Abstract
In the last few decades, there are some exciting developments in the field of lithium (Li)-ion batteries from small portable devices to large power system such as electric vehicles (EVs). However, the maximum energy density of lithium-ion batteries is insufficient for the extended range of EVs propulsion. On the other hand, metal-air batteries have a greater power storage capacity, a few times more than the best performing lithium-ion batteries. Mechanically rechargeable zinc (Zn)-, magnesium (Mg)-, and aluminum (Al)-air batteries are receiving increasing attention, due to the advantages of using safe, low cost and abundant materials. If successfully developed, these batteries could provide an energy source for EVs comparing that of gasoline in terms of usable energy density. Nevertheless, there are still numerous scientific and technical challenges that must be overcome, if this alluring promise can be turned into reality. This paper provides a comprehensive overview of recent advances and challenges of metal air batteries from various elements, including air cathode, electrolyte, and anode. In addition, this review outlines the fundamental principles and understanding of the electrochemical reactions in the areas of lithium-air batteries. Finally, a summary of future research directions in the field of the metal-air batteries is provided.
Export citation and abstract BibTeX RIS
The world is moving toward green energy that produces less CO2 emissions. However, there is a dichotomy between power production and CO2 emissions. Burning hydrocarbon releases a significant amount of CO2 into the environment. Therefore, several renewable energy sources are being developed as alternative energy resources, such as solar and wind energy, hydropower and other renewable energy sources. The development of high energy storage systems is essential to save extra power for the increased demands and delivery to where it is required. The energy storage technologies available for large-scale applications can be divided into four types: mechanical, electrical, electro-chemical and chemical.1 Among these, electrochemical energy storage approach is popular due to the mechanisms used to store energy.2 In general, electrochemical energy storage possesses a number of desirable features, including pollution free operation, high round trip efficiency, a long life cycle, low maintenance and energy characteristics such as quick response when the contingency occurs to meet different grid functions. Batteries represent excellent energy storage technology for the integration of renewable resources. Fuel cells and super capacitors are also excellent mediums for electric energy with high power or energy density. Consequently, they are also receiving considerable attention.3 There are some basic parameters that are vital to energy storage systems, such as energy density (Wh/L), specific energy (Wh/kg), power density (W/L), specific power (W/kg), cycle life, almanac life, safety and cost.
The energy sources as for powering EVs require both high specific energy and high specific power. The feature of high specific energy is favorable for long driving range, while the high specific power is desirable for high acceleration rate and hill climbing capability.4 The development of electric vehicles will decrease the dependency on fossil fuels and increase the deployment of renewable energy resources. Lead acid batteries are commonly used battery for electric vehicles propulsion in the 90s, but its applications are limited by relatively low energy density.5 Other advanced battery systems such as nickel (Ni)-cadmium (Cd), nickel-metal hybrid, lithium-polymer, sodium-sulfur, and sodium (Na)-metal chloride batteries are also being actively pursued for vehicle propulsion applications.6,7 Most of today's EVs, especially cars, are moving toward using lithium-ion batteries for propulsion.8 However, lithium-ion batteries powered EVs have a driving range limited to 160 kilometers on a single charge and the batteries account for nearly 65% of the total cost.9,10 It is known that the theoretical energy density of gasoline is 13,000 Wh kg−1 and the energy density of lithium-ion batteries is around 100–200 Wh kg−1. Considering the energy conversion efficiency of tank-to-wheel of the fleet is 12.6%; therefore, the practical energy density of gasoline is 1700 Wh kg−1, which is still much higher than that of lithium-ion batteries. Therefore, novel energy systems with higher energy densities are prominently desired.11,12
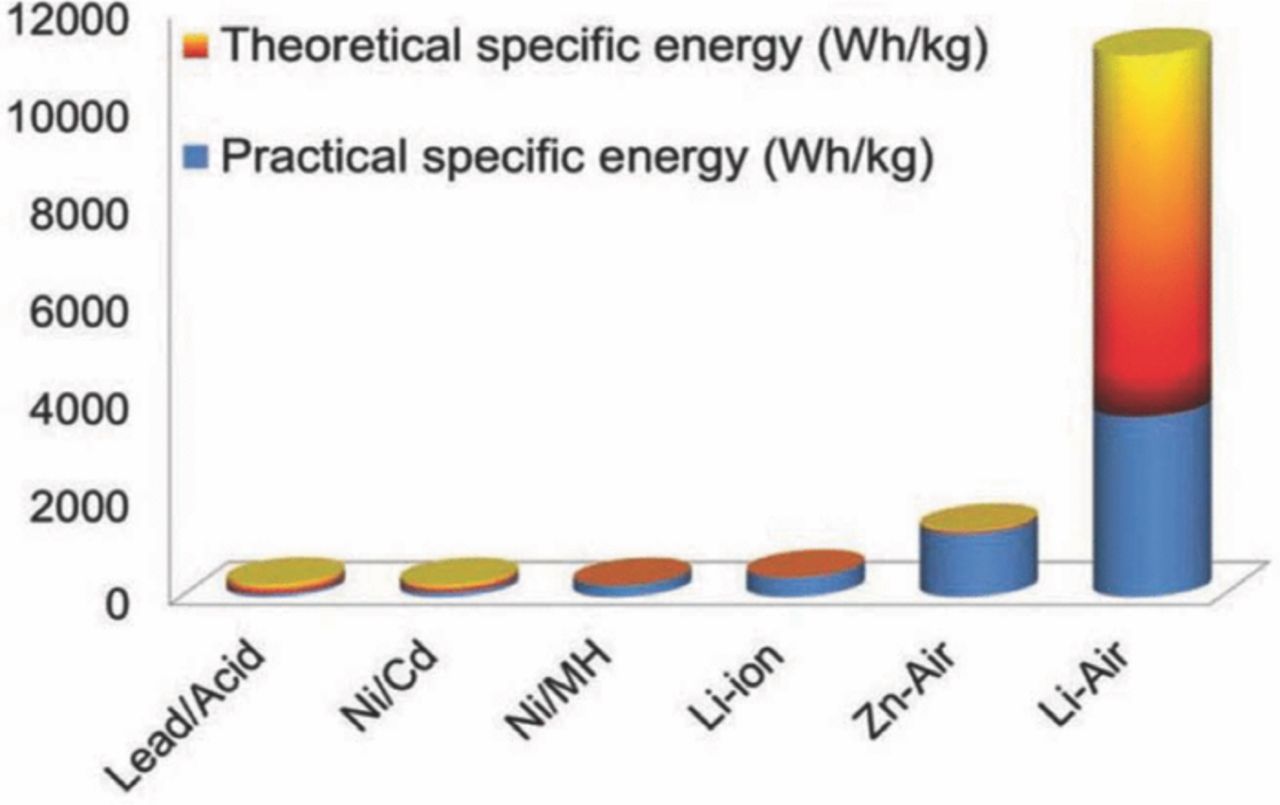
Metal-air batteries such as lithium-air, zinc-air, magnesium-air, and aluminum-air batteries are promising for future generations of EVs because they use oxygen from the air as one of the battery's main reactants, reducing the weight of the battery and freeing up more space devoted to energy storage. Among all these metal-air batteries, lithium-air battery shows the highest theoretical energy density, rivaling the gasoline engine (13000 Whkg−1). It has a much greater energy density than other rechargeable batteries,13,14 as shown in Fig. 1. But, there are many challenges facing the design of rechargeable lithium-air batteries such as incomplete discharge as porous carbon cathode blocking by discharge products, unstable anode in atmospheric moisture,15 inadequate understanding of catalysts effect,16 low electrical efficiency due to higher charge overpotential than discharge overpotential,17 carbonate based electrolytes decompose during discharge and produce lithium alkyl-carbonates and Li2CO3,18 which severely affects the rechargeability and cycle life of lithium-air batteries19 etc. There are also challenges for secondary Zn-air batteries such as requirement of closely controlled zinc precipitation, zinc anode dendrite formation, non-uniform zinc dissolution and limited solubility in electrolytes, higher charge overpotential than discharge overpotential, and necessity of bi-functional air cathode to liberate oxygen from discharge reaction products20 etc. In addition, both Mg-air and Al-air batteries are early in development and have received research attention from researchers. It is a matter of fact that Mg-air batteries are rechargeable but not the Al-air batteries. These two batteries are confronting several challenges such as corrosion problems of metal Mg and Al as anode, sluggish discharge products, high self-discharge rate, cell irreversibility, and low shelf life21,22 etc. Therefore, there are still numerous scientific and technical challenges of metal-air batteries that must be overcome before this promising technology is to turn into reality.
Figure 1. Theoretical and practical energy densities of batteries (adapted from).13
This review only briefly discusses the challenges of lithium-air batteries as there are already a number of excellent review papers in this area published to date.12,15,23 In addition, this paper focuses on the recent developments of zinc-air, aluminum-air, and magnesium-air batteries since these batteries are currently receiving increasing considerations in solving present energy demands and concerns.
Metal-Air Batteries
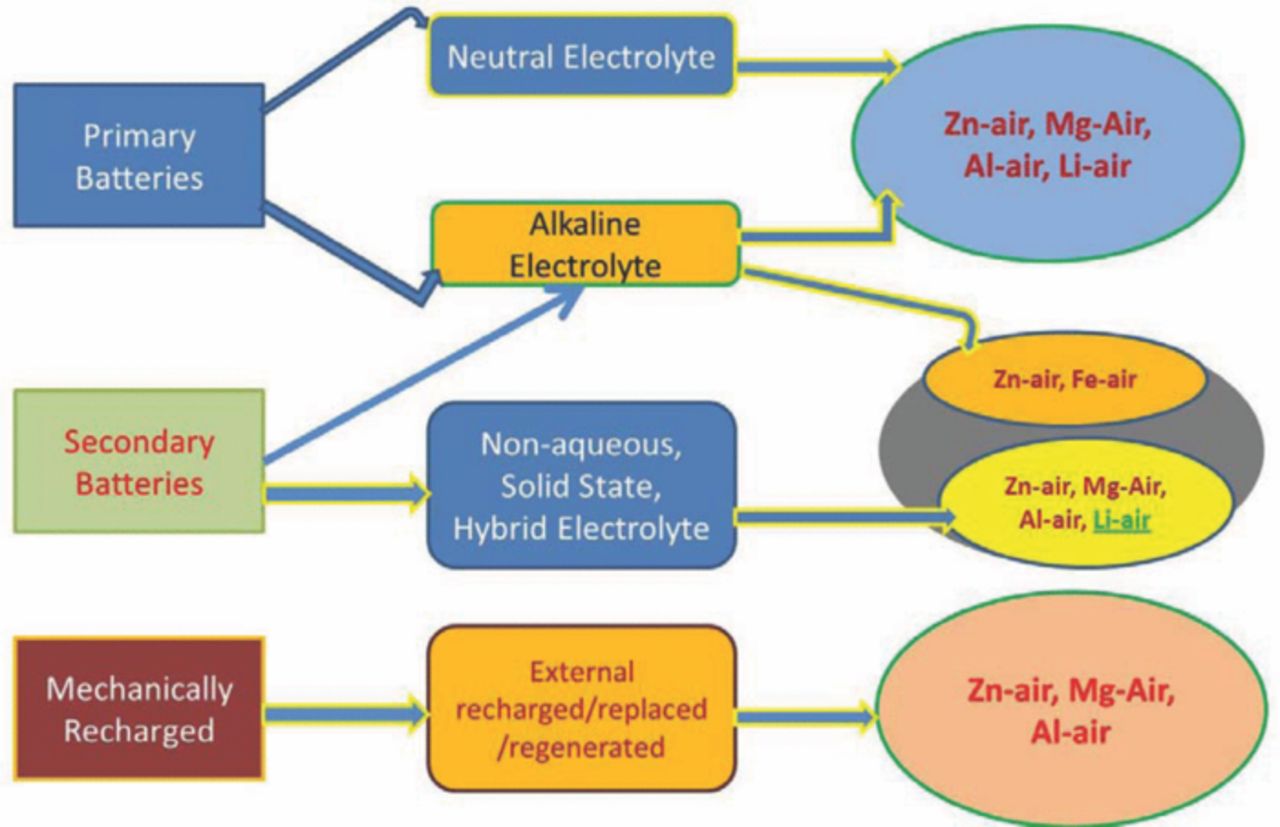
Metal-air batteries are the compact and potentially less expensive batteries available. Although many manufacturers offer refuelable units where the consumed metal is mechanically replaced and processed separately, not many developers offer an electrically rechargeable battery. Rechargeable metal-air batteries, especially lithium-air batteries, that are under development, have a life of only a few hundred cycles and an energy efficiency of about 50%.24 The notable characteristics of these metal air batteries is their open cell structure, using oxygen gas accessed from the air as their cathode material. There are several kinds of metal air batteries available based on different metal species and their reaction mechanisms. Fundamentally, metal-air batteries can be classified into two types according to their electrolytes. One is a cell system using an aqueous electrolyte, which is not sensitive to moisture. The other is a water sensitive system using an electrolyte with aprotic solvents, which is sullied by moisture.13 Fig. 2 presents the schematic diagrams of various types of metal-air batteries.
