g, 290.0 mAh/g, 274.6 mAh/g, 257.2 mAh/g and 186.1 mAh/g under the current levels of 0.1C, 0.5C, 1C, 2C and 5C, respectively. (Theoretical capacity is set at 515.5 mAh/g.) However, for a higher current rate, the reversible capacity is higher as the reaction time per cycle is shorter, and less reduced product dissolute. What's more, it is noted that the cell of TAPQ electrode can obtain a higher capacity compared with the electrode of a similar structure 1,4,5,8-tetraaza-9-10-anthraquinone (TAAQ).18 Two more benzene rings in TAPQ make it a molecule of higher weight, yet a bigger conjugated backbone enable it to support more charges and accommodate more lithium ions.19 In addition, the maximum discharge/charge capacity of the TAPQ/Li cell is 320.0/309.0 mAh/g, that is to say, the electrode could take up and release about four lithium atoms per molecule at most. This can be explained by lithium ions correlate with the organic compound via chelating with the carbonyl group and four neighboring conjugated -N = groups. Studies of similar structures can also account for this explanation.18,20 More interestingly, the cell showed a remarkable cycle performance under a high current loading of 10C, which could maintain a specific capacity of about 150 mAh/g for over 300 cycles with a stable coulombic efficiency of nearly 100% (Fig. 4c).
Figure 4. (a), (b) Galvanostatic discharge/charge curves of the current level of 0.1C, 0.5C, 1C, 2C and 5C. (c) The long-term cyclic performances of TAPQ/Li cell at 10C.
Density functional theory calculations
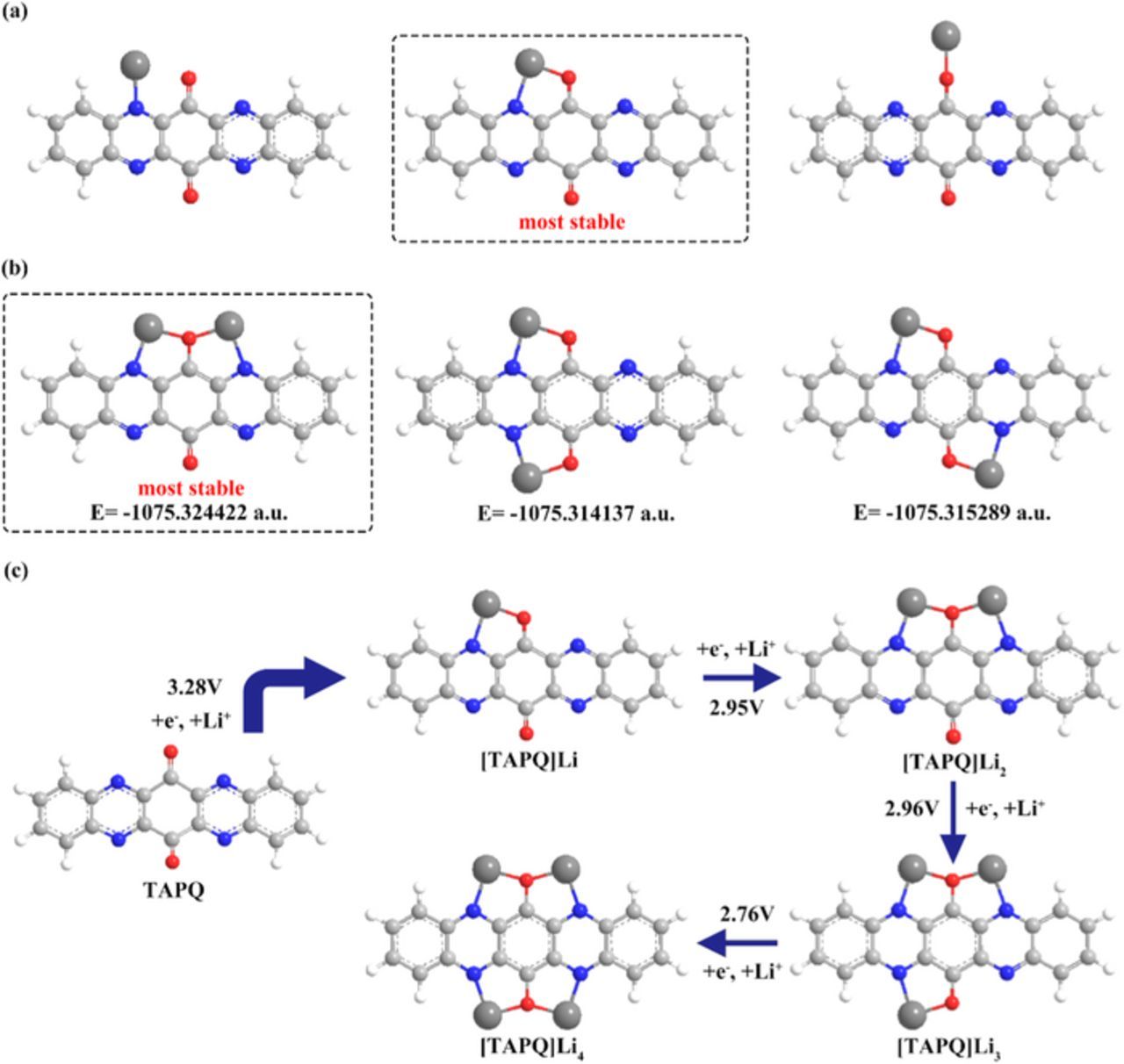
To begin with, we make use of the computational studies to help us get a preliminary understanding of the redox mechanism. Firstly, we did calculations for three situations of [TAPQ]Li and the geometry optimization results confirm that lithium will not singly bound with any atoms. (Fig. 5a) Nitrogen and oxygen atoms work as collaborative centers during the lithium intercalation. They can form a stable five-membered chelate ring when react with alkyl metal ions. This arrangement has been widely used in the coordination chemistry.21 Here we take advantage of this model to produce electrochemistry reactions and the formation of chelate cannot only facilitate the reduction progress but stabilize the molecule structure as well.18 Secondly, we compared the