Abstract
The oxygen reduction reaction (ORR) at the carbon-conductive polymer-silver (C-cp-Ag) composite electrode in non-aqueous electrolyte with small amounts of added water is the subject of this study. The contributions of the various components of the composite electrode were assessed by employing four electrodes: (1) glassy carbon (C), (2) polypyrrole coated glassy carbon (C-cp), (3) silver disk (Ag), and (4) carbon-polypyrrole-silver composite (C-cp-Ag). Notably, with 5000 ppm of water in non-aqueous solution, the ORR reaction at Ag and C-cp-Ag shows an n = 4 reduction, while ORR at C and C-cp display an n = 1 reduction. The results show that the use of a multilayer C-cp-Ag composite electrode provides the opportunity to achieve the four electron reduction of one O2 molecule, with a low precious metal (Ag) loading.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
In metal air batteries, the cathode consists of the electroactive cathode material, oxygen (O2) and the remainder of the inert electrode consisting of the current collector and oxygen reduction reaction (ORR) catalyst. Metal air batteries fall into a special category as the electroactive cathode material, oxygen (O2), is available in excess from outside the battery. While the cathode current collector, the ORR catalyst and the oxygen reduction products all add mass,1,2 metal air batteries still provide the opportunity for high energy densities relative to sealed battery technologies.3,4
Notably, the structures and chemistries of the inert electrode (air electrode) can be varied to address ORR kinetic issues. Typically, a composite air electrode consists of an electrical conductor mixed with an ORR catalyst, often strengthened with a binder5,6 and a support such as a metal mesh.7,8 A disadvantage of the conventional air electrode fabrication strategy is that catalyst particles positioned within the electrode interior often have limited access to oxygen.
With an earlier article, we introduced a new composite electrode paradigm for metal air batteries, and reported the preparation, characterization, and electrochemical activity of a carbon current collector-conductive polymer-silver (cc-cp-Ag) composite electrode.9 Enhanced oxygen reduction activity for our composite electrode was observed relative to coated glassy carbon or silver disk electrodes, at a low silver loading of < 0.3 mg cm−2. Specifically, the role of the current collector toward the electrochemical reduction of oxygen, the role of the conductive polymer in improving the structural integrity of the composite electrode, and a quantitative study of the silver loading effect on ORR activity were all investigated. A notable advantage of the electrodeposition based strategy we developed is the ability to easily generate silver coated three-dimensionally structured composites via use of three dimensional electrically conductive substrates. Three dimensional electrodes can increase the active surface area, enabling reduction of more oxygen per unit planar area. This approach was utilized to prepare three-dimensionally structured carbon-conductive polymer-silver (C-cp-Ag) composites, yielding composite electrodes with ∼4X the oxygen reduction capacity of their planar counterparts.10 This study complements the prior report by providing mechanistic insight into the ORR process occurring at the C-cp-Ag composite electrode surface. While the data are collected on a planar C-cp-Ag composite, they will be relevant to the 3D C-cp-Ag composite.
The three-phase electrode-electrolyte-oxygen interface is a critical determinant of ORR activity, where each component plays an important role. In order to realize the opportunity for high operating voltage and high energy density offered by utilization of alkali and alkaline earth anodes in metal air batteries, a nonaqueous electrolyte is required. However, a water-free electrolyte is difficult to maintain under practical operating conditions for a metal air battery functioning in ambient air. Therefore, a mixed electrolyte based on primarily non-aqueous solvent which incorporates a small percentage of water may represent a realistic optimal compromise. While not the subject of this report, studies of the role of water at anodic alkali and alkaline earth metal surfaces in non-aqueous electrolytes have been recently reported and confirm the role of water at electrode surfaces to remain a scientifically relevant topic.11–14
This systematic study investigates the ORR activity of carbon (C), carbon-polypyrrole (C-cp), and carbon-polypyrrole-silver (C-cp-Ag) composite electrodes in hybrid nonaqueous-aqueous electrolytes (acetonitrile-water, tetrabutylammonium tetrafluoroborate). Two water loadings are selected, a moderate-water condition (500 ppm, 22 mM) and a higher-water condition (5000 ppm, 220 mM) more representative of operation in ambient air environment without rigorous exclusion of water.