Figure 2. Types of metal-air batteries.
Since advanced electronic equipments are being rapidly introduced, this has resulted in an ever-increasing demand for high energy density and power density power sources. As a result, researchers have intensely focused on the metal-air batteries but it is still a work in-progress. Some metals are considerably affected by aqueous electrolyte, yet others are not. Metals such as Cd, Al, calcium (Ca), iron (Fe) and Zn are most appropriate for an aqueous electrolytic system. A comparison of various metal air batteries is shown in Table I.
Table I. Metal oxygen energy densities (Summarized from).25
| Metal | Cell Voltage | Energy density (weight basis) | Energy density (volume basis) |
|---|---|---|---|
| Oxygen | (Volts) | (Wh/kg) | (Wh/L) |
| CaO | 3.11 | 2972 | 9960 |
| MgO | 3.03 | 4032 | 14400 |
| Li2O2 | 2.98 | 3487 | 8050 |
| Li2O | 2.93 | 5252 | 10600 |
| Al2O3 | 2.75 | 4332 | 17300 |
| Na2O | 1.97 | 1703 | 3870 |
| ZnO | 1.68 | 1109 | 6220 |
Lithium-Air Batteries
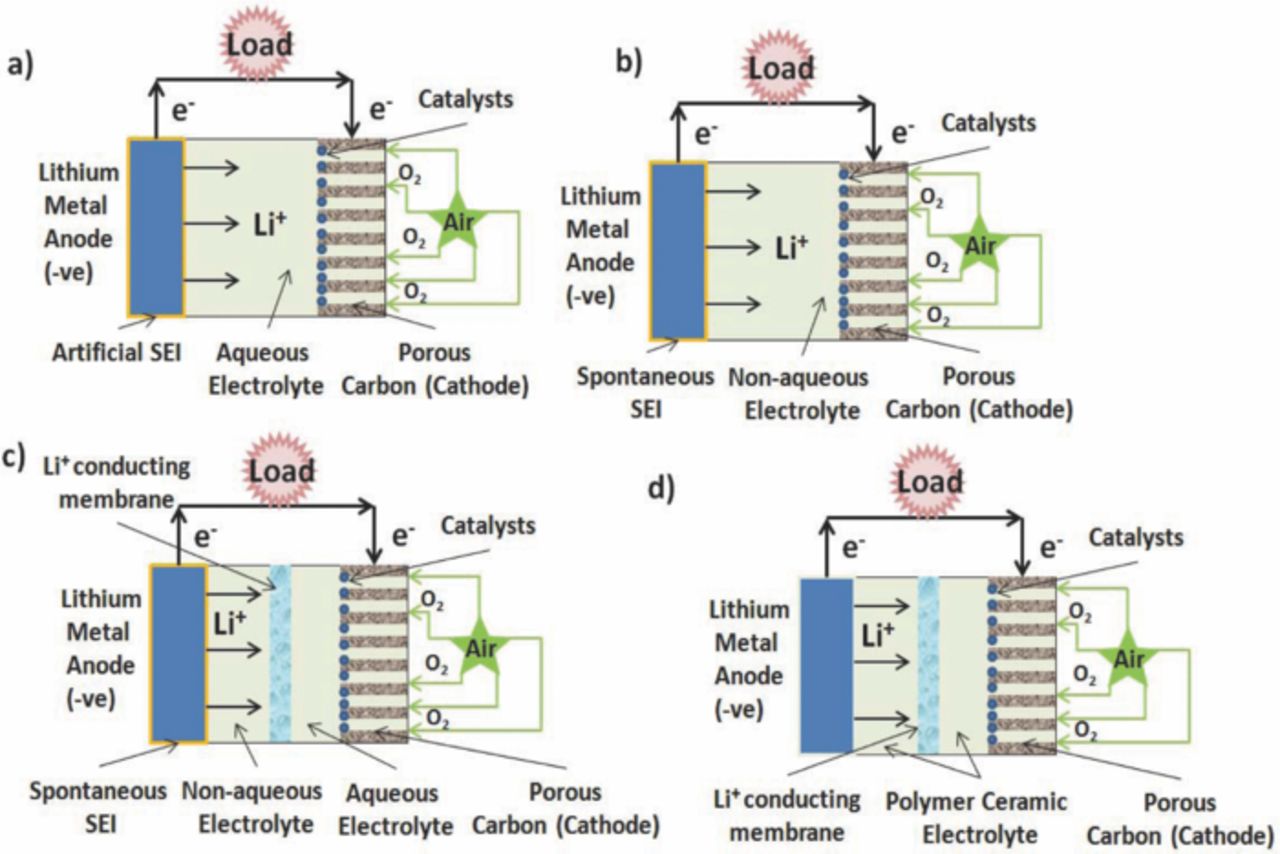
Lithium-air batteries have shown 5–10 times more energy density than a standard Li-ion battery. The specific energy density of a Li-air battery is 5200 Whkg−1 or 18.7 MJkg−1 when the mass of oxygen is included. However, Li-air batteries did not attract wide attention when they were introduced, although they have higher energy densities than those of conventional Li-ion battery systems (200–250 Whkg−1).26 The estimation of theoretical capacity and energy of Li-air batteries is in debate. Theoretical gravimetric and volumetric energy densities using aqueous electrolytes have been investigated based on Li metal anode, air electrode, and electrolyte.27 It was determined that the maximum theoretical gravimetric and volumetric energy densities are 1300 Whkg−1 and 1520 WhL−1 in basic electrolyte, and 1400 Whkg−1 and 1680 WhL−1 in acidic electrolyte, respectively. The above specific capacity and energy projections are the theoretical maximum limitation and are based on active materials including only Li metal, air electrode, and electrolyte. However, in practical cells, some excess electrolyte must be in the cell in order to provide ionic conductive media during the entire discharge process. In addition, other necessary materials, including current collectors, membrane, and package materials, will further reduce the cell specific capacity and energy by 20–30%.27 Li-air battery is comprised of a lithium metal foil anode, a Li+ conductive electrolyte and a thin carbon composite air cathode.28 There are four types of Li-air batteries based on electrolytes as shown in Fig. 3: i) aprotic/non-aqueous electrolytic type, ii) aqueous electrolytic type, iii) mixed/ hybrid electrolytic type, and iv) solid-state electrolytic type. All Li-air battery systems use lithium metal as the anode and oxygen gas as the cathode material, but their electrochemistry differs according to the electrolyte types.12
Figure 3. Types of lithium-air batteries: a) Aqueous electrolytic type, b) Aprotic/non-aqueous electrolytic type, c) Mixed (aprotic-aqueous) type, and d) Solid-state type.