Gibbs Free Energy for the three possible configurations of [TAPQ]Li2 and found that two lithium atoms prefer to react with the nitrogen and oxygen of the same side so as to obtain the lowest energy (Fig. 5b). For [TAPQ]Li3 and [TAPQ]Li4, there are only one possibility of the structure and we find that the planar structure of TAPQ is being kept when reduced. We also noted that Gibbs Free Energy deviations of molecules suddenly elevate after inserting four lithium atoms (Fig. 6, Table I), so the lithiation will be more difficult afterwards. This can be explained by the broken planar structure and decreased stability of the product molecules. The result is also in concern with the mostly four electrons transforming of TAPQ electrode we observed. In addition, we calculated the theoretical potentials of TAPQ upon lithiation and match them with the four reduced processes. (Table II) The redox potentials can be corresponded with the CV curves, though polarization should be considered to some extent. The complete redox mechanism is depicted in Fig. 5c and more details are shown in Table III.
Figure 5. (a) Three molecule configurations upon the first lithium inserting. (b) Gibbs Free Energy comparison of various forms of [TAPQ]Li2. (c) The complete molecule revolution of the lithiation process.
Figure 6. Gibbs Free Energy deviations when inserting Li atoms.
Table I. Gibbs Free Energy deviations after inserting Li atoms.
| Molecule | Gibbs Free Energy (a.u.) | Energy Deviation (a.u.) |
|---|---|---|
| TAPQ | −1060.055581 | / |
| [TAPQ]Li | −1067.696046 | −7.640465 |
| [TAPQ]Li2 | −1075.324422 | −7.628376 |
| [TAPQ]Li3 | −1082.953142 | −7.628720 |
| [TAPQ]Li4 | −1090.574606 | −7.621464 |
| [TAPQ]Li5 | −1098.129945 | −7.555339 |
| [TAPQ]Li6 | −1105.686139 | −7.556194 |
Table II. The calculated potentials according to the Gibbs Free Energy.
| Molecule | TAPQ | [TAPQ]Li | [TAPQ]Li2 | [TAPQ]Li3 | [TAPQ]Li4 |
|---|---|---|---|---|---|
| Gibbs Free Energy (a.u.) | −1060.055581 | −1067.696046 | −1075.324422 | −1082.953142 | −1090.574606 |
| Reaction process | TAPQ→[TAPQ]Li | [TAPQ]Li→[TAPQ]Li2 | [TAPQ]Li2→[TAPQ]Li3 | [TAPQ]Li3→[TAPQ]Li4 | |
| Calculated potential vs. Li/Li+ (V) | 3.280752526 | 2.9517929 | 2.961153651 | 2.763707125 |
(ELi = −7.5199 Hartee)
Table III. DFT calculations and optimizations for various lithiated molecules.
