Abstract
An efficient new method was investigated for preparing smooth manganese (Mn) doped V2O5 films. This method uses cathodic electrodeposition to dope Mn ions into V2O5 films. The doped Mn concentration was in proportion to the applied voltage. X-ray diffraction patterns and Raman spectra were measured to detect the structure change of V2O5. The influence of Mn concentration on morphology, electrical, and optical properties of the films were also studied. Repeated chronocoulometry was applied to investigate the cycling stability of films with different Mn concentration. The results showed that the charge capacity of Mn-doped film was more stable than pure V2O5 film during cycling process. Moreover, the method will be useful in doping various ions into V2O5.
Export citation and abstract BibTeX RIS
V2O5 has been widely investigated because of its typical layered structure and because various species of ions and molecules can be reversibly intercalated and extracted between the layers. Amorphous V2O5 thin film has been known to intercalate larger amounts of lithium ions (Li+) than crystalline ones. This high capacity makes it useful for a wide variety of device application, such as batteries,1–3 sensors,4–6 and electrochromic devices.7–12 V2O5 displays a minor color change during the insertion and extraction of ions as described previously.13 It is often used as the counter electrode in electrochromic devices. Much work has been focused on the manufacturing and device performance of the V2O5 thin film with a variety of deposition methods.14–16 However, the development of V2O5 has been limited due to its poor cycling stability, which is essential for practical applications.
Much recent research haa been focused on improving the cycling stability of V2O5 films. Nanostructure,1,2,17–20 aimed at improving electrolyte accessibility and shortening the ion diffusion length, is beneficial to accelerating the electrochemical process. However, nanostructured V2O5 films still suffer from unsatisfactory cycling stability.21–23 Ion doping is another way of enhancing the electrochemical properties of V2O5 films. For example, the electronic conductivity of amorphous V2O5 was increased by doping with Ag, Cu, and Zn.24–29 While work on Mn doping is seldom reported due to the difficulty of doping with high concentrations, which is generally synthesized via a sol-gel process to improve the cycling durability of V2O5.30–32 In the sol-gel process, the mixed solution is clear for preparing smooth films at low Mn doping concentration. The precipitation occurs with an increase in Mn concentration and it is difficult to make smooth and uniform doped V2O5 films. Other methods such as the electron beam evaporation technique and hydrothermal reaction have also been adopted to prepare Mn-doped V2O5.33–35 These methods also have disadvantages for making smooth Mn-doped V2O5 films, such as rigorous experiment conditions and precipitation.
Here, we put forward a simple and low-cost electrochemical deposition method for preparing Mn-doped V2O5 films with great stability. Mn ions are inserted into V2O5 films by applying a negative voltage. Uniform doped films with different Mn concentration are obtained by adjusting the voltage. The effect of doping Mn ions on structure, morphology, and electrochemical properties of V2O5 films has been investigated in detail.
Experimental
All chemicals used in the experiment were analytical grade and used without further purification. The vanadium solutions were synthesized using a method reported by Livage et al.36 V2O5 powder (Alfa Aesar) was dissolved in 30 wt% H2O2 aqueous solution with a V2O5 concentration of 0.15 M to prepare the precursor solution for V2O5 films, the process details were described in our previous report.37 Chronoamperometry was performed for the deposition of V2O5 films on ITO glass in a conventional three-electrode cell, using a platinum (Pt) sheet as the counter electrode and a silver (Ag) wire as the reference electrode. Before deposition, the ITO glasses were ultrasonically cleaned in ethanol, then in deionized water, and finally dried at 110°C. The prepared V2O5 films were dried at room temperature.
The solution for Mn doping was prepared by dissolving manganese chloride tetrahydrate (MnCl2·4H2O) into deionized water forming a concentration of 0.1 M solution. The freshly prepared V2O5 films as working electrodes were immersed in manganese solution, with the Pt sheet working as the counter electrode and the Ag wire as the reference electrode. The Mn ions were intercalated into films by applying constant negative voltage for 15 s. Different negative voltages (−0.1, −0.3, −0.6, −0.8, and −1 V) were used to prepare Mn-doped V2O5 films with different concentration. These films were washed with deionized water and annealed at 300°C for 30 min. The Mn/V2O5 mole ratios of doped films were, respectively, 0.27:1, 0.4:1, 0.54:1, 0.64:1, and 0.94:1, which were measured by an inductively coupled plasma mass spectrometer (ICP-MS, X Series 2, Thermo Fisher Scientific Inc.,Waltham, MA, USA).