An important objective of this report is the study of ORR with the carbon-conductive polymer-silver (C-cp-Ag) composite electrode under conditions consistent with use in humidity (i.e. water present in the electrolyte, air as reactant). Further, in order to probe the role of each composite component in the presence of water, a series of electrodes was studied, including the uncoated glassy carbon (C), polypyrrole coated glassy carbon (C-cp), silver disk (Ag), and carbon-polypyrrole-silver composite (C-cp-Ag) electrodes. Each electrode was tested, both in low water electrolyte (500 ppm, 22 mM), and higher water containing electrolyte (5000 ppm, 220 mM), where the water content of the electrolyte was measured at the conclusion of each ORR measurement. Additionally, the ORR activity was tested under pure oxygen as well as under air in order to elucidate the effect of oxygen concentration.
Experimental
Glassy carbon and silver disk working electrodes were obtained from CH Instruments (Texas, USA). Glassy carbon working electrodes were also used as substrate for the depositions. Platinum auxiliary electrodes were used for all measurements. Reference electrodes were purchased from CH Instruments. For aqueous measurements, a silver/silver-chloride reference was used, while for nonaqueous measurements a silver/silver nitrate reference electrode was used. Potentials are reported versus the reference electrodes used. Acetonitrile was selected for the study as it has a wide potential window of electrochemical stability15 and is an appropriate electrolyte solvent for non-aqueous ORR16 study. While acetonitrile undergoes condensation reactions in the presence of some active metals (such as Li and Na), it has been used successfully in studies related to magnesium based batteries.15,17 Notably, carbonate-based electrolytes react with superoxide18–21 and are not suitable for metal air batteries.
Three-electrode electrochemical cells were used for the deposition and oxygen reduction experiments. All electrochemical experiments were conducted at 25 ± 1°C. The carbon current collector-conductive polymer (C-cp) and carbon current collector-conductive polymer-silver (cc-cp-Ag) composite electrodes were prepared using previously described methods.22,23 Oxygen reduction was measured using an electrolyte of 0.1 M tetrabutylammonium tetrafluoroborate (TBABF4) in acetonitrile (MeCN), at potential sweep rates of 50, 100, 180, 225, 325 and 500 mV s−1.
CH Instruments potentiostats were used for the deposition, oxygen reduction experiments. Pine Research Instrumentation (North Carolina, USA) modulated speed rotators and electrodes were used for the rotating disk experiments. The oxygen reduction analysis was based on peak coulomb flux, based on the planar geometric area of the substrate of each electrode. To reach desired concentrations of water, a microsyringe was used to add deionized water into the electrolyte solution. After oxygen reduction experiments, the water concentration in electrolyte solution was determined using a Mettler Toledo C20 coulometric Karl Fischer titrator. For the moderate-water condition, labeled as 500 ppm in the body of the text, actual measured values were 450 ± 50 ppm. For the higher-water condition, labeled as 5000 ppm, in the body of the text, actual measured values were 4950 ± 100 ppm.
Results
Determination of diffusion coefficient
Reduction of molecular oxygen (O2) in 0.1 M TBABF4/MeCN solution at a glassy carbon electrode was measured at rotational rates ranging from 200–1600 rpm. The limiting currents were fit using the Levich equation,
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn1.jpg)
where the limiting current, ilim, is a function of electrons transferred n, the Faraday constant F, electrode area A, diffusion coefficient DO, angular rotation rate w, kinematic viscosity v, and concentration of the analyte C.24 The curve of Coulomb flux (A/cm2) vs. w1/2 (rad/s)1/2 exhibited high linearity (R2 = 1.00). From prior reports, n was assumed to be 1.0,16 the kinematic viscosity was assumed to be 4.4 × 10−3 cm2 s−1,16 and the solubility of O2, C, in acetonitrile under 1 atm O2 atmosphere was assumed to be 8.1 mM at 25°C.25 Based on these assumptions, the diffusion coefficient was calculated to be (6.09 ± 0.08) × 10−5 cm2 s−1 based on our measurements. This value is in good agreement with previously reported values of 4.87 × 10−5 cm2 s−1, 5.74 × 10−5 cm2 s−1 and 7.12 × 10−5 cm2 s−1, and 7.03 × 10−5 cm2 s−1 in acetonitrile solutions containing 0.9 M TEABF4, 0.1 M TEAClO4, 0.1 M TEAClO4, and 0.1 M TBAClO4, respectively.26–29
Effect of water on reversibility and rate: Cyclic voltammetry (CV)
Glassy Carbon (C) Electrode
Cyclic voltammograms were recorded using a glassy carbon working electrode at a series of scan rates in oxygen saturated electrolyte containing ≤ 500 ppm of water (Figure 1A). Cathodic peaks were observed between −1.3 to −1.4 V, while the corresponding anodic peaks appeared at −1.1 to −1.2 V. The data in oxygen saturated electrolyte containing ≥ 5000 ppm of water were generally consistent (Figure 1B), with slight increases in the corresponding ΔEp values in the higher water content electrolyte (Table I), demonstrating that the ORR became less reversible due to addition of water. Both the cathodic and anodic peaks showed minor shifts in the peak position as a function of scan rate, with increasing ΔEp with increasing scan rate for both water contents.