| Molecule | Before optimization | After optimization | Gibbs Free Energy (a.u.) |
|---|---|---|---|
| TAPQ | −1060.055581 | ||
| [TAPQ]Li-1 | −1067.695796 | ||
| [TAPQ]Li-2 | −1067.695976 | ||
| [TAPQ]Li-3 | −1067.696046 | ||
| [TAPQ]Li2-1 | −1075.324422 | ||
| [TAPQ]Li2-2 | −1075.314137 | ||
| [TAPQ]Li2-3 | −1075.315289 | ||
| [TAPQ]Li3 | −1082.953142 | ||
| [TAPQ]Li4 | −1090.574606 |
X-ray photoelectron spectroscopy analysis
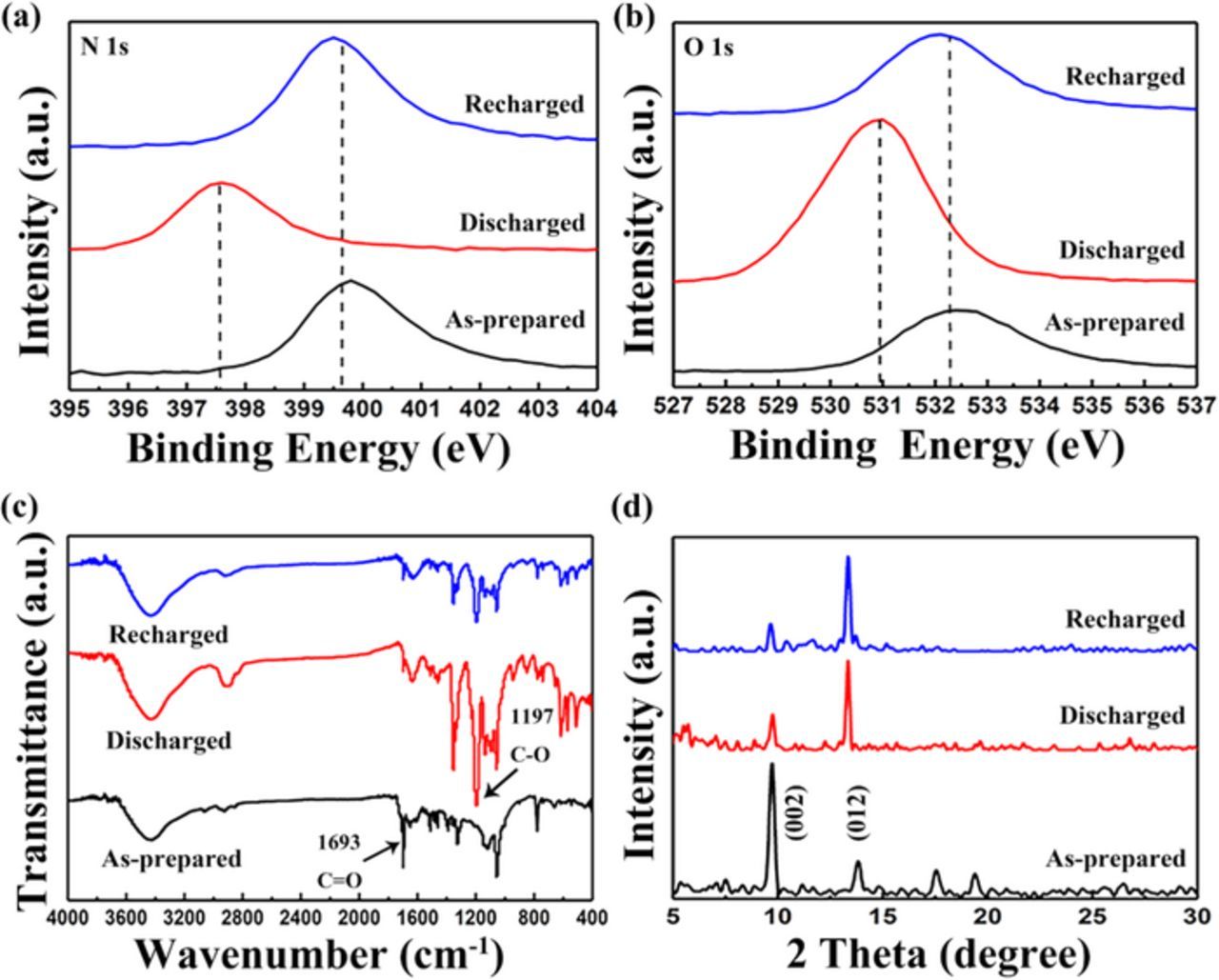
With the computational results obtained in hand, XPS was then employed to explore the changes of chemical bonding in TAPQ during the battery cycling. In the N1s spectrum(Fig. 7a), the as-prepared electrode showed a peak at 399.6 eV assigned to C=N-C bond.22 After discharging, the original peak diminished and an additional peak with a lower binding energy at 397.5 eV evolved, which could be identified as N-Li bond.20 This indicates that the C=N-C bond also participate in the lithiation process with the formation of lithium-chelate. For the recharged state of the cell, the C=N-C peak recovered and replaced the N-Li peak, implying the reversible electrochemistry of the battery. The O1s spectrum (Fig. 7b) confirmed that the peak of C=O bond (532.2 eV) in the pristine electrode shifted to the position of the C-O bond (531.0 eV) when discharged and then returned to the initial state after the cell was recharged, which are about the same as the commonly observed.23 The two spectra above testify that nitrogen and oxygen atoms work together to coordinate with the lithium ion, which is in agreement with the previous DFT calculations. In addition, as XPS can only detect the surface characteristic of the sample, we can infer that the surface substance of the electrode is highly reversible during the cycling process. The irreversibility of the cell is largely owning to the incomplete reaction of inner part of the electrode as well as the dissolution problem.