The structural characterization of the films was investigated by XRD on a diffractometer with Cu Kα (Kα = 1.5418 Å, 40 kV, 200 mA) radiation (TTR-III, Rigaku, Japan). XPS (ESCALAB 250, Thermo-VG Scientific, East Grinsted, West Sussex, UK) spectra were recorded using Al-Kα radiation as the excitation source. Raman Spectroscopy (LABRAM-HR, JobinYvon, France) was used to analyze the structural change of V2O5 by doping Mn2+ ions. The surface morphology was examined using scanning electron microscope (SEM, Sirion200, FEI, Hillsboro, Oregon, USA). The electrochemical and optical properties of films were investigated using UV/visible/near-infrared spectrophotometer (V-670, JASCO, Tokyo, Japan) and electrochemical analyzer (CHI 650D, CH Instruments, Shanghai, China). The electrochemical properties were measured in a three-electrodes cell. Pt sheet was used as the counter electrode and Ag wire as the reference electrode, and 0.1 M LiClO4 in propylene carbonate (PC) as the liquid electrolyte.
Results and Discussion
Structural characterization of Mn-doped V2O5 films
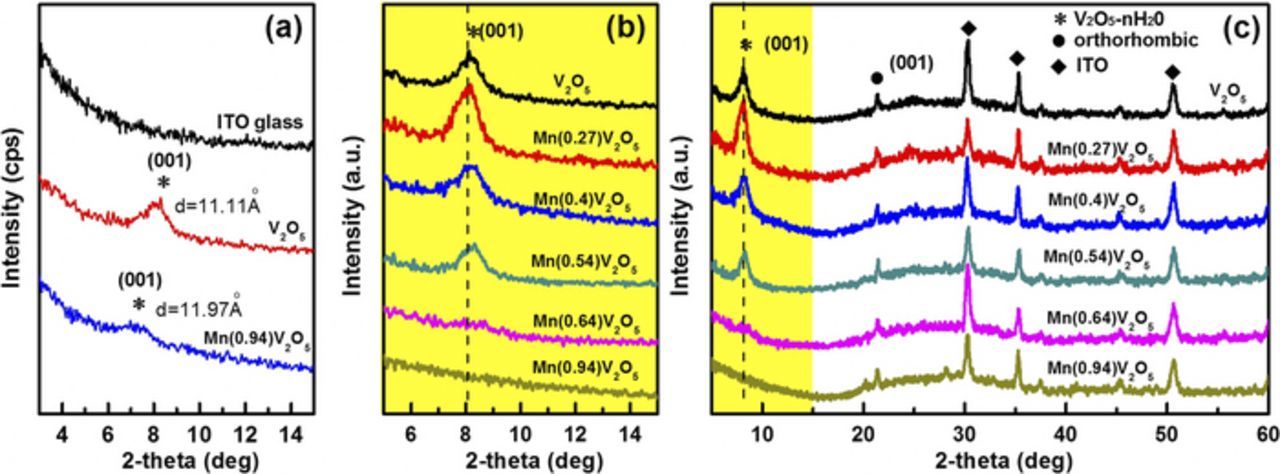
Figure 1 shows the XRD of pure and Mn-doped V2O5 films dried at room temperature for 12 h (Fig. 1a) and heated at 300°C for 30 min (Fig. 1b) separately. V2O5 has a typical layer structure, each layer being built up from VO5 square pyramids sharing edges and corners. The peak centred around 2θ = 8° indicates a predominant amorphous nature similar to those of V2O5•nH2O xerogels,30 suggesting that pure V2O5 films and Mn/V2O5 films with low molar ratio are hydrous vanadium pentoxide. The (001) diffraction plane is related to the V2O5 interlayer structure. For the unheated films shown in Fig. 1a, they are amorphous and the interlayer distance are calculated to be 11.97 Å for Mn-doped V2O5 films (Mn/V2O5 = 0.94), larger than 11.11 Å for pure V2O5 films, which could be due to the doping of Mn ions into V2O5 interlayer space.30 For the heated film shown in Fig. 1b, the diffraction peak of (001) plane gradually declines and then disappears with the increasing Mn content. The interlayer distance for different Mn/V2O5 molar ratio films (0:1, 0.27:1, 0.4:1 and 0.54:1) is 10.69 Å, 10.98 Å, 10.92 Å, and 10.75 Å, respectively. Compared with films dried at room temperature, the interlayer space shrinks for all heated films, because some water molecule intercalated between V2O5 layers is removed during the heating process. As the doping content increases, more interlayer water is squeezed out during the annealing process, causing the gradually decreased interlayer distance of the doped films. The XRD pattern also shows that the peak of (001) plane disappears at high doped Mn concentration, which illustrates that the layered structure of the doped films is disordered. In addition, the orthorhombic V2O5 (2θ = 21°) is found to coexist in all of the samples, indicating that the phase transition is not inhibited by the introduction of manganese ions into hydrous vanadium structure. The larger 2 theta window of XRD results are shown in Fig. 1c.