Table I. Redox potentials on a glassy carbon working electrode by cyclic voltammetry. All potentials vs. Ag/Ag+ reference.
| O2 | O2 | air | air | |
|---|---|---|---|---|
| scan rate,V | 500 ppm water | 5000 ppm water | 500 ppm water | 5000 ppm water |
| (mV/s) | ΔEp (V) | ΔEp(V) | ΔEp(V) | ΔEp(V) |
| 50 | 0.170 | 0.205 | 0.095 | 0.120 |
| 100 | 0.205 | 0.260 | 0.105 | 0.135 |
| 180 | 0.205 | 0.310 | 0.125 | 0.155 |
| 225 | 0.250 | 0.335 | 0.135 | 0.170 |
| 325 | 0.305 | 0.380 | 0.155 | 0.190 |
| 500 | 0.350 | 0.430 | 0.175 | 0.220 |
Figure 1. A. Cyclic voltammogram of pure oxygen at a glassy carbon working electrode, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 500 ppm. B. Cyclic voltammogram of pure oxygen at a glassy carbon working electrode, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 5000 ppm.
At 100 mV s−1, our CV in electrolyte with 500 ppm water showed a peak separation of 205 mV (Figure 1A). The peak separation, ΔEp, increased slightly with increasing potential scan rate (Table I). When the amount of water was increased from 500 to 5000 ppm, the value of ΔEp increased from 205 to 260 mV (Figure 1B).
The oxygen concentration in the air-saturated solutions was determined to be 1.7 mM at 25°C in the TBABF4/MeCN electrolyte solution using Henry's Law.30 The CVs at the glassy carbon working electrode under air demonstrated very similar behavior to those observed under pure oxygen. In CV measurements, the ΔEp value at 100 mV s−1 increased from 105 to 135 mV when the amount of water increased from 500 to 5000 ppm. Again, the increase in the ΔEp values suggested that the ORR became less reversible due to addition of water.