In aprotic/ non-aqueous electrolytic type of Li-air battery, a liquid organic electrolyte is used. Lithium salt such as LiPF6, LiAsF6, LiN(SO2CF3)2, and LiSO3CF3 in organic solvent such organic carbonates, ethers, and esters are commonly used electrolytes.29 In aqueous electrolytic type of battery has the same structure as an aprotic type Li-air battery except that electrolyte used is aqueous lithium salt. Lithium as an anode reacts excessively with water and corrodes. Therefore, it is necessary to have an artificial solid electrolytic interface (SEI) over the surface of the lithium anode. Usually lithium ion conducting ceramic or glass is used as the SEI. Several attempts have been made to introduce this effective Li+ conductive solid electrolyte interface. A protective glass ceramic layer of LiSICON, such as Li2+2x Zn(1-x)GeO4 (−0.36 < x > 0.87), LiM(PO4)3 like LiTi2(PO4)3 for Li was introduced for the metal anode.30,31 In mixed (aprotic-aqueous) type of battery, both aprotic and aqueous type electrolytes are used to overcome the limitations of the aprotic type and aqueous type Li-air battery. A lithium ion conducting membrane is used to separate the two types of electrolytes. Typically, a lithium anode is in contact with an aprotic type electrolyte and a cathode of porous carbon is in contact with an aqueous type electrolyte. There is no cathode clogging and moisture problem in this type of Li-air battery and less production of spontaneous SEI layers due to aprotic electrolyte used.32,33 Usually polymer ceramic or glass with a Li+ conducting membrane separating the anode and cathode as the electrolyte is used. This cell exhibited excellent thermal stability and rechargeability in the range of 30 to 105°C.34 However, the main drawback of this type of battery is lower ionic conductivity of the glass-ceramic electrolytes such as NASICON structure of Li1+x Ge2-x Al(PO4)3 electrolyte compared to liquid electrolytes.35
During the last few years, most of the research carried out on Li-air batteries has focused on the development of novel catalyst and cathode materials. In contrast, less progress has been made on the electrolyte, which represents the actual challenge in the development of rechargeable Li-air batteries. It is not practical for the Li-air battery to use an aqueous electrolyte unless the anode can be protected from parasitic degradation.36 One solution to this issue is to form an entirely solid-state battery or mixed electrolyte battery as mentioned earlier. This review only covers non-aqueous electrolyte system. Currently, the electrolytes in non-aqueous Li-air batteries can be divided into two types based on the solvent used. One is organic carbonate based (Propylene carbonate, ethylene carbonate, diethyl carbonate, and dimethyl carbonate) and the other is ethers based (tetrahydrofuran, dioxolane, dimethoxyethane, tetraethylene glycol dimethyl ether), which solvate lithium salts, such as LiBF4, LiPF6, LiN(SO2CF3), and LiSO3CF3.12,37,38 The organic carbonate-based electrolytes are well proved systems in conventional Li-ion batteries. Their advantages are low volatility, compatible with Li anode, high ionic conductivity and oxidation stability with respect to the Li/Li+ couple. In the case of Li-air battery, several Li salt/organic carbonate combinations were investigated, generally based on propylene carbonate (PC) and different co-solvents, such as ethylene carbonate, ethers or glymes, in order to control the oxygen solubility, the solution viscosity and ionic conductivity, the evaporation rate, and the polarity.38,39 However, it has been reported that such electrolytes decompose in Li-air batteries during discharge, and forms Li2CO3, rather than electrochemically reversible Li2O2.18 Ethers are also attractive for Li-air batteries for highly stable with Li metal anode, stable to a high oxidation potential over 4.5 V versus Li/Li+, safe, inexpensive and low volatility.18,40 Bruce et al.41 reported that although the ethers are more stable than organic carbonate, Li2O2 only forms on the first discharge and ether-based electrolytes undergoes decomposition, giving a mixture of Li2CO3, HCO2Li, CH3CO2Li, polyethers/esters, CO2 and H2O. Especially after only five cycles, there was little or no evidence of Li2O2 and replaced by Li2CO3. Therefore, neither carbonate nor ether-based electrolyte is suitable for Li-air batteries. This remains as a major challenge in the development of rechargeable Li-air batteries. The demonstrated unsuitability of the organic carbonate-based and ether-based electrolytes for Li-air batteries diverts the research interest toward the ionic liquids-based Li-air batteries. The use of ionic liquids (ILs) in no-aqueous Li-air batteries is attractive due to their properties such as nonflammability, low toxicity, high boiling points, high lithium salt solubility and a high oxidation potential of 5.3 V versus Li+/Li.42,43 It was reported that high discharge capacities greater than 5000 mAh/gcarbon at very low discharge currents of 0.01 mA/cm2 was observed by using hydrophobic ionic liquid as electrolyte.42 Abraham et al.44 investigated the oxygen electrode (glassy carbon (GC) and gold (Au) electrodes) rechargeability in a neat and Li+-containing room-temperature ionic liquid (RTIL), 1-ethyl-3-methylimidazolium bis (triflouromethanesulfonyl) imide (EMITFSI). The result indicated that the nature of the electrode affects the reaction mechanism, with Au showing the ability for high efficiency recharging of the oxygen electrode, as evidenced by multiple cycles without passivation. Although ILs may be employed as electrolyte solvents in Li-air batteries, they have to be further modified in order to exhibit both higher electrochemical stability and lower viscosity for better ion diffusion. Currently, a discharge voltage ranging from 2.0 to 2.5 V were observed for ILs, while it was recorded at a higher potential range between 2.7 to 2.8 V for the carbonate-based electrolytes. However, sometimes it is difficult to satisfy simultaneously requirements of electrochemical stability and low viscosity. Therefore, ionic liquids are promising electrolytes for rechargeable Li-air batteries, and selection of an electrolyte that is stable to both the Li and oxygen electrode is recognized as a major future direction of research.
A recapping summary of the aforementioned four types of Li-air batteries is presented in Table II.
Table II. Summary of lithium-air batteries.
| Types | Cell reactions | Electrolytes | Remarks | Ref. |
|---|---|---|---|---|
| Aprotic/ Non-aqueous Electrolytic Type | 2Li + O2→Li2O2 E0 = 3.10 V vs. Li/Li+ 4Li + O2→2Li2O E0 = 2.91 V vs. Li/Li+ | Lithium salt such as LiPF6, LiAsF6, LiN (SO2CF3)2 & LiSO3CF3 in organic solvent such organic carbonates, ethers, and esters. | Spontaneous solid electrolytic interface (SEI) produced and that is why anode is free from dendrite formation. This battery is extremely flammable at high temperatures, as an organic electrolyte used. Clogged the pores of the carbon cathode and prohibits the introduction of O2 by Li2O2. | 28,29 |
| Aqueous Electrolytic Type | 4Li + O2 + 2H2O→4LiOH (Basic electrolyte) 4Li + O2 + 4H+→4LiOH + 2H2O (Acidic electrolyte). | Lithium salt in water. | Artificial solid electrolytic interface (SEI) is essential to resist the violent reaction of lithium anode with water. Protective glass ceramic layer of LiSICON, such as Li2 + 2x Zn(1-x)GeO4 (−0.36 < x > 0.87), LiM(PO4)3 like LiTi2(PO4)3 or LTP has been using as SEI layer. No cathode clogging and moisture effect | 25,30,31 |
| Mixed (Aprotic-Aqueous) Type | Aforementioned | Aforementioned | Effective lithium ion conducting membrane is essential to conduct Li+ and separating two types of electrolytes. No cathode clogging and moisture effect. Spontaneous SEI layer produced. | 32,33 |
| Solid-State Type | O2 + 2e + 2Li+→Li2O2 3.10 V | Polymer ceramic or glass. | No lithium dendrite formation, excellent thermal stability, and rechargeability. Low ionic conductivity compared to liquid electrolytes. | 34,35 |
Zinc-Air Batteries
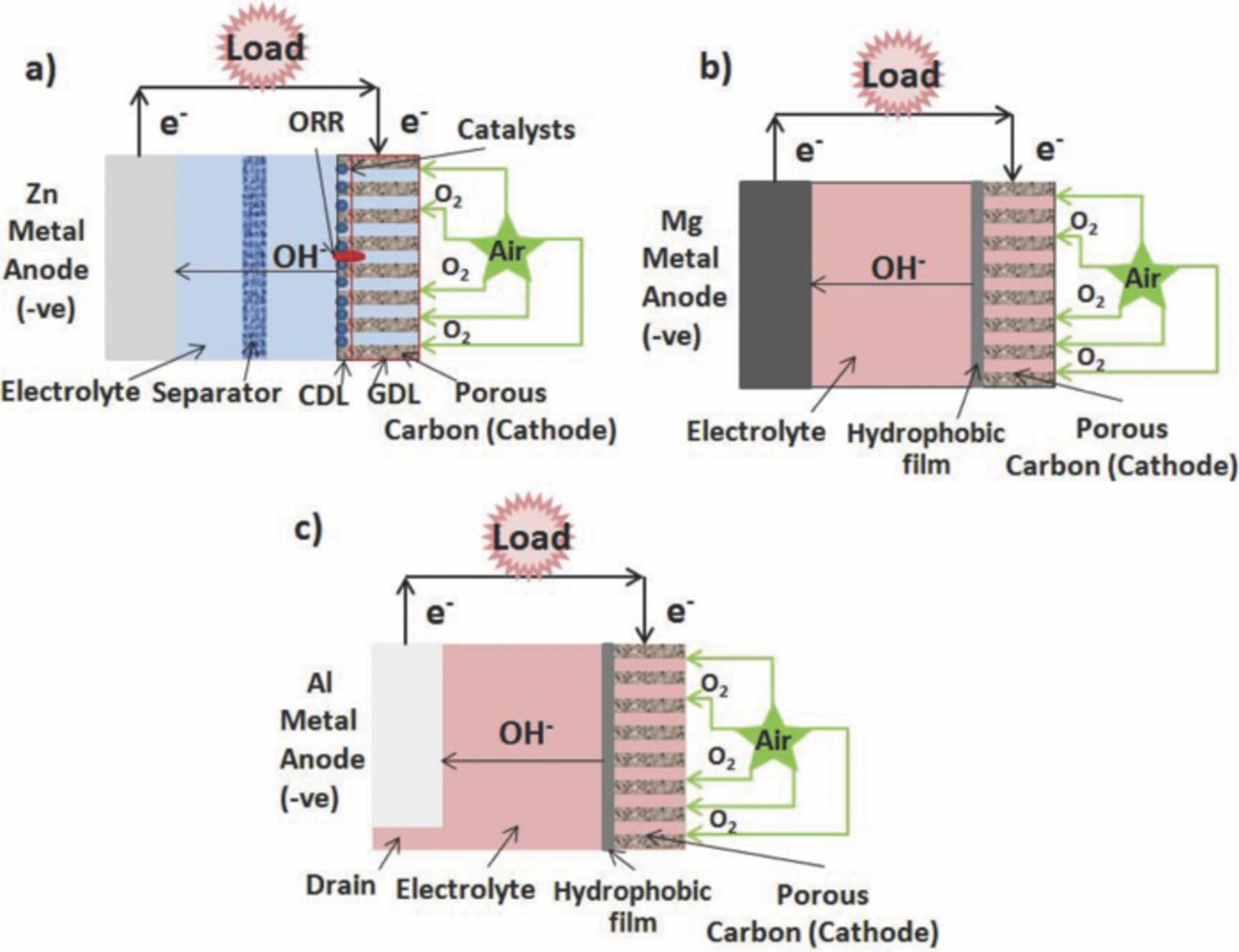
Zinc-air batteries are composed of three parts: zinc metal as anode, an air electrode as the cathode which is divided into catalytic active layer (CAL) and gas diffusion layer (GDL), and a separator, as shown in Fig. 4a. The theoretical specific energy density of zinc-air battery is 1350 Whkg−1.45 Oxygen from the atmosphere diffuses into the porous carbon electrode by difference in pressure of oxygen between the inside and outside of the cell, and then catalyst facilities the oxygen reduction reaction (ORR) to hydroxyl ions in alkaline electrolyte with electrons generated from the zinc metal as anode reaction. This process is a three-phase reaction: catalysts (solid), electrolyte (liquid), and oxygen (gas). The generated hydroxyl ions migrate from air cathode to the zinc metal anode, and complete the cell reaction.
Figure 4. Schematic illustration: (a) zinc-air battery, (b) magnesium-air battery, and (c) aluminum-air battery.
The overall electrochemical reactions during discharge in alkaline solution of zinc air cell are as follows:46
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn1.jpg)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn2.jpg)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn3.jpg)
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn4.jpg)
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn5.jpg)
However, zinc corrosion in alkaline electrolyte can produce potentially explosive hydrogen gas according to the reaction: Zn + 2H2O→ Zn(OH)2 + H2.47 This hydrogen evolution reaction (HER) is not supposed to occur during discharge and suppression of hydrogen evolution is necessary for primary and secondary Zn-air batteries.48 The practical working voltage of zinc-air battery is less than 1.65 V due to the internal loss of the cell, resulted from the activation, the ohmic and concentration loss.49 The major drawback of this type of battery is the low lifetime when recharged electrically. In addition, rechargeable zinc-air battery suffers two critical weaknesses which do not affect the primary battery: the stability of the air electrode when used to charge the battery and the formation of dendrites on the zinc electrode, leading to short circuits and shedding of zinc.50 However, mechanically rechargeable zinc-air batteries have been developed by the Electric Fuel limited company for military application and fleet of electric vehicles.45 In this concept, the discharged zinc anodes have to be mechanically replaced by fresh ones. However, this procedure necessitates a huge logistic and robot intervention.