Figure 1. XRD pattern of pure and Mn-doped V2O5 films dealt at room temperature (a), 300°C temperature for 30 min (b) and its larger 2-theta window (c).
XPS analysis of Mn-doped V2O5 films
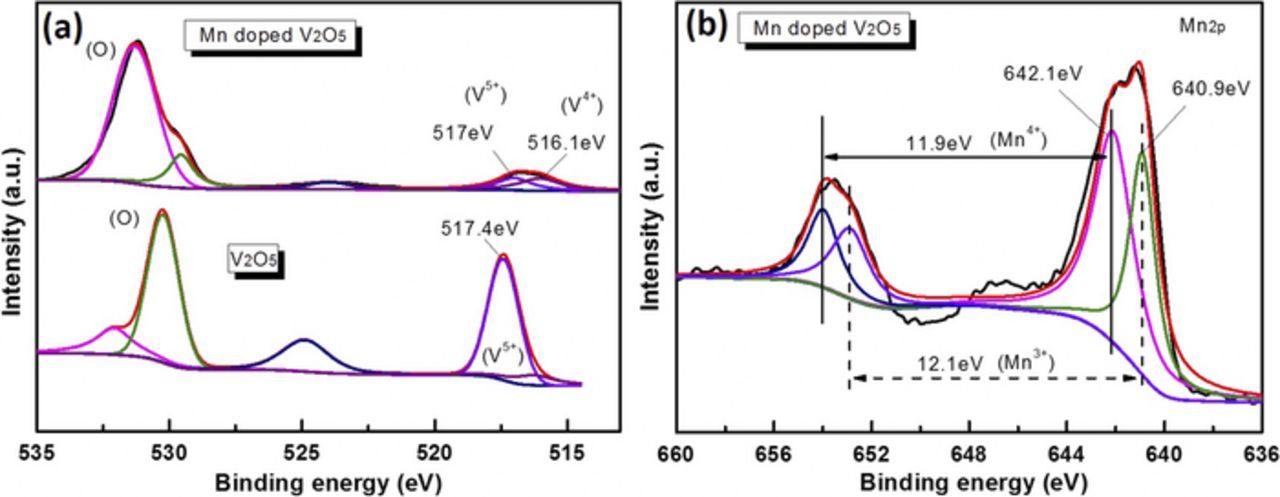
To investigate the valence change of vanadium in doped films, and get more information about the doping process, all the films are examined by XPS. Figure 2a shows V2p spectra of pure V2O5 film and Mn-doped V2O5 film at room temperature with high resolution. For pure V2O5 films, the V2p3/2 peak values are 517.4 eV,7 which is related to the states V5+. And one new peak at 516.1 eV (V4+) appeared near 517 eV (V5+) for Mn-doped V2O5 film and the intensity is similar, which means one new V = O bond appeared. The XPS spectra of Mn2p peaks for Mn-doped V2O5 film are also analyzed as shown in Fig. 2b. There are two main peaks which can be assigned to Mn4+ (642.1 eV) and Mn3+ (640.9 eV) with a 12.1 and 11.9 eV separation, respectively.40 The doping process is illustrated in
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/7/H541/revision1/d0001.gif)
During the doping process, negative voltage is applied, Mn ions and electrons are simultaneously doped into V2O5 films causing the low valence state of V (V4+), and the existence of Mn3+ and Mn4+ proves that the oxidation of Mn2+ occurred and that the ions do dope into the V2O5 interlayer space instead of depositing on its surface. When V5+ ions are partially replaced by Mn ions, [MnO6] octahedral30 may form in the framework of V2O5, introducing the three-dimensional character, which makes the structure stronger in dealing with the deformation of the material structure during Li-ion intercalation and de-intercalation cycling.30
Figure 2. XPS spectra of O1s, V2p3/2 (a) and Mn2p (b) energy regions of pure V2O5 films and Mn doped V2O5 films dealt at room temperature.