Glassy Carbon-Polypyrrole (C-cp) electrode
Glassy carbon electrodes were coated with polypyrrole following a previously reported procedure to form carbon-conductive polymer (C-cp) composite electrodes.22 The estimated thicknesses of the polypyrrole coatings were ∼4–7 microns. Under these deposition conditions, the polypyrrole forms a conformal layer which completely masks the electrochemistry of the underlying substrate during ORR.31
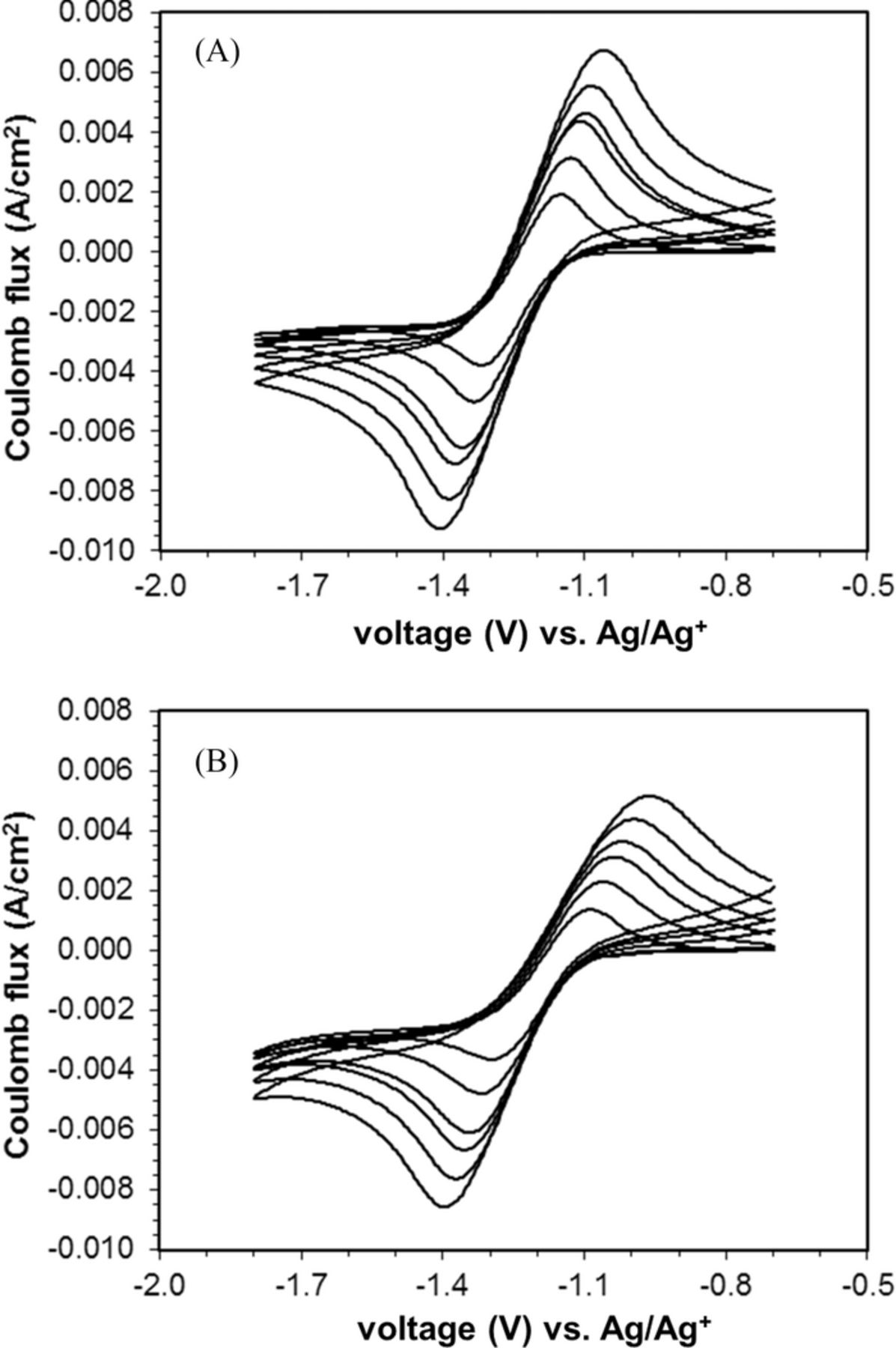
The CV in solution showed one major cathodic peak and one major anodic peak, with increasing ΔEp with increasing scan rate (Figure 2). For the CV in electrolyte containing 500 ppm water, the main cathodic peak was observed at −1.35 to −1.45 V, accompanied by the corresponding anodic peak at −1.02 to −1.15 V (Figure 2A). Under some scan rates, a second minor cathodic peak was seen as a shoulder near −1.8 V, as discussed in more detail below. When the amount of water was increased to 5000 ppm, only one cathodic peak and one corresponding anodic peak was found at the potentials of −1.54 to −1.69 V and −0.80 to −1.05 V, respectively (Figure 2B).
Figure 2. A. Cyclic voltammogram of pure oxygen at a glassy carbon-polypyrrole (C-cp) composite working electrode, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 500 ppm. B. Cyclic voltammogram of pure oxygen at a glassy carbon-polypyrrole (GC-PPy) composite working electrode, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 5000 ppm.