Zinc metal anode
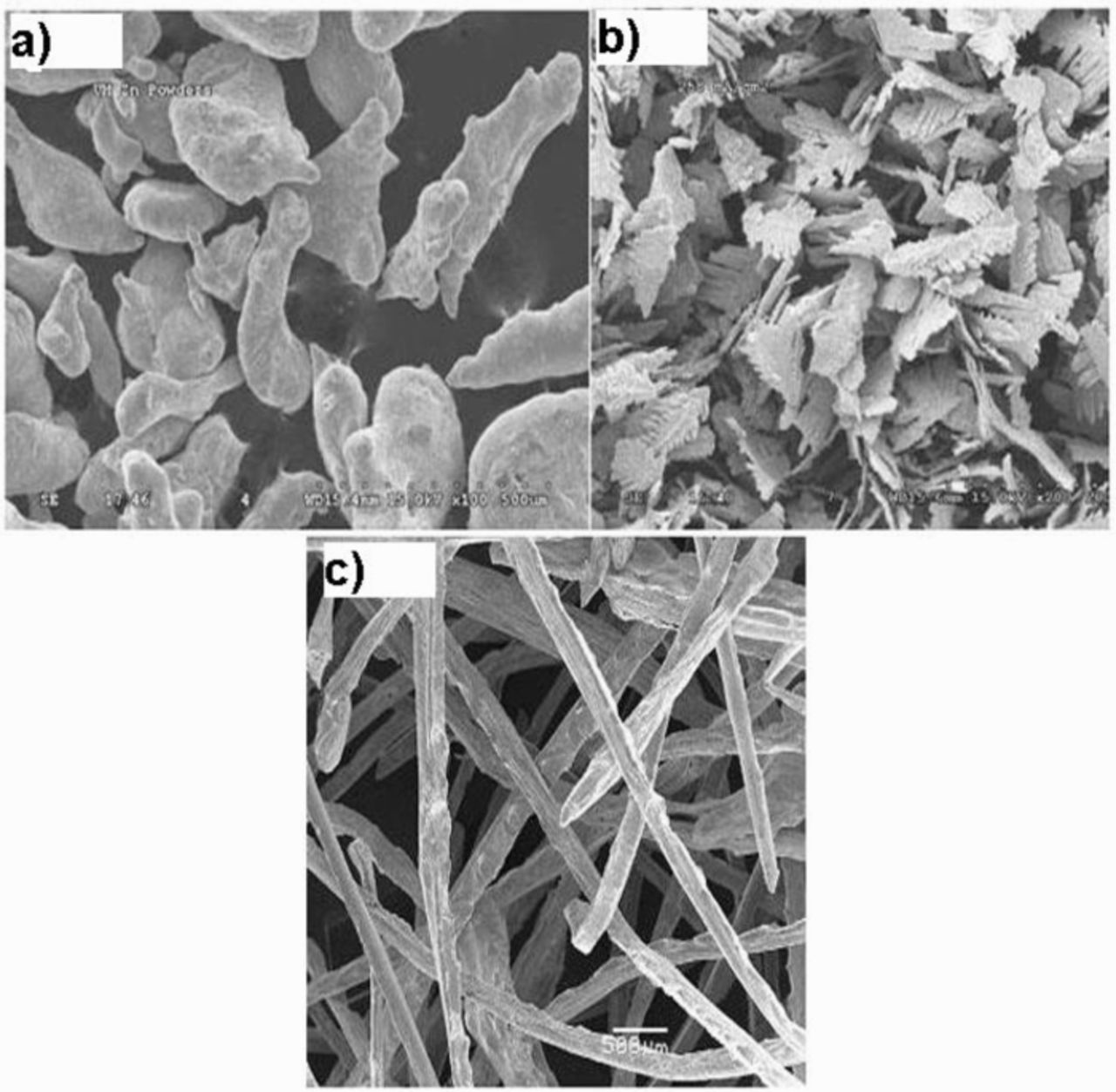
Zinc has enormous advantages such as low cost, abundance, environmental friendliness, its constant discharge voltage and long shelf life etc. Pure zinc metal is used in zinc-air battery and oxidation of zinc metal occurs during discharge through zinc corrosion in alkaline electrolyte. In addition, the shape change of zinc electrode, which occurs during repeated discharge/charge cycles, is mostly resulted from the uncontrolled dissolution of zinc metal electrode in electrolytes. Moreover, the zinc does not necessarily deposited from where it has consumed. This process ultimately produces shading and dendrites which can break-off, or worse, grow to form a short-circuit with positive electrode.50,51 Zinc metal participates in the anode reaction during discharge. The most efficient method to improve the performance of zinc anode is to increase the surface area or to tailor the surface morphology for high reaction rate with alkaline electrolyte.52,53 Controlled surface morphology of molten-zinc powder, dendritic-zinc powders in alkaline zinc-MnO2 cell,54 and fibers have been used as zinc anode for high surface area, shown in Fig. 5.55
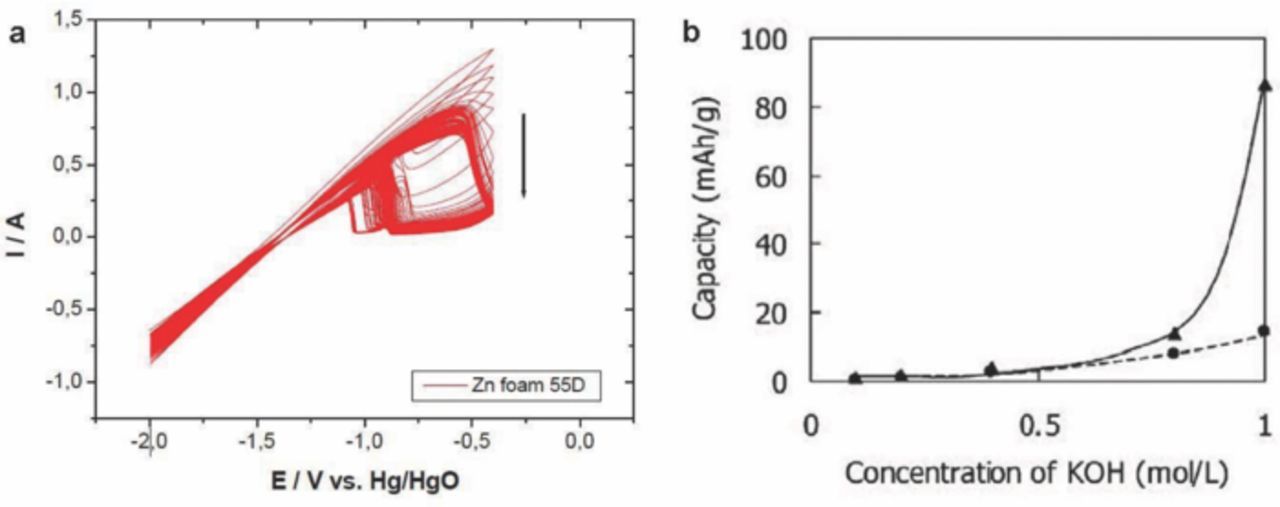
When a porous zinc anode reacts with alkaline electrolyte, the stability of zinc electrode is a concern due to hydrogen gas evolution, resulting in a later increases in the pressure of the battery cell and water electrolytes.56 These side reactions eventually decrease the cycle life of a zinc-air battery. Zinc alloy with other metals such as mercury (Hg), lead (Pb), lead(II) oxide (PbO), and Cd have been studied to reduce the hydrogen evolution and to improve the electrochemical behavior.57,58 For example, Hg has been added to Zn to improve the electrical conductivity,59 however, these metals are toxic and may cause environmental problem. In contrast, zinc, nickel (Ni), and indium (In) alloys have been used to improve the electrochemical behavior of zinc anodes for zinc-air battery.60 This alloy showed reversibility even after 100 cycles of the cyclic voltammogram and a clear indication of less dendrite formation and low shape change tendency.60 In addition, a zinc-air cell has been investigated with zinc metal foam which is free from Hg and Pb, and got a specific energy of about 500 mWhg−1 at a discharge current of 5 mA.51 But, this anode showed poor mechanical stability and electrochemical properties, as the anodic zinc oxidation peak decreased from about 1.25 A down to 0.7 A with increasing cycle number up to 100 cycles, as shown in Fig. 6a.
Separator
A separator used in zinc-air battery for transporting the hydroxyl ion (OH−) not hydrogen ion (H+) from the air electrode to the zinc electrode.62 The basic requirements of a proper separator are stability in alkaline electrolyte solution, appropriate pore size, high ionic conductivity, and electrically nonconductive. Besides the basic requirements, rechargeable zinc-air battery's separator should be inert to oxidation, and should remain stable during charging and discharging. Furthermore, it needs high absorption for electrolytes and fine porous structure to sustain electrolytes in pores and retard zinc dendrite formation.47 Separators are made of polyethylene, polyvinyl alcohol (PVA), polyolefin, polyethylene oxide (PEO), asbestos, and polypropylene.51,63–67 Among these, PEO and PVA have already shown the promising properties for rechargeable alkaline battery applications. Dewi et al. reported that an ion selective polysulfonium-1, poly(methylsufonio-1,4-phenylenethio-1,4-phenylene triflate) membrane, has acceptable separator properties, increasing the discharge current capacities (polysulfonium-1: 86.4 mAhg−1, and polypropylene:14.5 mAhg−1) of the Zn-air batteries by almost six times (Fig. 6b).61 This polysulfonium-1 membrane showed a high ionic selectivity and prevented the cation permeation to the cathode that increased the overall capacity of Zn-air batteries.
In addition, mesoporous materials such as MCM-41 membrane have been proposed as new separator material for electrochemical zinc-air cell.68 This Zn/MCM-41/air cell showed the improvement of power density from 13 mWcm−2 to 32 mWcm−2 and possesses a volumetric energy density of 300 WhL−1 at discharge capacity of 15 mAh, which are comparable to product data sheet for commercial zinc-air button cells such as Duracell's DA675. These observations suggest that MCM-41 materials serve its intended functions as the ion exchange membrane and electrolyte reservoir, thus viable as a new separator material.