Figure 7. (a), (b) XPS spectra of N 1s and O 1s regions for three cycling conditions. (c), (d) FTIR spectra and X-ray patterns of the electrodes in three states.
Fourier transform infrared spectroscopy analysis
Ex situ FTIR analysis was also performed to verify the changes in molecule structures (Fig. 7c). For the discharged state of the battery, the peak at 1197 cm−1 comes into being, corresponding to the emerging of the C-O bond, while the intensity of the C=O peak (1693 cm−1) decreases. This implies that the C=O bond takes part in the redox reaction of TAPQ, forming a chelation between the C-O bond and lithium atoms. This is consistent with XPS data. When the cell was recharged, the vibration mode of C=O bond relatively strengthened while the C-O bond become weak but did not totally disappear as the as-prepared electrode shown, giving rise to the slight irreversibility of the battery.
X-ray diffraction analysis
Alteration in the crystal structure was also investigated by comparing the ex situ X-ray diffraction patterns of the electrodes retrieved in three different cycle conditions (Fig. 6d). We found that the initial crystal structure partially lost upon the first discharge and remained as this afterwards. As we can see in the pattern of the two cycled states, there are only two peaks can be detected obviously. One peak is at 9.7°, corresponding to the (002) plane, which also exist in the as-prepared state but the intensity is weaker. The other is at 13.3°, which is newly evolved and slightly different from the (012) plane in the pristine. Therefore, the crystallinity being kept is owing to the relatively strong intermolecular force of π-π stacking, providing electrochemical activity to the reaction during the battery cycling. While the losing part may be attributed to the distortion of weak hydrogen bonds among π stacks in the lithium interaction progress, in consistent with the poor cycle performance in the former several cycles. The observed amorphousness of the crystalline is very common for organic active materials.8,20,24
Sodium and potassium storage in TAPQ
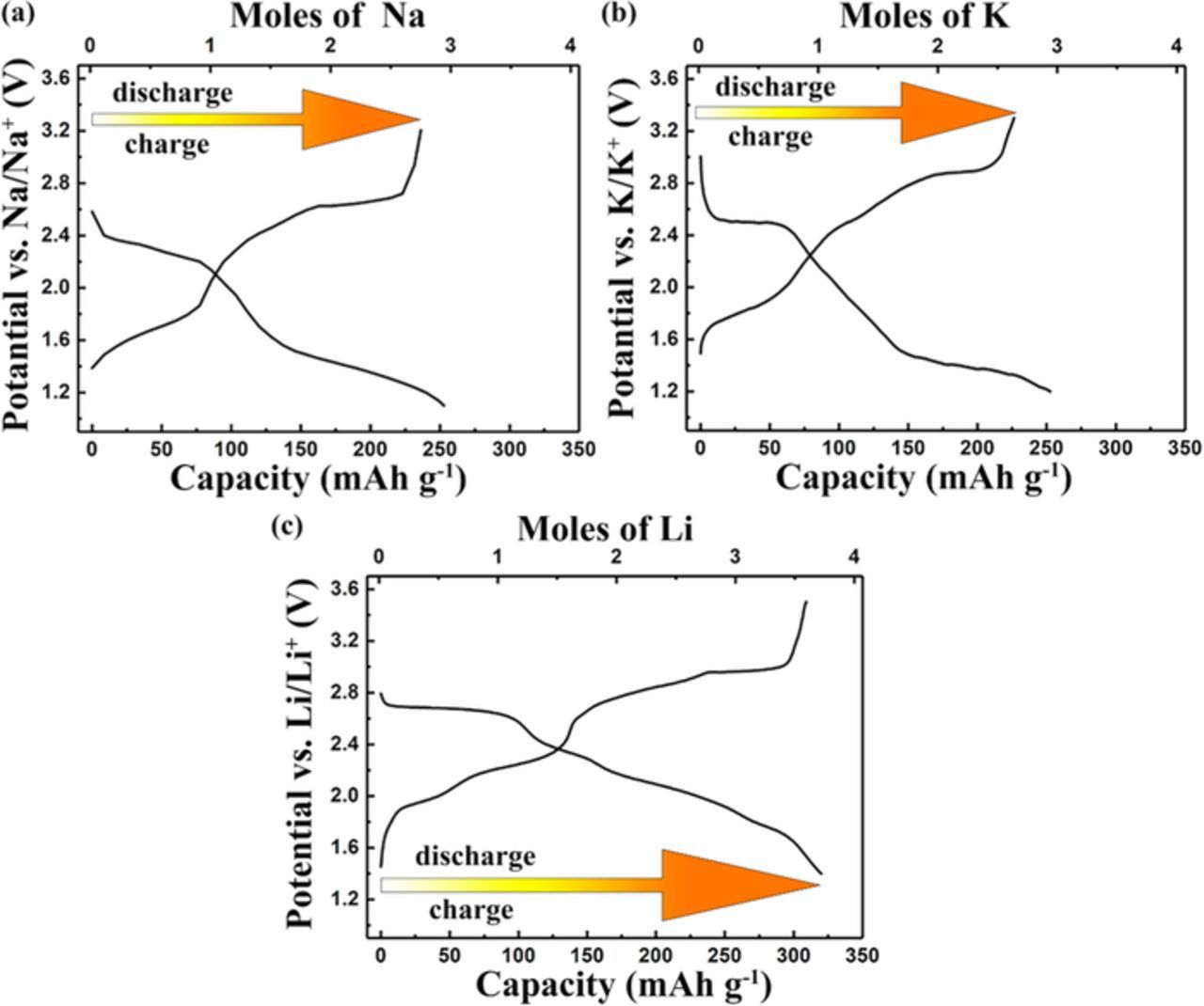