Raman spectra of Mn-doped V2O5 films
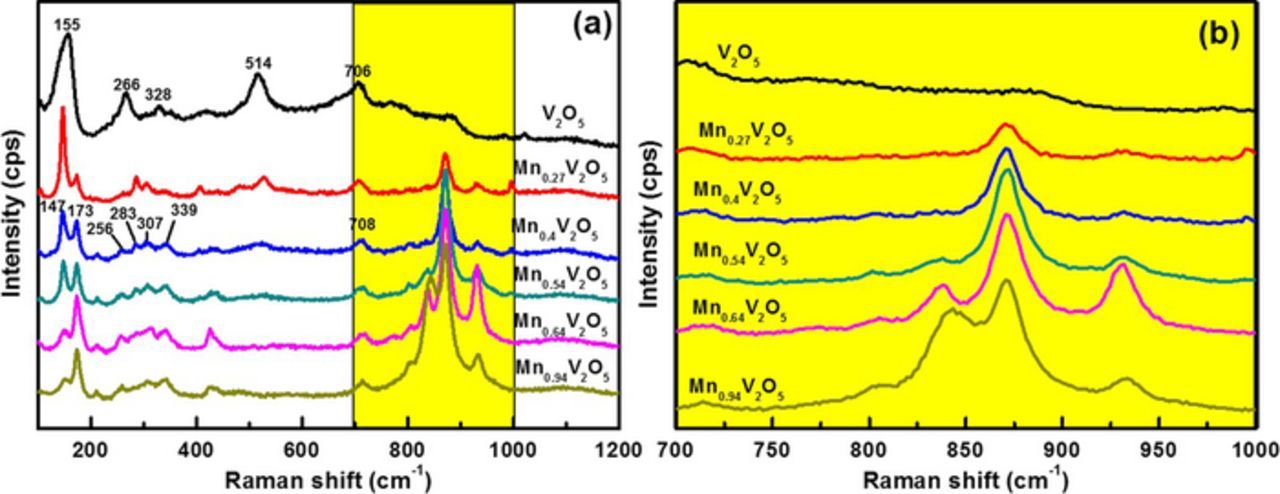
Figure 3 shows the Raman spectra of V2O5 films by Mn doping. The Raman modes of V2O5 can be classified into external and internal vibrations. Most of the low wavenumber modes can be described in terms of external modes. The external modes can be considered as relative motions of the V2O5 units with respect to each other. The internal modes, on the other hand, which are observed in the medium and high wavenumber, can be described in terms of O-V-O and V-O-V bending modes and V-O stretching vibration modes.
Figure 3. (a) Raman spectra of heat-treated films with different Mn concentration. (b) Enlarged plot of films with different Mn concentration.
The Raman spectra is collected for different Mn-doped amorphous V2O5 films (Fig. 3). For pure V2O5 films, the peak at 155 cm−1 describes the translational mode of V2O5 units.38 The peaks between 250 and 750 cm−1 correspond to the bending and stretching modes of V-O bond. The Raman spectra recorded for Mn-doped V2O5 films exhibits several changes, the wavenumber of the translational mode shifts from 154 to 147 cm−1, and a new peak appears at 173 cm−1. These changes in the translational mode illustrate the relative position changes of V2O5 unites, which influence the layer structure of V2O5 films. The peaks corresponding to V-O bond between 250 and 750 cm−1 declines with an increasing concentration of Mn. The peak located at 514 cm−1 disappear and new peaks appear between 800 and 1000 cm−1 (805, 843, 870, and 932 cm−1).15,39 These changes prove the existence of different types of V = O bonds,39 which are generated by the insertion of Mn ions into the V2O5 structure.