Electrolyte
There are still challenges in developing bifunctional catalysts, reversible zinc anode and proper electrolytes for electrically rechargeable zinc-air batteries.69 An alkaline composite PEO–PVA–glass-fiber-mat polymer electrolyte with high ionic conductivity (10−2 S/cm) at room temperature has been prepared and applied to solid-state primary zinc-air batteries.70 The electrolyte showed excellent mechanical strength and electrochemical stability (±1.2 V). The results demonstrate that the electrochemical performance of zinc-air cells with composite polymer electrolytes is much better than that of conventional of cells with PE/PP or cellulose separator.70 The alkaline electrolytes such as potassium hydroxide (KOH), sodium hydroxide (NaOH), and lithium hydroxide (LiOH) were used in zinc-air batteries.49 Among these, KOH is being widely used in zinc-air battery for its high ionic conductivity. Ionic conductivity can be increased by increasing the concentration of KOH, but high concentrated KOH can led to an increase in viscosity of the electrolyte and the formation of ZnO.71 A zinc-air battery is operated in an alkaline solution and exposed to air. The electrolyte is very sensitive to atmospheric CO2, which can react with hydroxyl ions to form carbonates. The carbonation of the alkaline electrolyte could cause decreased battery capacity.71,72 To reduce the CO2 concentration in a zinc-air battery, absorption in a rotating packed bed has been investigated.73 The results indicated that the proposed compact device could effectively reduce CO2 to a level below 20 ppm, as required by a zinc-air battery, using piperazine (PZ) and its mixture with 2-(2-aminoethylamino) ethanol (AEEA) and monoethanolamine (MEA) as the absorbents.73
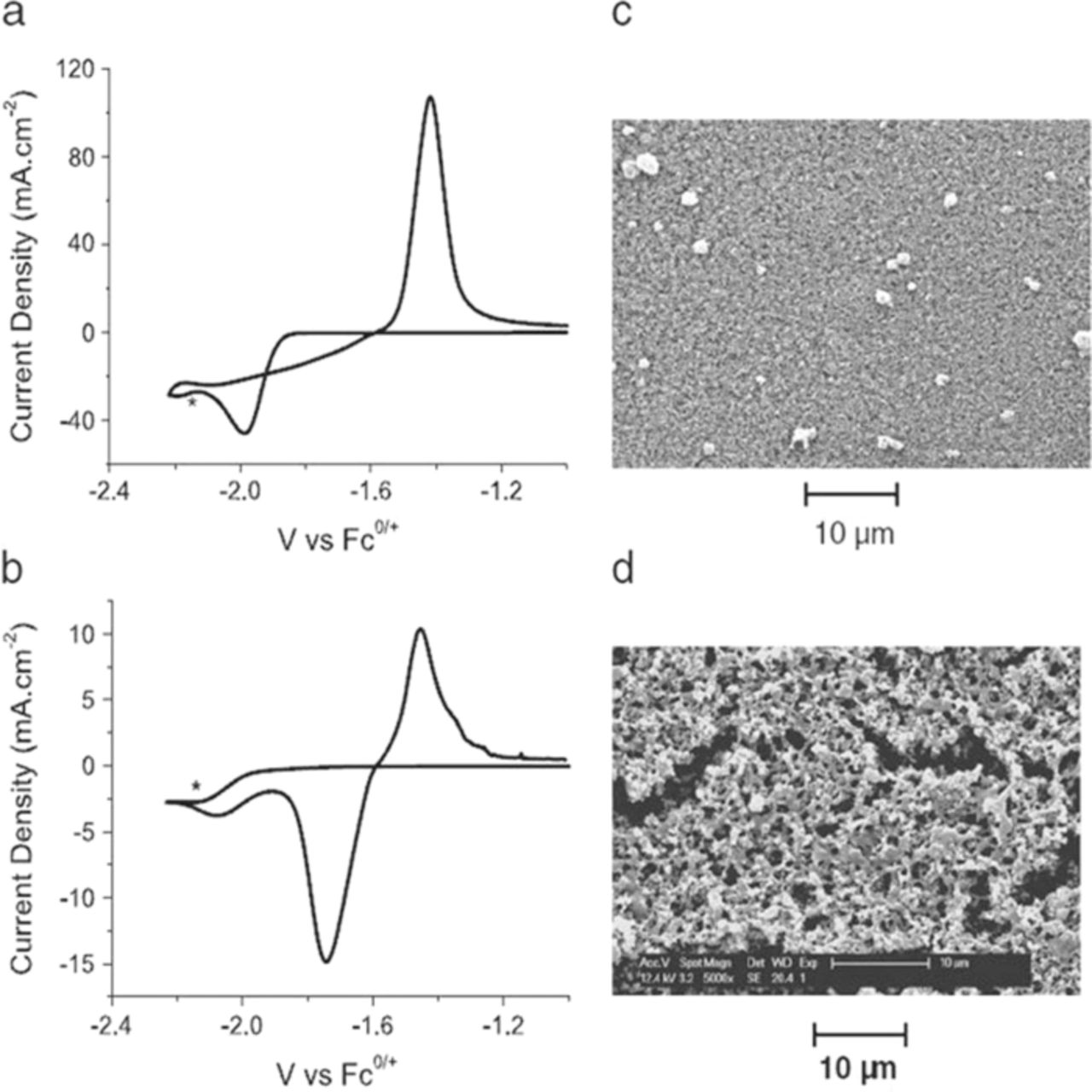
Ionic liquids (ILs) have been extensively explored as electrolytes in lithium-ion and Li-air battery applications; but their use in other metal-based battery systems is still in its infancy.74 A novel zinc ion conducting polymer gel electrolytes (PGEs) consisting of ionic liquids 1-ethyl-3methylimidazolium triffluoromethanesulfonate (EMITf) and 1-ethyl-3methylimidazolium bis(trifluoromethanesulfonyl)imide (EMITFSI), with a zinc salt dissolved in it, blended with a polymer matrix, poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) have been investigated for non-aqueous zinc-air battery.75 This PGEs possess high ionic conductivity on the order of 10−3 Scm−1 at room temperature, wide electrochemically anodic stability limit at 2.8 V vs. Zn2+/Zn and cathodic limit at zinc deposition, exceptional thermal stability from −50 to 100°C, free of volatile solvents, and excellent mechanical strength. In addition, the deposition morphology of 10 mol% Zn(dicyanamide)2 in 1-ethyl-3methylimidazolium dicyanamide [emim][dca] ionic liquid on glassy carbon has been investigated.76 It is important that the deposition of zinc should be non-dendritic in nature, occur at a potential that prevents solvents breakdown and be possible in solutions containing high levels of water produced during cell discharge. Zn(dca)2 in [emim][dca] containg 3 wt% H2O held at −2.15 V is a smooth coating of zinc as shown in Fig. 7a and 7c; whereas 0.05 wt% H2O held at −2.15 V is a condition of spongy, porous morphology of zinc as shown in Fig. 7b and 7d. However, 0.05 wt% H2O IL displayed poor adhesion to the GC compared to IL containing 3 wt% H2O.
Figure 7. Cyclic voltammetry and SEM of 10 mol% Zn(dca)2 in [emim][dca] on a GC working electrode at −2.15 V vs. Fc0/+(marked by *) for 10 min at different water contents: (a) CV of 3 wt% H2O, (b) CV of 0.05 wt% H2O (scan rate 100 mV/s in both cases),(c) SEM of 3 wt% H2O, and (d) SEM of 0.05 wt% H2O (adapted from).76
Air electrode
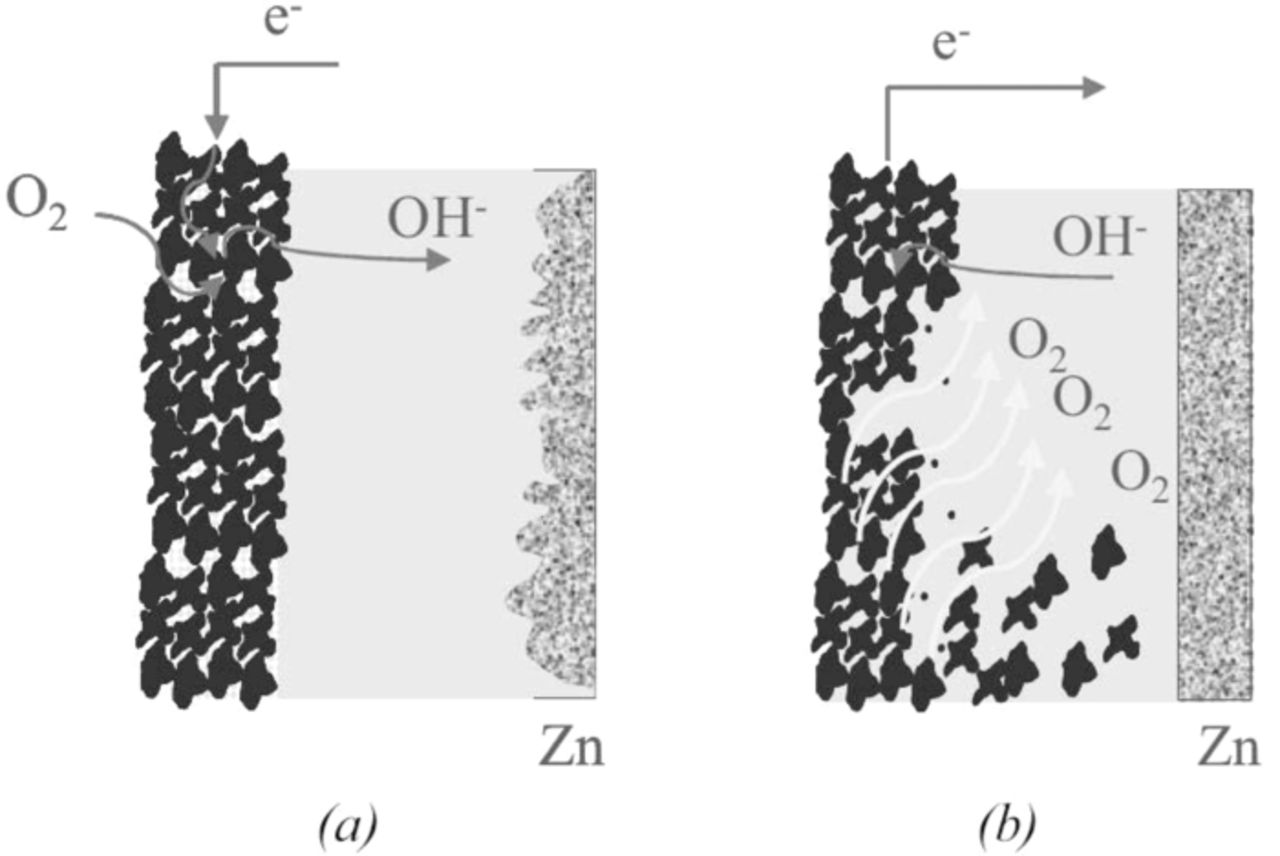
Zinc-air battery requires the air electrode to have both proper catalyst for ORR and a highly porous structure. The highly porous structure makes the diffusion path for oxygen and functions as a substrate for the catalysts to work with a triple phase interface composed of a gas (O2), a liquid (electrolytes), and a solid (carbon cathode). This fragile structure, optimized for gas-liquid and gas-solid interfaces, resulted in a high current density of 1 Acm−2 when used as fuel cells,77 but was mechanically broke down for oxygen evolution during charging process of the battery, as shown in Fig. 8b.50 In addition, carbon dioxide from the air reacts with alkaline electrolytes such as KOH and produces potassium carbonate which precipitates inside the air electrode, blocks the pores of the electrode, also leading to irreversible failure of air cathode.50
Figure 8. (a) Air electrode during discharge and (b) charge (adapted from).50
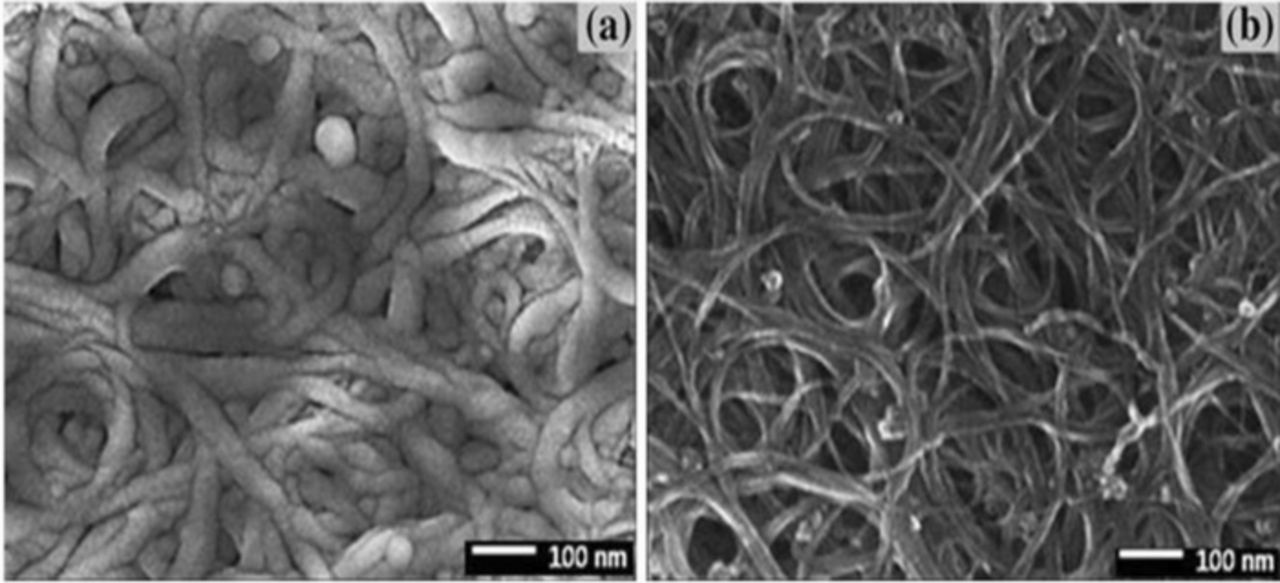
Carbon materials such as activated carbon and CNT can be used as air electrode.69,78 A carbon nanotube, composite material of calcium (Ca) and strontium (Sr) substituted rare earth cobaltate powders with the perovskite structure (ABO3) was prepared by a catalytic hydrocarbon dissociation reaction.79 It exhibited better cycling performance, higher thermal and chemical stability than that of graphite nanoparticles. In addition, SWNTs decorated with silver (Ag) nanoparticles(size 3–5 nm) shows the doubled specific capacity of 515 mAhg−1 and specific energy density of 300 Whkg−1 compared to the bare SWNTs specific capacity of 270 mAhg−1 and specific energy density of 125 Whkg−1, respectively; whereas, conventional gas-diffusion electrodes showed a specific capacity of 200–600 mAhg−1 and specific energy density of 250–400 Whkg−1.80 Fujiwara et al. reported a reversible air electrode for electrically rechargeable zinc-air battery using anion-exchange membrane (AEM) as polymer electrolyte membrane and Pt-Ir catalyst.81 This air electrode with an AEM inhibited the incorporation of CO2 in alkaline electrolytes, and suppressed the precipitation of carbonate in the pores of air electrode, and enhanced ORR. In addition, Pt-20 wt%-Ir catalyst remarkably reduced the overpotential of OER as much as 0.29 V at 20 mAcm−2. Gupta et al. fabricated a flexible air electrode (AEP2/P3) based on non-functionalized (P2) and functionalized (P3) SWNTs and demonstrated the fully interconnected uniform surface morphology, as shown in Fig. 9.82 The high active surface area, measured as 130.54 m2/g for AE60/40 and 158.76 m2/g for AE80/20, respectively, enhanced the OER and ORR of zinc-air cell. In addition, AE60/40 showed an ionic conductivity of ∼1 × 10−2 S/cm, higher discharge capacity of ∼300 mAhg−1, compared to AE80/20 with an ionic conductivity of 7.2 × 10−3 S/cm and 2–28% less of discharge capacity. It is clearly indicating an increase in the cell conductivity and a decrease in the discharge capacity for the increased P3-SWNTs content due to the increasing hydrophilic or smooth surface nature.