The possibility of utilizing TAPQ electrode is not limitedly based on LIB, it is also electrochemically active to sodium or potassium ion batteries (Fig. 8). The charge-discharge profiles reveal that there are two detectable pairs of voltage plateaus at 2.6/2.3 V and 1.6/1.3 V vs. Na/Na+ and also two pairs of voltage plateaus displayed at 2.9/2.5 V and 1.8/1.4 V vs. K/K+. This corresponds well with the redox potentials in lithium battery (3.0/2.7 and 2.1/1.7 V vs. Li/Li+). The similar curves indicate a similar redox mechanism of alkali ions in TAPQ electrode. While the cycling performance and capacity in sodium and potassium batteries are not as good as lithium battery. The limitation could be attributed to the non-optimized electrolytes and the larger radii of sodium and potassium ions compared with lithium. All in all, the insensitiveness to select the cation of counter electrode makes the TAPQ electrode more useful and its prospect more attractive.
Figure 8. (a) The first cycle galvanostatic discharge/charge curves of sodium battery at 1C with the cutoff voltage of 1.1–3.2V. (b) The galvanostatic discharge/charge curves of potassium battery at 0.5C with the potential between 1.2 and 3.3V for the first cycle. (c) The comparison of lithium battery galvanostatic discharge/charge curves at 0.1C in the potential range of 1.4–3.5V.
Conclusions
In summary, a conjugated aromatic compound with multiple fused redox centers of TAPQ was synthesized and investigated as a novel electrode of lithium battery. It can reach a reversible capacity up to 320.0 mAh/g at 0.1C and keep a capacity of about 150 mAh/g for over 300 cycles at 10C. In addition, both the computational and experimental measurements demonstrate the evolution of electrochemical reactions. The electrode can also be used in sodium or potassium ion batteries with a similar redox mechanism. In general, it is definitely a promising electrode to be developed.
Acknowledgments
This work was financially supported by and the National Natural Science Foundation of China (grant No. 21773037, 51722301 and 21674023), the National Key Scientific Research Project (grant No. 2016YFB0901500). In particular, the author also thanks Zhen-Yu Zhu in group of Xin Xu for his great contributions in DFT calculations very much.
ORCID
Zheng-Wen Fu 0000-0002-4649-0194