Surface morphology of Mn-doped V2O5 films
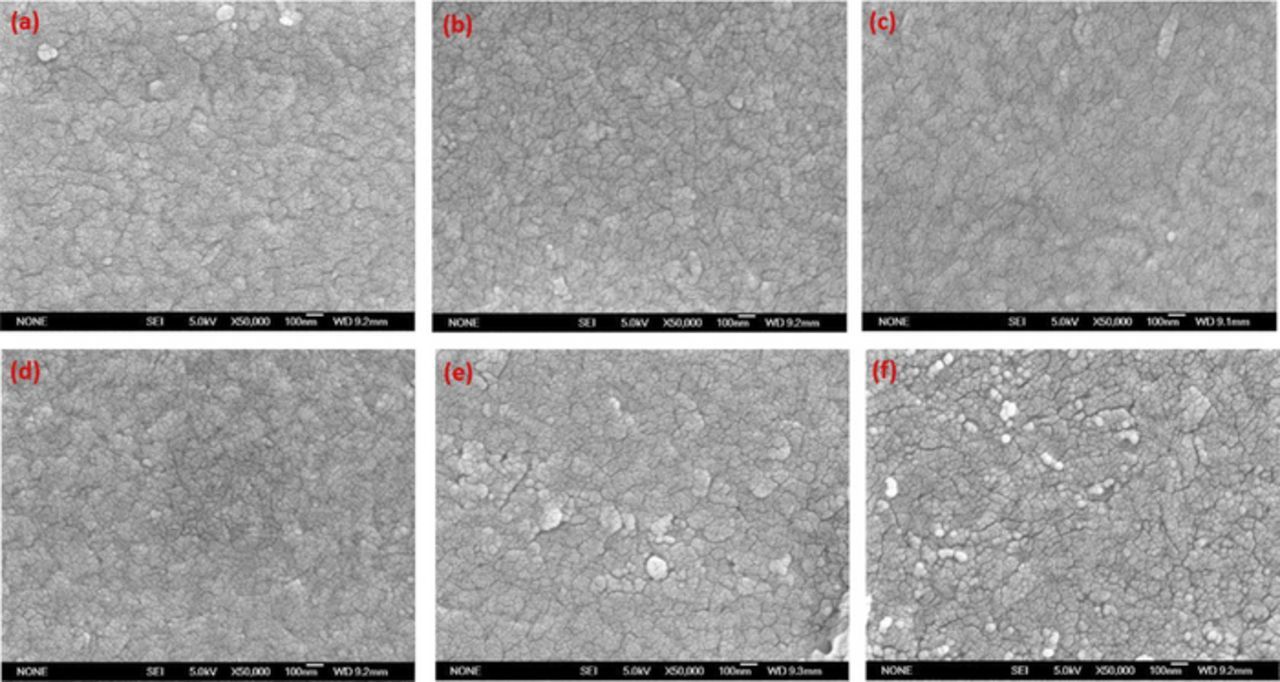
A scanning electron microscope (SEM) was used to study possible morphological changes for Mn-doped V2O5 films. Figure 4 shows the surface morphologies of Mn-doped V2O5 films with different Mn concentration. For pure V2O5 film, the surface is compact and contains many small particles (∼100 nm) with unclear boundaries (Fig. 4a). These particles are amorphous state confirmed by XRD results and are not crystalline grain. Compared with un-doped V2O5 films, there are many more small particles (∼50 nm) of Mn-doped films surface, and the boundaries become clearer and deeper. The situation appears obviously in the case of Mn/V2O5 = 0.64 and 0.94 is shown in Figs. 4e and 4f, which may be induced by the shrinkage of V2O5 structure with particles size decreasing, and similar results have been reported.30 The cracks on the surface reveal the influence of Mn doping, which changes the microstructure of the films directly and which will allow more flexibility in volume changes because of ions intercalation/deintercalation. The clear boundaries are beneficial to a better cycling performance.
Figure 4. SEM micrographs of films with different Mn/V2O5 composition: (a) 0:1, (b) 0.27:1, (c) 0.4:1, (d) 0.54:1, (e) 0.64:1 and (f) 0.94:1.
Electrochemical and optical properties of Mn-doped V2O5 film
To understand the oxidation and reduction behavior of the Mn-doped V2O5 films, cyclic voltammograms were recorded by a CHI 660D electrochemical workstation. The test was carried out using Pt sheet as the counter electrode and Ag wire as the reference electrode, and the electrolyte used is 0.1 M of LiClO4 in PC.