Figure 9. FE-SEM images of surface morphology of the freestanding bucky paper: (a) P2-SWNTs and (b) P3-SWNTs (adapted from).82
Catalyst
An air electrode is divided into two parts: one is the gas diffusion layer, composed of carbon material and a hydrophobic binder such as polytetrafluoroethylene (PTFE) as a wet proofing agent, which makes gas diffusion layer permeable to only air but not water.83 The second part is a catalytic active layer which consists of catalysts, carbon materials, and binder. ORR takes place in the catalytic active layer.84 A bi-functional catalyst is essential for a rechargeable zinc-air battery. Although, noble metal catalysts such as Pt have high activity for ORR, it will account for the high cost of manufacturing of an air electrode. Due to the high cost of noble metal catalysts, other types of catalysts such as metal oxides have been investigated. Tungsten carbide nanocrystals promoted Ag composite electro-catalyst was prepared and used for the electro-reduction of oxygen in alkaline solution. This Pt-free electro-catalyst showed high activities similar to those of Pt-based electro-catalyst.85 With the advantages of alkaline electrolyte in zinc-air battery, air cathodes are based on individual oxides, or mixtures of such, with a spinel, perovskite, or pyrochlore structure such as MnO2, Ag, Co3O4, La2O3, LaNiO3, NiCo2O4, LaMnO3 etc. providing an optimal balance of ORR activity and chemical stability in an alkali electrolyte.84,86,87 Among these, Mn oxide thin film showed higher activity for both ORR and OER, similar to that of noble metal catalyst.88 In contrast, MnO2, which was used as a catalyst, was not stable in alkaline solution at a large overpotential of 2 V for OER and was oxidized to MnO4− which went into the solution and decreased the cell performance.50 In addition, the conductivity of the metal oxides (MnO2) is relatively low, thus reducing the power density of the cell. To address this obstacle, it is necessary to reduce the size of the particles and increase the area of the catalytic active surface.89
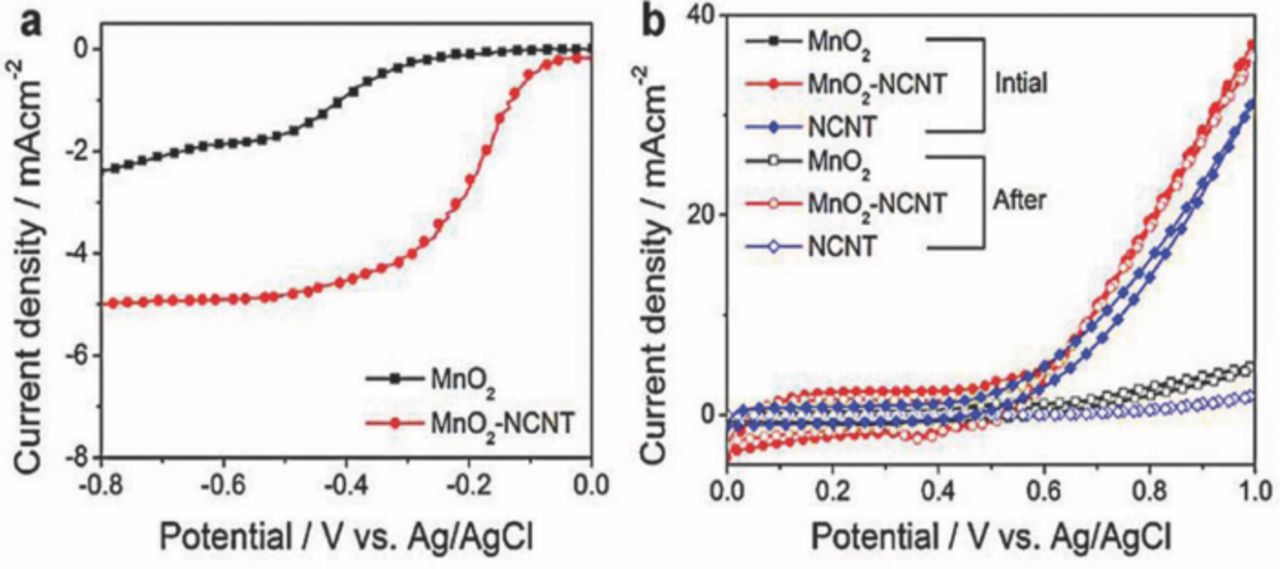
Nitrogen-doped carbon nanotubes (N-CNTs) have been investigated in different concentration of alkaline electrolytes as catalyst of zinc-air battery, and revealed excellent catalytic activity toward ORR.90 The highest cell power density of 69.5 mW cm−2 was obtained with an air-cathode catalyst loading of 0.2 mg cm−2 and an optimal KOH electrolyte concentration of 6M. This same research group has investigated a composite bifunctional catalyst of MnO2 and NCNTs for rechargeable zinc-air batteries and a significant positive shift (+0.22 V) in the half wave potential and a doubled limiting current have been observed for the MnO2-NCNTs (−5.0 mA/cm2) in comparison to the pure MnO2 as shown in Fig. 10a. It has been demonstrated that the cell performance has been enhanced due to the addition of NCNTs, and showing enhancements in both the electrical conductivity and overall activity for ORR.91 Furthermore, the MnO2-NCNTs composite has retained 96% of the OER current density at 1 V after 50 cycles as shown in Fig. 10b. This is significantly higher than that of NCNTn (only 4% remained), indicating excellent OER stability of the proposed composite bifunctional catalyst.
Figure 10. Comparison of RRDE voltammetry results showing at 900 rpm rotational speed: (a) ORR polarization curves of bare MnO2 and MnO2-NCNT composite, (b) OER polarization curves of bare MnO2, NCNT and MnO2-NCNT composite (adapted from).91
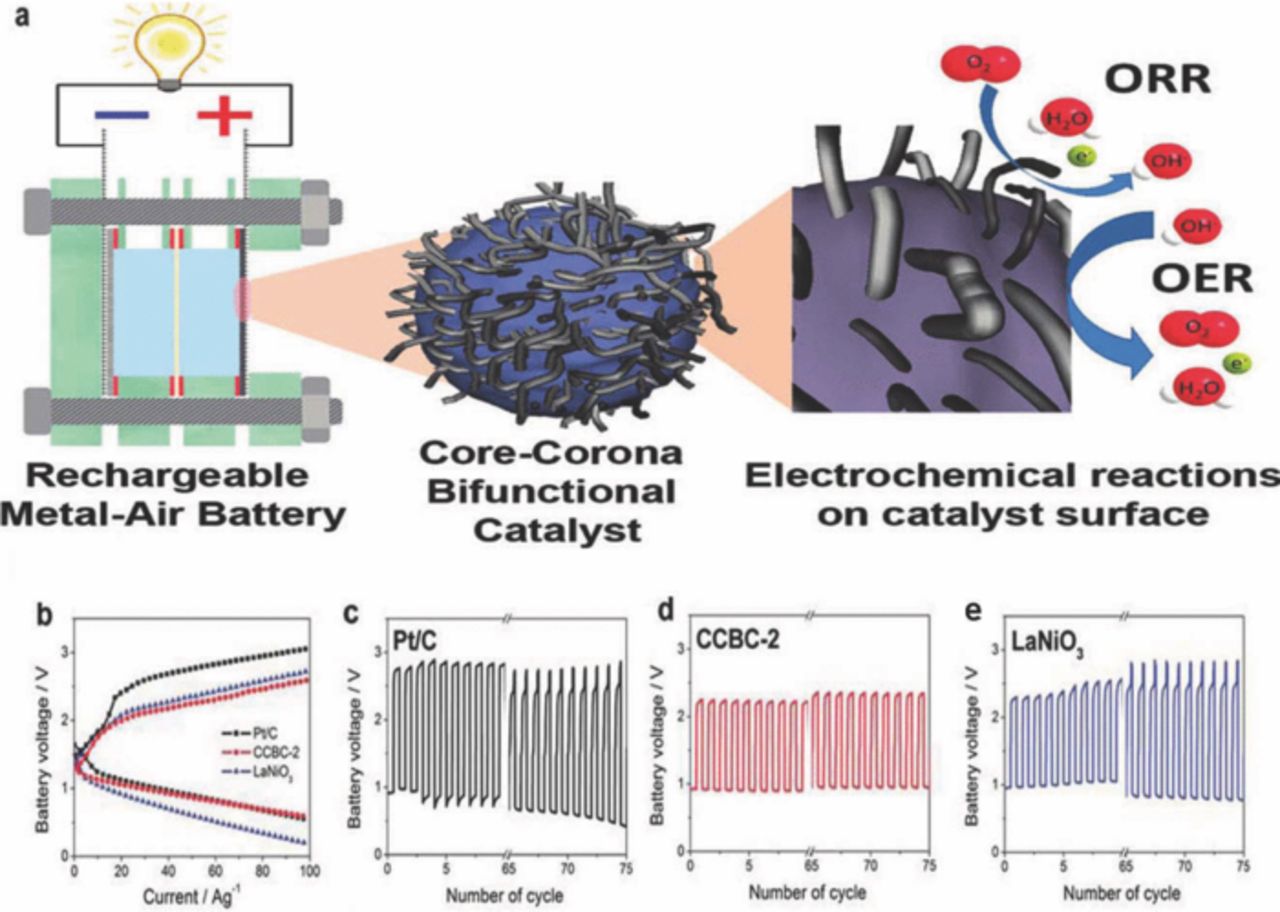
Recently, the same research group has investigated highly active and durable core-corona structured bifunctional catalyst (CCBC) consisting of lanthanum nickelate (LaNiO3) at core and NCNTs at corona for rechargeable metal air battery application especially for zinc-air battery, as shown in Fig. 11a.92 This CCBC with 2 mL precursor solution that is CCBC-2 has been compared with most acceptable benchmark electrocatalysts materials such as LaNiO3 (for OER) and Pt/C (for ORR).