The ΔEp values increased with scan rate for both water concentrations, and the data collected under the same scan rate showed larger ΔEp values in the high water content electrolyte relative to the low water content electrolyte (Table II). While these general observations were consistent with those observed with the uncoated glassy carbon (C) working electrode, the effect of increased water concentration was much more pronounced for the polypyrrole coated (C-Cp) electrode. For example, at 100 mV s−1, when the water concentration increased from 500 to 5000 ppm, theΔEp for C-PPy increased from 240 mV to 605 mV, while the ΔEp increased from 205 to 260 mV for C.
Table II. Redox potentials for the ORR at carbon-polypyrrole (C-cp) by Cyclic Voltammetry. All potentials vs. Ag/Ag+ reference.
| C-cp, under O2 | C-cp, under O2 | C-cp, under air | C-Cp, under air | |
|---|---|---|---|---|
| scan rate,V | 500 ppm water | 5000 ppm water | 500 ppm water | 5000 ppm water |
| (mV/s) | ΔEp (V) | ΔEp (V) | ΔEp(V) | ΔEp(V) |
| 50 | 0.205 | 0.495 | 0.185 | 0.415 |
| 100 | 0.240 | 0.605 | 0.210 | 0.435 |
| 180 | 0.285 | 0.660 | 0.230 | 0.450 |
| 225 | 0.305 | 0.700 | 0.245 | 0.455 |
| 325 | 0.345 | 0.785 | 0.265 | 0.505 |
| 500 | 0.395 | 0.890 | 0.300 | 0.565 |
Effect of water on rate: Linear Sweep Voltammetry (LSV)
Silver Disk (Ag) Electrode
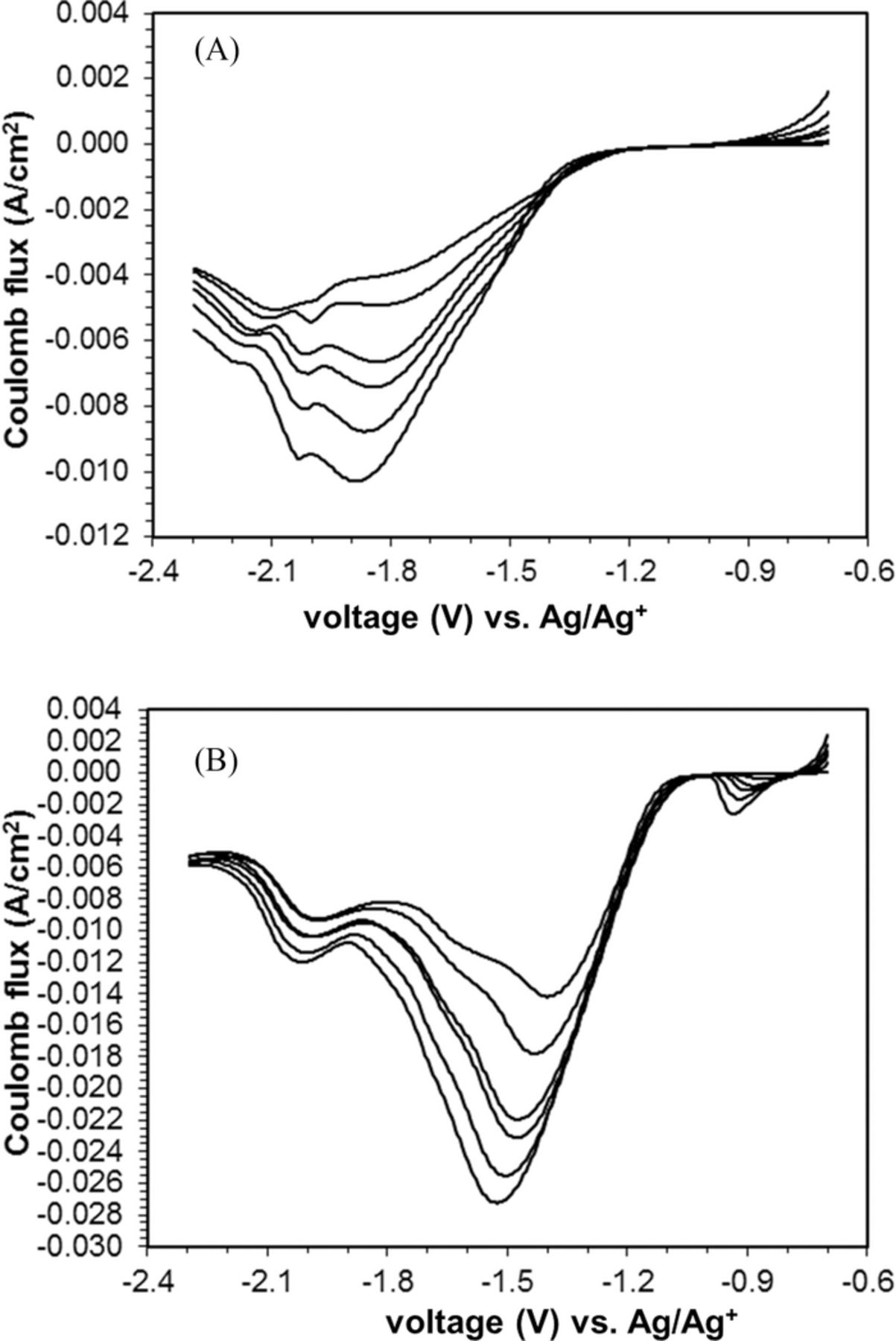