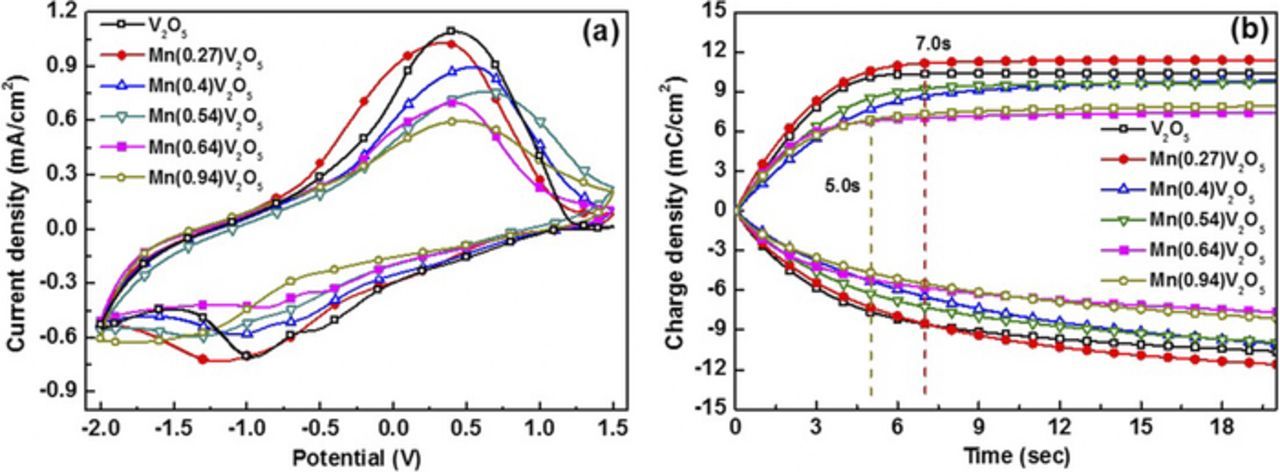
Figure 5 demonstrates the reduction and oxidation process with 10 mVs−1 scan speed for pure and Mn-doped V2O5 electrodes. Figure 5a reveals that the pure V2O5 film has an obvious anodic peak (0.42 V. vs. Ag/Ag+) and two cathodic peaks (−0.53 V, −0.97 V). For low doped content (Mn/V2O5 = 0.27), the intensity of the redox peaks increases with Mn content. In the doped V2O5 films with higher ratios, the intensity of both the cathodic and anodic peaks decrease as the increasing doped content, revealing a decrease in charge capacity. The anodic peak decreases to 0.33 V of the low doped content (Mn/V2O5 = 0.27), because of the enlarged interspace (10.98 Å). However, as more Mn is doped into V2O5 film, the layered structure is distorted and the interspace starts to decrease from 10.98 Å to 10.75 Å (Fig. 1), which makes the anodic peak increase to 0.67 V (Mn/V2O5 = 0.54). For the high doped content (Mn/V2O5 = 0.64/0.94), the anodic peak recovers back to 0.46 V because the layered structure is totally distorted (Fig. 1b) and more free space for the Li+ is provided. Figure 5b shows consistent results measured with the method of Chronocoulometry under ±1.2 V (vs. Ag/Ag+) for 20 s in a conventional three-electrode cell. Compared with pure V2O5 films, the charge capacity of the case (Mn/V2O5 = 0.27) slightly increases, mostly due to the enlarged interlayer space (Fig. 1b). However, the higher ratio also results in a decline in the charge capacity, which may be caused by the doped Mn occupying the efficient Li+ insertion sites and distorting parts of the original structure. The distorted structure will facilitate response time (the charge capacity reached 90% value of its max) that changes from 7.0 s (Mn/V2O5 = 0.27) to 5.0 s (Mn/V2O5 = 0.94), which is consistent with the result of anodic peak in Fig. 5a.
Figure 5. Cyclic voltammetry (a) and charge capacity (b) of Mn-doped V2O5 films with different Mn concentration. Supporting electrolyte (a and b): 0.1 M LiClO4 in PC; scan rate (a): 10 mVs−1.