Figure 11. (a) Core-corona structured bifunctional catalyst for rechargeable metal air battery, (b) C-D polarization curves of Pt/C,CCBC-2, and LaNiO3. 75 C-D cycling of (c) Pt/C, (d) CCBC-2, and (e) LaNiO3 (adapted from).92
However, CCBC-2 catalyst demonstrated similar discharge current of 60.5 Ag−1 compared to Pt/C discharge current of 62.0 Ag−1, and two times of LaNiO3 discharge current of 29.4 Ag−1. In addition, CCBC-2 demonstrated 1.5 times in charge current of 20.2 Ag−1 compared to Pt/C of 1.33 Ag−1, which indicated that CCBC-2's performance was close to the benchmark electrocatalysts for ORR and OER. The rechargeability of the CCBC-2 catalyst has been evaluated by charge-discharge (C-D) cyclic experiment, as shown in Fig. 11. It was shown the discharge potential (Edischarge) of CCBC-2 (Fig. 11d) remain unchanged after 75 cycles, and Pt/C (Fig. 11c) and LaNiO3 (Fig. 11e) suffer decreasing of 20% and 56%, respectively. In contrast, CCBC-2 exhibited 22% lower charge potential (Echarge) compared to Pt/C and LaNiO3. These characteristics of CCBC-2 exhibited the similar requirements of an active bifunctional catalyst for good rechargeability such as low charge potential, high discharge potential and minimal fluctuation.
Magnesium-Air Batteries
Among various metal-air batteries, rechargeable magnesium-air batteries show a high theoretical voltage (3.09 V), theoretical specific capacity (2205 mAhg−1), energy density (3910 Wh/kg), low cost, light weight, environmental friendly,20 and abundant in both the earth's crust and sea water.93
Magnesium-air battery consists of magnesium as anode, electrolyte, and oxygen from air as cathode.94,95 The cathode represents a layer of active carbon containing catalyst and hydrophobic additives (TEFLON suspension in a quantity of 10–25%) contacting with free electrolyte. An external waterproof layer of the cathode is fabricated from hydrophobic acetylene carbon black, permeable for air and impermeable for electrolyte. The electrode is reinforced by metal grid for imparting mechanical strength which also serves as current collector.96 Fig. 4b is a schematic illustration of a magnesium-air battery. The electrochemical reactions of the magnesium-air battery can be expressed as follows:97
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn6.jpg)
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn7.jpg)
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn8.jpg)
Magnesium metal anode
The major problems of magnesium-air batteries are the low coulombic efficiency of the traditional magnesium plate using as electrode, irreversible polarization characteristics during discharge process, and high self-discharge rates. In addition, the discharge product is sluggish which masks the bottom portion of magnesium anode and difficult to replace after discharge of magnesium anode in alkaline electrolytes.22 The corrosion problem of magnesium electrodes, which reduces the shelf life and operation life of the battery, is much more acute in electrolytes of mixtures of Mg(ClO4)2 and 14 wt% of NaCl by the chemical reaction Mg + 2H2O → Mg(OH)2 + H2↑.98 Packaging materials such as Vexar expanded polyethylene and glycerinated cellulose were investigated to prevent the sludge falling to the bottom of the cells and to minimize magnesium hydroxide formation on the cathodes.97 Heat generation due to anode self-discharge and cell irreversibility is another problem in magnesium-air batteries. Thus magnesium-air batteries require a water reservoir and/or forced air cooling system.22,96 It is therefore great scientific and technological challenge to improve the properties of magnesium electrode so as to enhance the performance of magnesium-air batteries. The high specific surface areas of the magnesium sea-urchin like nanostructures has been reported as to reduce the real current density for electrode during discharge, low anodic polarization/corrosion of magnesium and fast sedimentation of the anodic product Mg(OH)2 in electrolytes.98 Furthermore, in most polar organic electrolytes, the bulk magnesium anode does not function as a reversible electrode due to the formation of a passivizing barrier during the electrochemical magnesium deposition and dissolution. However, graphene like MoS2 (G-MoS2) and ultra-small magnesium nanoparticles was synthesized.99 The combination of G-MoS2 as cathode and ultra-small magnesium nanoparticles (N-Mg) as anode achieved a high operation of 1.8 V and a first discharge capacity of 170 mAhg−1, of which 95% was kept after 50 C-D cycles.100 Mg alloy with other metals such as Al, Zn, Li and cerium (Ce), have been studied to reduce self-corrosion rate and to improve the electrochemical behavior. A comparison of the corrosion behavior and discharge performance of Mg, AZ31 and Mg–Li–Al–Ce in 3.5 wt% NaCl solution has been investigated and results showed that Mg–Li–Al–Ce had higher electrochemical activity and lower self-corrosion rate.99
Electrolytes
The proper electrolyte choice is a major issue for magnesium-air batteries. The objectives that must be satisfied by the electrolyte for magnesium-air batteries are: i) a low and uniform corrosion rate of magnesium anode, ii) low anodic polarization of magnesium at useful current densities, iii) and fast coagulation of anodic product Mg(OH)2 in the electrolytes. Electrolytes have been reported for magnesium-air batteries such as NaCl, KHCO3, NH4NO3, NaNO3, solution of NaNO3 and HNO3, NaNO2, Na2SO4, MgC12, MgBr2, and Mg(C1O4)2.101 The excessive passivation of magnesium anode and evaporation of electrolyte is the main draw back as earlier mentioned. A boron based electrolyte which has been developed through the reaction of tri (3,5-dimethylphenyl) boron (Mes3B) and PhMgCl in THF.102 Magnesium anode was stable in this electrolyte. However, an improved electrolyte comprises a magnesium salt, preferably magnesium perchlorate dissolved in an organic solvent with additive such as 1-butyl-3-methylimidazolium has been proposed.103 This electrolyte retarded the buildup of deleterious passivation coating on magnesium anode and enhanced the cell performance.
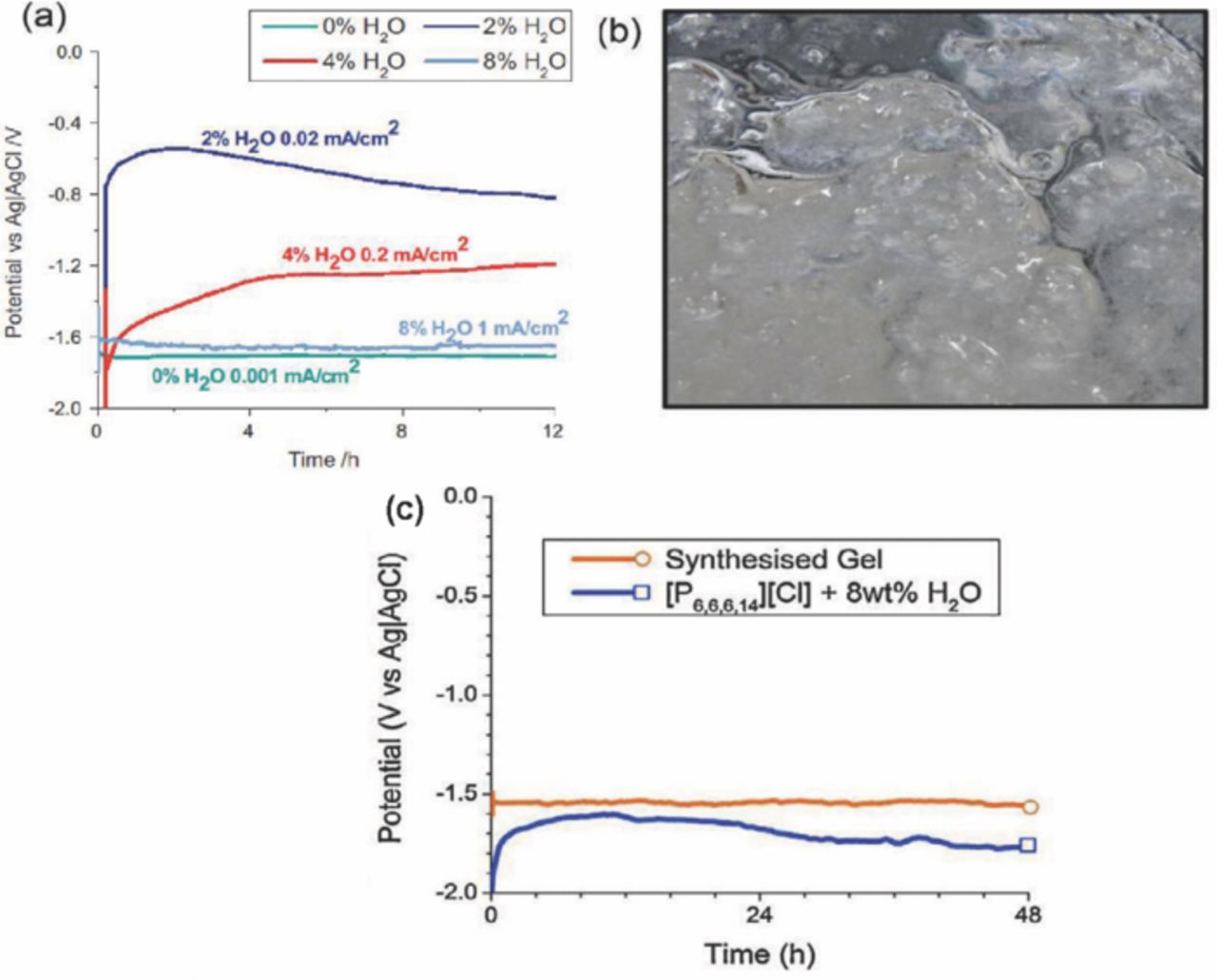
An ionic liquid phosphonium chloride ionic liquid ([P6,6,6,14][Cl]) and water mixture based electrolyte to stabilize the metal/electrolyte interface has been proposed.104 The saturated IL (8 wt% H2O) electrolyte exhibited a stable discharge current of 1 mA cm−2 at a stable potential of −1.65 V vs. Ag/AgCl compared with 0, 2, and 4 wt% H2O. Furthermore, the higher amount of water content of the ionic liquid electrolyte played an important role for possible high rate of discharge, as shown in Fig. 12a.104
Figure 12. (a) Glvanostatic discharge curves of a Mg electrode in a trihexyl (teradecyl) phosphonium chloride ionic liquid electrolyte containing 0, 2, 4 and 8 wt% H2O (adapted from),104 (b) Image of the synthesized gel, (c) comparison of 0.05 mA cm−2 galvanostatic discharge potential of the synthesized gel and ionic liquid electrolytes (adapted from).105
This same research group has reported a synthesized gel type electrolyte such as hydrated organophosphonium–magnesium (hydroxyl) chlorides, and compared with [P6,6,6,14][Cl].105 The cell using synthesized gel exhibited low discharge voltage (−1.52 V vs. Ag/AgCl) compared to [P6,6,6,14][Cl] (−1.65 V vs. Ag/AgCl),104 as shown in Fig. 12b and 12c, most likely due to the reduced conductivity of the gel. However, they also reported that the synthesized gel type electrolyte magnesium-air battery reduced the side reaction such as H2 gas formation.