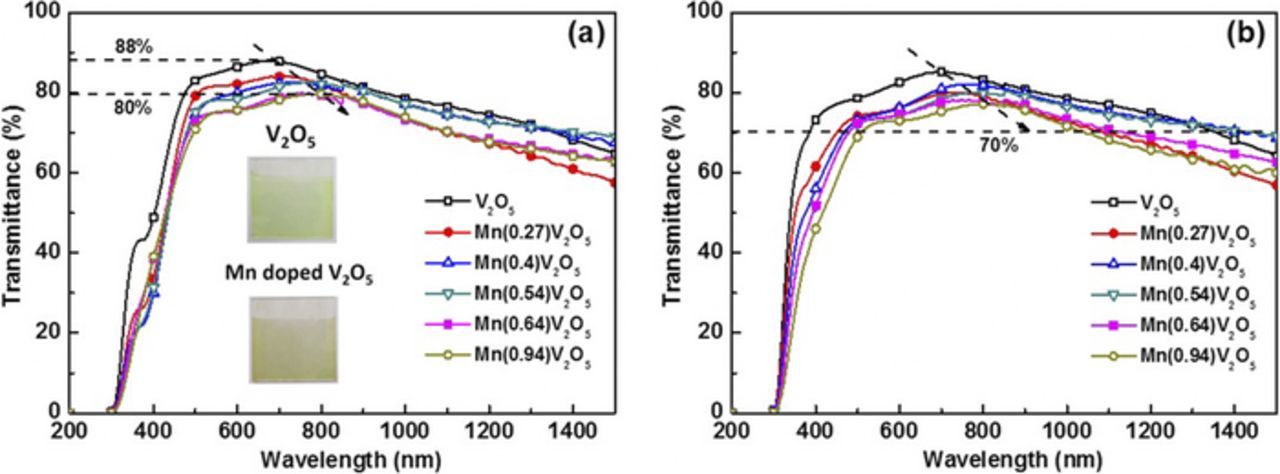
To discuss the application of Mn-doped films in electrochromic devices, the optical transmittance spectra as a function of wavelength range from 300 to 1500 nm are shown in Fig. 6. This experiment was carried out under 1.2 V (vs. Ag/Ag+). In the visible spectra, it is observed that the maximum transmittance for doped films decreases from 88% to 80% as Mn content increases (Fig. 6a), which is caused by the doping of Mn inserted into the V2O5 interlayer structure and formed Mn-O oxide (brown), and parts of vanadium ions (V5+) are transferred to V4+ (Fig. 2) leading to a lower transmittance of Mn-doped film. The photos of V2O5 film and Mn doped V2O5 film are inset in Fig. 6a. Meanwhile, the peak position of maximum transmittance in both reduced and oxidized states express a gradual redshift. The transmittance of doped films is larger than 70% in the visible spectra shown in Fig. 6b, which is high enough that it can be used as a counter electrode in an electrochromic device.
Figure 6. Transmittance spectra of Mn doped films with different Mn concentration in oxidized state (a) and reduced state (b).
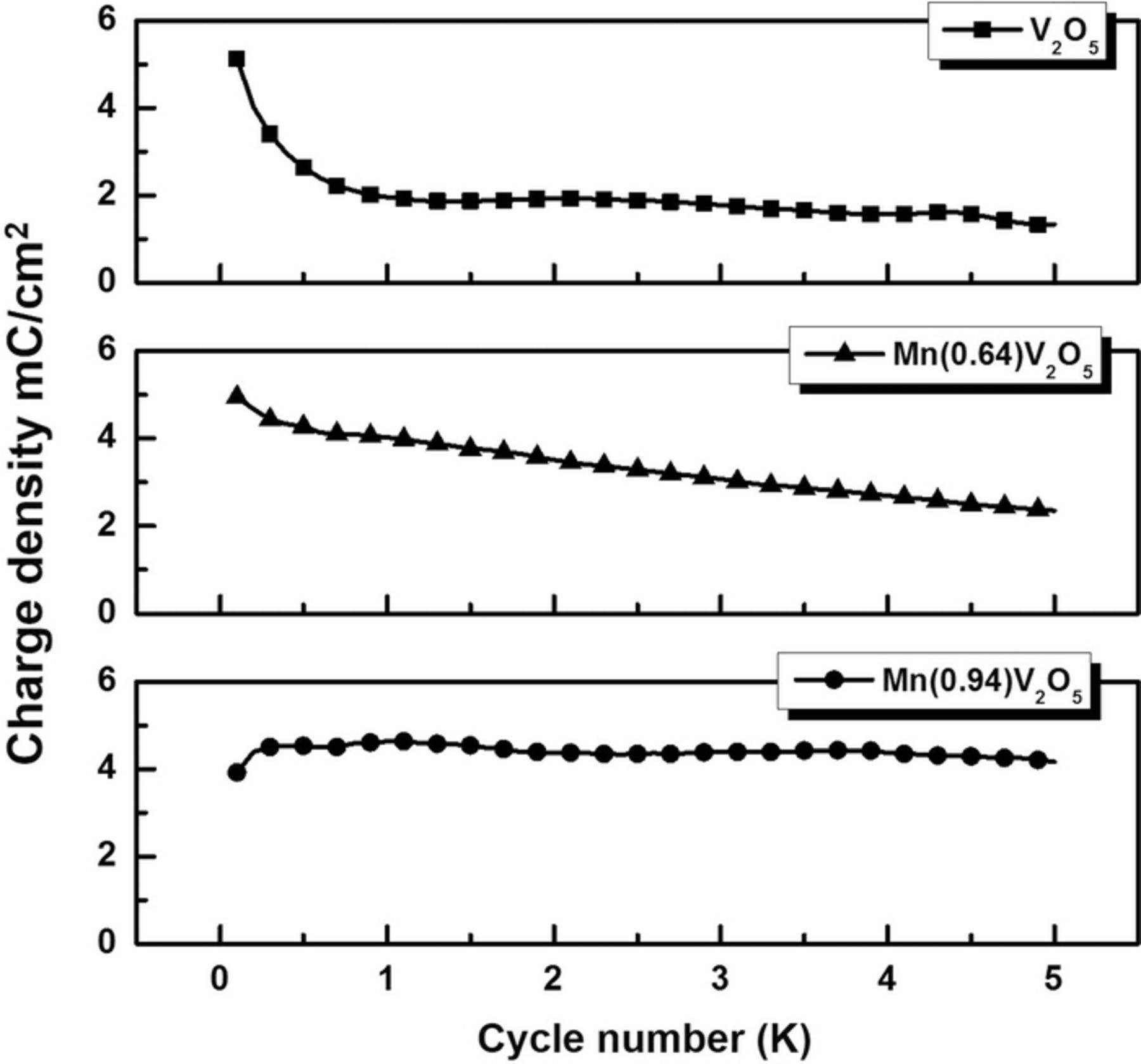
Finally, the Mn-doped V2O5 films were subjected to repeated insertion and release cycles to obtain cycling performance. Figure 7 describes the cycling characteristics of films, which is charged/discharged by applying constant voltage of 1.2 V. During the cycling process, the charge capacity of pure and low Mn-doped ratio (Mn/V2O5 = 0.64) V2O5 films decreases continually. The charge capacity of the pure films decreased to 38.3% of the initial capacity within 1000 cycles. In contrast to the pure films, the films with the molar ratio Mn/V2O5 = 0.64 exhibited palliatory charge fade, the charge capacity decreasing to 79.8% of the initial capacity. The addition of Mn in molar ratios Mn/V2O5 = 0.94 dramatically improved the cycling stability of the films, with almost no loss of