Future research in electrolytes field is necessary to develop the novel electrolytes because their properties govern the choice of cathode. While some major progresses have already been made, the road is still long to go.
Cathode
There is no significant progress on cathode side of Mg-air batteries. In present-day practice, the air cathode is commonly constituted of active carbon (with or without an added dissociation-promoting catalysts) containing a finely divided hydrophobic polymer material and incorporating a metal screen as the conductive element.106 So, it is too early to say that a magnesium-air battery is ready to be fitted into the practical applications.
Aluminum-Air Batteries
Aluminum-air batteries consist of metal aluminum as anode, electrolyte, hydrophobic separator, and porous carbon as cathode with catalysts, as shown in Fig. 4c. The basic chemistry of this battery is the reaction of oxygen with metal aluminum. However, aluminum-air battery is non-rechargeable. The cell stops producing electricity when aluminum metal anode is consumed in electrolytes by the reaction of oxygen to form hydrated aluminum oxide.21,107 It is highly dense energy of 4302 Whkg−1, with the potential for up to eight times the range of the lithium-ion battery and significant lower total weight. The cost of aluminum as anode material is very low as long as the reaction product can be recycled. Fuel efficiency during the cycle process in aluminum-air battery EVs can be 15% (present stage) or 20% (targeted), comparable to internal combustion engines.21 The electrochemical cell reaction of aluminum-air cell is as follows:108
![Equation ([9])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn9.jpg)
![Equation ([10])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn10.jpg)
![Equation ([11])](https://content.cld.iop.org/journals/1945-7111/160/10/A1759/revision1/jes_160_10_A1759eqn11.jpg)
Aluminum metal anode
The main advantages of aluminum-air batteries are: i) anode is made of cheap sheets of aluminum alloy, and ii) CO2 rejecting electrolytes which can be handled easily by the consumers. However aluminum-air batteries have two major problems: i) the discharge of aluminum anode is irreversible, and ii) the self-discharge of aluminum anode is very rapid. Aluminum-air batteries use either alkaline or brine electrolytes. And anode aluminum metal corroded in electrolytes to forms gel like hydrated alumina,109 and reduces the cell electricity capacity.110 It is possible to mechanically recharge the battery with new aluminum anode made from recycling the hydrated aluminum oxide.111 Aluminum alloys containing small addition of both tin (0.1 wt%) and gallium (Ga) (0.05 wt%) has been shown dissolve in sodium chloride media at high rates. Current density has been obtained greater than of 0.2 Acm−2 at potentials close to the open circuit potential of −1500 mV vs. saturated calomel electrode (SCE).112 Aluminum-air cell generates heat due to the high self-discharge rate of aluminum alloy anode and the cell irreversibility. This heat generation is the main reason for high rate of water loss. It creates hazardous and runaway conditions and severely decreases the anode self-life during both idle and discharge periods.111,113 A composite aluminum anode has been proposed with a selectively reactive coating on a surface of aluminum anode core. The selectively reacting coating was capable of chemically reacting with the electrolyte during discharge of the aluminum-air battery. This reactive coating is comprised of zinc alloy and may also include an anode corrosion inhibitor or material consisting of one or more of In, gallium (Ga), Pb, thallium (Tl) or Hg.114 The electrochemical properties of pure aluminum (99.999%), technical grade aluminum (99.8%) and the alloys with In and tin (Sn), i.e. Al-0.1%In, Al-0.2%Sn and Al-0.1%In-0.2%Sn have been investigated in 2 mol/L NaCl solution.110 Among these, Al-In alloy showed a low anodic polarization, minimum hydrogen evolution rate, minimum negative difference effect (NDE), a morphology which was consistent with general dissolution and high-anodic efficiency, as shown in Table III.110 This result indicated that aluminum alloys can be used as electrode of aluminum-air batteries.
Table III. Properties of aluminum alloys (reproduced from).110
| Samples | Anodic polarization order | Hydrogen evolution during galvanostatic test at current density 100 mA/cm2 (about ml/cm2) | NDE in 2 mol/dm3 NaCl solution | Anodic efficiency at current density 100 mA/cm2 and 2 mol/dm3 NaCl solution (about%) | Morphology |
|---|---|---|---|---|---|
| Al (99.999%) | Al (99.999%) > Al (99.8%) > Al-In > Al-In-Sn > Al-Sn | 2.95 | 0.171 | 85 | Material lost its reflective surfaces and subsequent extensive damage ensued which shows the effects of strong general. |
| Al (99.8%) | 3.8 | 0.188 | 86 | Same as Al (99.999%). | |
| Al-In | 2.0 | 0.121 | 91 | Rough morphology which is consistent with general dissolution. | |
| Al-Sn | 6.35 | 0.235 | 77 | Rough surface with metallic luster which indicates that general dissolution. | |
| Al-In-Sn | 4.2 | 0.211 | 82 | Extensive surface damage and pitting with general dissolution. |
Electrolyte
The choice of electrolytes used in aluminum-air batteries is fairly flexible. The electrolyte can be common salt (NaCl), sea water, and alkaline electrolytes such as potassium hydroxide (KOH).20 The concentration of electrolyte is an important fact for aluminum-air batteries. Aluminum electrodes dissolve rapidly on open circuit in alkaline electrolytes with the evolution of hydrogen and polarize excessively. The aluminum polarization curve has been investigated in KOH electrolyte with a half cell at concentration of 0.1, 1.0, 2.0, 4.0 M.115 Aluminum polarization curve was measured in 0.1 M and 4.0 M KOH electrolyte containing additives, such as ethylene diamine tetra acetic acid (EDTA), gluconic acid, and NaCl. The corrosion current of aluminum increased as the concentration of KOH increased from 0.1 M to 4.0 M.115 However, aluminum-air battery with gelled 0.6 M KOH solution electrolyte with a hydroponics gelling agent has been investigated.116 This cell exhibited the highest capacity and power density of 105.0 mAhg−1 and 5.5 mWcm−2, respectively. In contrast, the corrosion potential in 4 M KOH aqueous electrolyte of alloys Al-Mn-Sn and Al-Mn-Ga as anode has been investigated.117 It was exhibited that the corrosion potential of these alloys was lower than that of pure aluminum anode. Therefore it is considered to be advantageous to use Al-Mn-Sn or Al-Mn-Ga alloy as anode.117
Cathode
The cathode structure used in most metal air batteries consists of catalyst and Teflon supported on a hydrophobic film with the current collector.118 The hydrophobic film prevents the seepage of electrolyte from the cell and contributes a fast and uniform supply of air (oxygen) into the cell. Modern air electrode consists of activated carbon porous structure with catalyst such as cobalt and hydrophobic film PTFE. The electrical rechargeable aluminum-air battery is still in progress at laboratory based.
Summary
Metal-air batteries have the advantage of high volumetric and gravimetric energy density. Metal-air batteries offer great benefits if they are to be harnessed to their fullest potential. Lithium-air batteries could be found new applications in technologies such as electric vehicles, plug-in hybrid electric vehicles, robots, and electric power storing systems. However, commercial lithium-air batteries still do not offer sufficient practical energy density for these high power consumption devices and have a short life cycle. The current lithium-air battery is not moisture stable in the atmosphere as other metal air batteries such as zinc-air, aluminum-air, and magnesium-air batteries do. It is associated with higher manufacturing costs and complexity as compared with magnesium-air, aluminum-air, and zinc-air batteries. However, the reversibility of lithium-air batteries is better than that of zinc-air and magnesium-air batteries whereas aluminum-air batteries are not rechargeable. Finally, to summarize the metal air batteries:
For zinc-air batteries
- (1)Zinc anode with high surface area that react efficiently with alkaline electrolytes, resulting in hydrogen evolution reaction and poor capacity of zinc-air batteries. To overcome this problem, alloying, coating, and adding additives to the electrolytes have been investigated.
- (2)The pore size of the separator should be optimized to control the migration of Zn2+ ions from anode to cathode.
- (3)The lifetime of the zinc-air batteries are greatly affects by carbonation in alkaline electrolytes which decreases the capacity. A CO2 remover needs to be considered.
- (4)The architecture of air electrode, sluggish oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) are important criterions affecting the overall performance of zinc-air batteries. Noble metal such as Pt is used as catalyst, but it has high activity for ORR only. Non-noble metal catalyst such as MnO2 is effective for ORR and OER, however it is not stable in alkaline electrolytes. Therefore, it is important to find a chemically stable and bifunctional catalyst for secondary zinc-air battery.
For magnesium-air batteries
- (1)Magnesium has been proven to cycle with high efficiency in laboratory-scale cells. In contrast to lithium, magnesium metal anodes can achieve high cycle life and efficiency.
- (2)Magnesium-air batteries are rechargeable but low columbic efficiency of the traditional magnesium plate as electrode, irreversible polarization characteristics during discharge process, high self-discharge rates, and produces sluggish discharge product in alkaline electrolytes. To overcome the falling of sluggish products, packaging materials have been proposed.
- (3)Heat generation for anode self-discharge has been reported as an acute problem of magnesium-air batteries. The high surface area nanostructured magnesium alloy anode has been proposed to reduce the self-discharge.
- (4)Novel electrolytes such as ionic liquids and boron based electrolyte has been investigated to reduce the corrosion rate of Mg anode.
For aluminum-air batteries
- (1)The aluminum-air batteries are mechanically rechargeable with new Al anode. This type of battery is promising due to the advantages of using safe, low cost and abundant materials.
- (2)Due to self-discharge, huge amount of heat generate, which result in a high rate of water loss and reduce the anode shelf life. To overcome this problem, the use of aluminum alloy has been proposed.
Acknowledgments
The authors acknowledge financial support for this research through the Australia-India Strategic Research Fund (AISRF, ST 060048).