the charge capacity. Such an excellent electrochemical stability of Mn-doped V2O5 films could be attributed to poor crystallinity or an amorphous-like nature and the presence of oxygen vacancies. The poor crystallinity makes it stronger in dealing with the deformation of the material structure during Li-ion intercalation/de-intercalation cycling. In addition, the presence of oxygen vacancies may also alleviate the stress or strain during Li-ion transference. The Mn content distorts the layer structure of V2O5 and provides more free space for the Li+ intercalation/deintercalation process. The cracks on the surface of films allow more flexibility in volume changes, which also leads to a better cycling performance.
Figure 7. Charge cycling stability of pure and Mn doped V2O5 films. The films were cycled between +1.2 and −1.2 V in a 0.1 M LiClO4/PC liquid electrolyte.
Conclusions
We used a novel eletrochemical method to prepare doped V2O5 films with different Mn concentration, and prepared one Mn-doped V2O5 film with good stability. XRD and XPS data showed that with Mn doping, the V2O5 interlayer space increased and the intensity of (001) plane was reduced or even disappeared. In Raman spectra, the appearance of new peaks in the high wavenumber region proved the existence of different types of V = O bonds. These results demonstrate that Mn ions were inserted into and distorted the V2O5 interlayer structure, which provided more free space for the Li+ intercalation/de-intercalation. Compared with pure V2O5 films, Mn-doped films (Mn/V2O5 = 0.94) had a better cycling performance. Other metal ions can also be doped into V2O5 layer structure by using this method, which can increase the charge capacity to match with working electrode (such as WO3) in electrochromic devices.
Acknowledgments
This work received financial support from the National Natural Science Foundation of China (21273207, 21274138 and 21474096); and the "Hundred Talents Program" of CAS.