Oxygen reduction at a silver surface was measured under a series of scan rates and two differing water contents in nonaqueous electrolyte. Higher oxygen reduction current (increased peak Coulomb flux) was observed relative to ORR at the glassy carbon (C) or polypyrrole coated (C-PPy) working electrodes. The oxidation peaks were not observable, indicating an electrochemically irreversible process at the silver surface. The linear sweep voltammograms (LSVs) of ORR in electrolyte containing 500 ppm of water exhibited a major cathodic peak at −1.80 to −1.85 V followed by a minor cathodic peak at −2.00 to −2.10 V (Figure 3A). At some scan rates, a third minor cathodic peak was discernable near −2.15 V.
Figure 3. A. Linear sweep voltammetry of oxygen at a silver disk (Ag) working electrode, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 500 ppm. B. Linear sweep voltammograms of silver (Ag) electrode in pure oxygen, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. B. Water content is 5000 ppm
The main reduction peak shifted positively by ∼400 mV when the amount of water present in the electrolyte solution was increased from 500 to 5000 ppm (Figure 3B). In addition to the positive potential shift, the peak current values almost doubled when the water content was increased from 500 to 5000 ppm. In the air-saturated TBABF4/MeCN solution containing water content of 500 ppm, the ORR at Ag exhibited similar electrochemical behavior to that under oxygen. The main ORR peaks were observed at the potential between −1.7 to −1.8 V with lower peak current values. When the water amount was increased to 5000 ppm, the peaks potentials shifted positively to −1.2 to −1.3 V and the peak current almost doubled after the increasing water content from 500 to 5000 ppm.
Glassy Carbon-Polypyrrole-Silver (C-cp-Ag) electrode
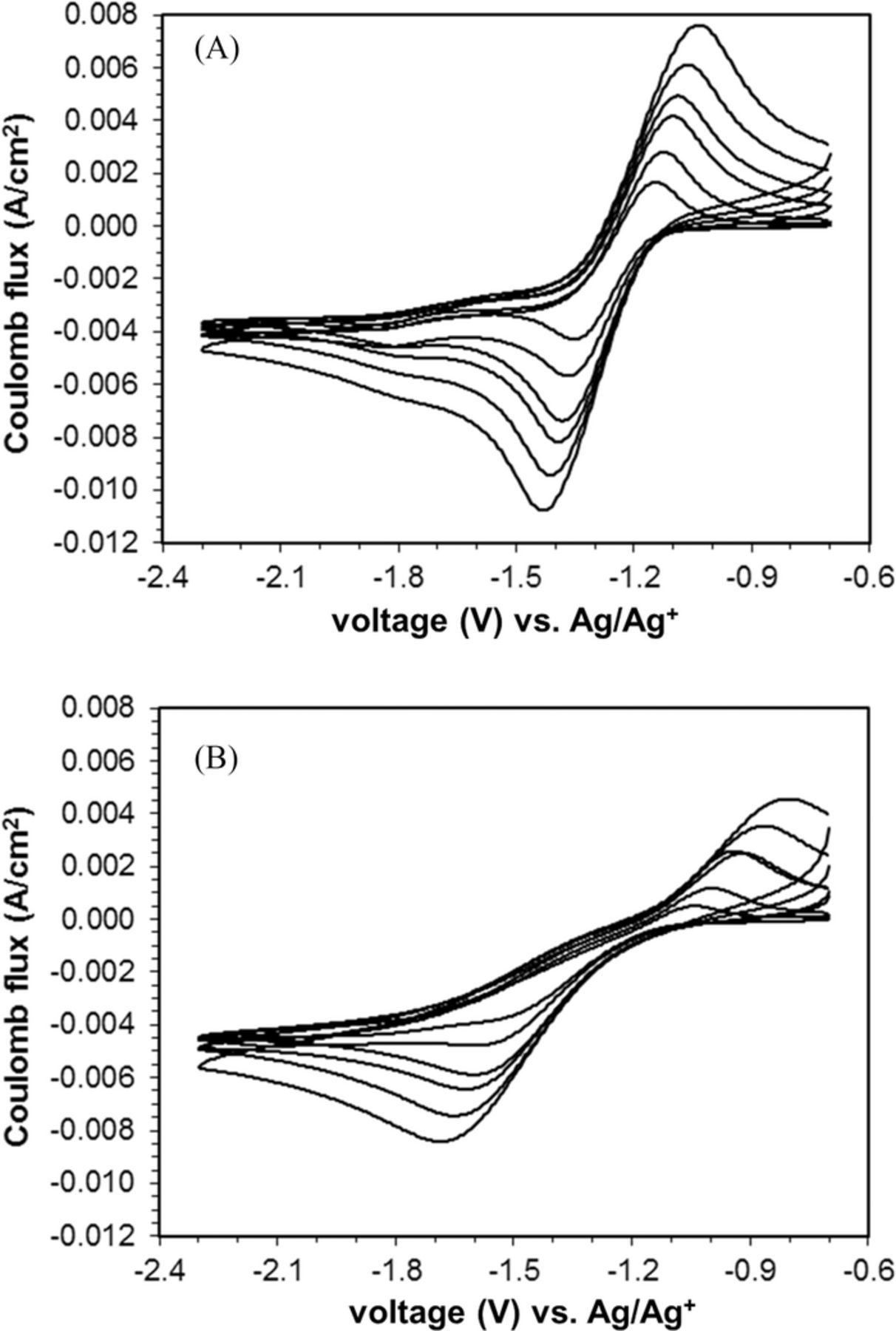
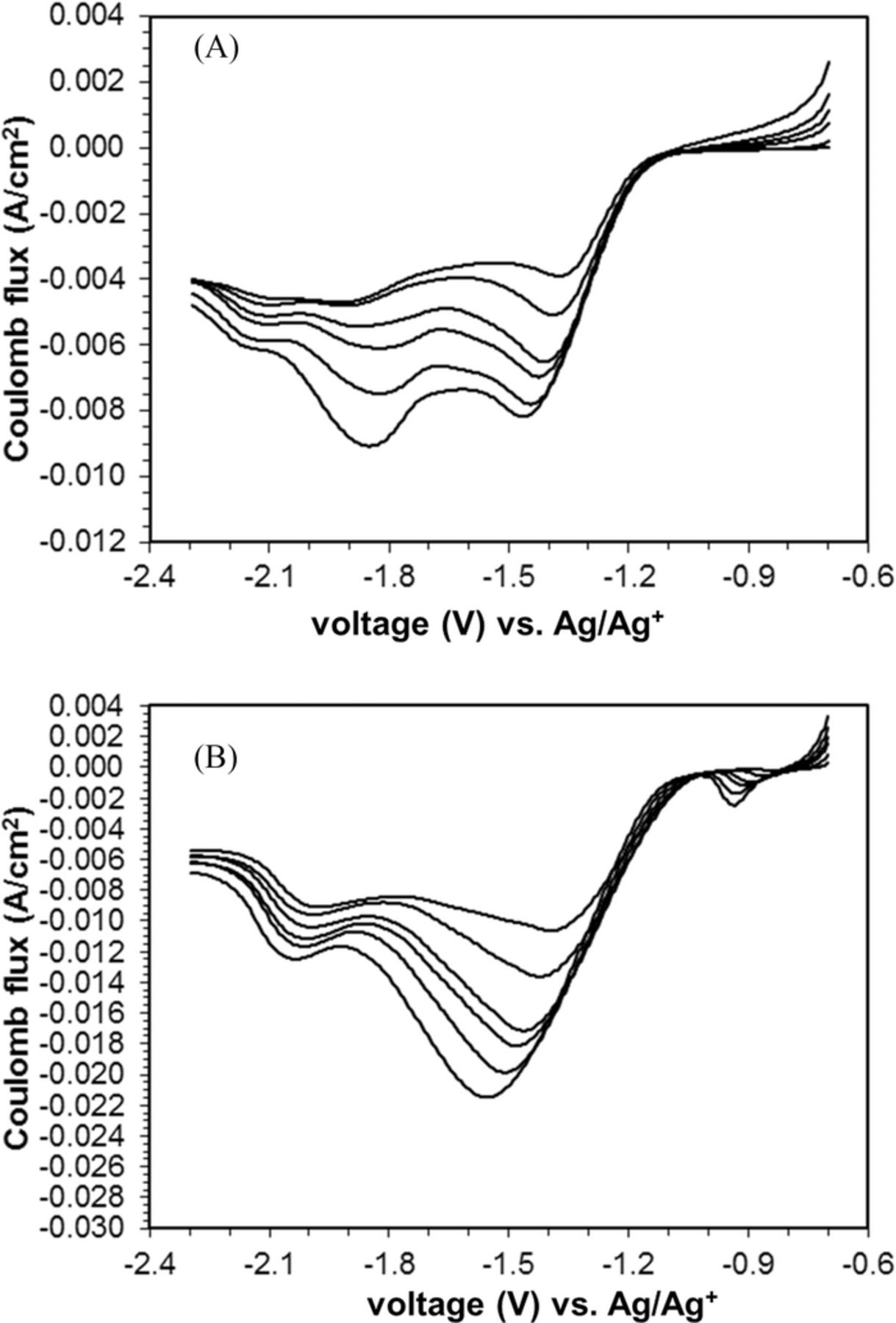
The LSVs of the ORR at C-cp-Ag composite electrode in 0.1 M TBABF4/MeCN system containing water at 500 ppm showed two main reduction peaks with comparable peak currents (Figure 4A). The first reduction peak occurred at potentials from −1.37 to −1.47 V, which were very close to the ORR peak potential observed at a C-cp electrode in the same electrolyte system. The second reduction peak appeared at potential of −1.8 V to −1.9 V, similar to the potentials for ORR at Ag disk electrode in the same electrolyte solution.
Figure 4. A. Linear sweep voltammograms of glassy carbon-polypyrrole-silver (C-cp-Ag) composite electrode in pure oxygen, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 500 ppm. B. Linear sweep voltammograms of glassy carbon-polypyrrole-silver (C-cp-Ag) composite electrode in pure oxygen, at scan rates of 50, 100, 180, 225, 325, and 500 mV s−1. Water content is 5000 ppm.
With 5000 ppm of water present in the solution, the electrochemistry of ORR at C-cp-Ag electrode changed from a combination of partial C-cp and partial Ag behavior to the behavior more consistent with that of an Ag electrode (Figure 4B). Both the C-cp-Ag composite electrode and the Ag electrode showed similar LSV in terms of shape and the peak potential, while the LSV for C-PPy-Ag electrode showed slightly less current amplitude, ∼ 94% of that of the Ag electrode.
When the TBABF4/MeCN solution was saturated with air, the ORR at C-cp-Ag composite electrode demonstrated LSVs with similar shapes to those under oxygen. Similarly, the increase in the water concentration in the TBABF4/MeCN solution had strong effects on the ORR behavior at the Ag-PPy-GC composite electrode. Like the LSV for Ag-PPy-GC composite electrode under oxygen, the LSVs for that electrode under air could be considered as a combination of the ORR activities of a PPy-GC and an Ag disk electrode under air.
Mechanistic determination: Electron equivalents (n) associated with oxygen reduction
Glassy Carbon (C) Electrode
The Randles-Sevcik equation describes the relationship between peak current, Ip, and potential scan rate, V (eq. 2).
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn2.jpg)
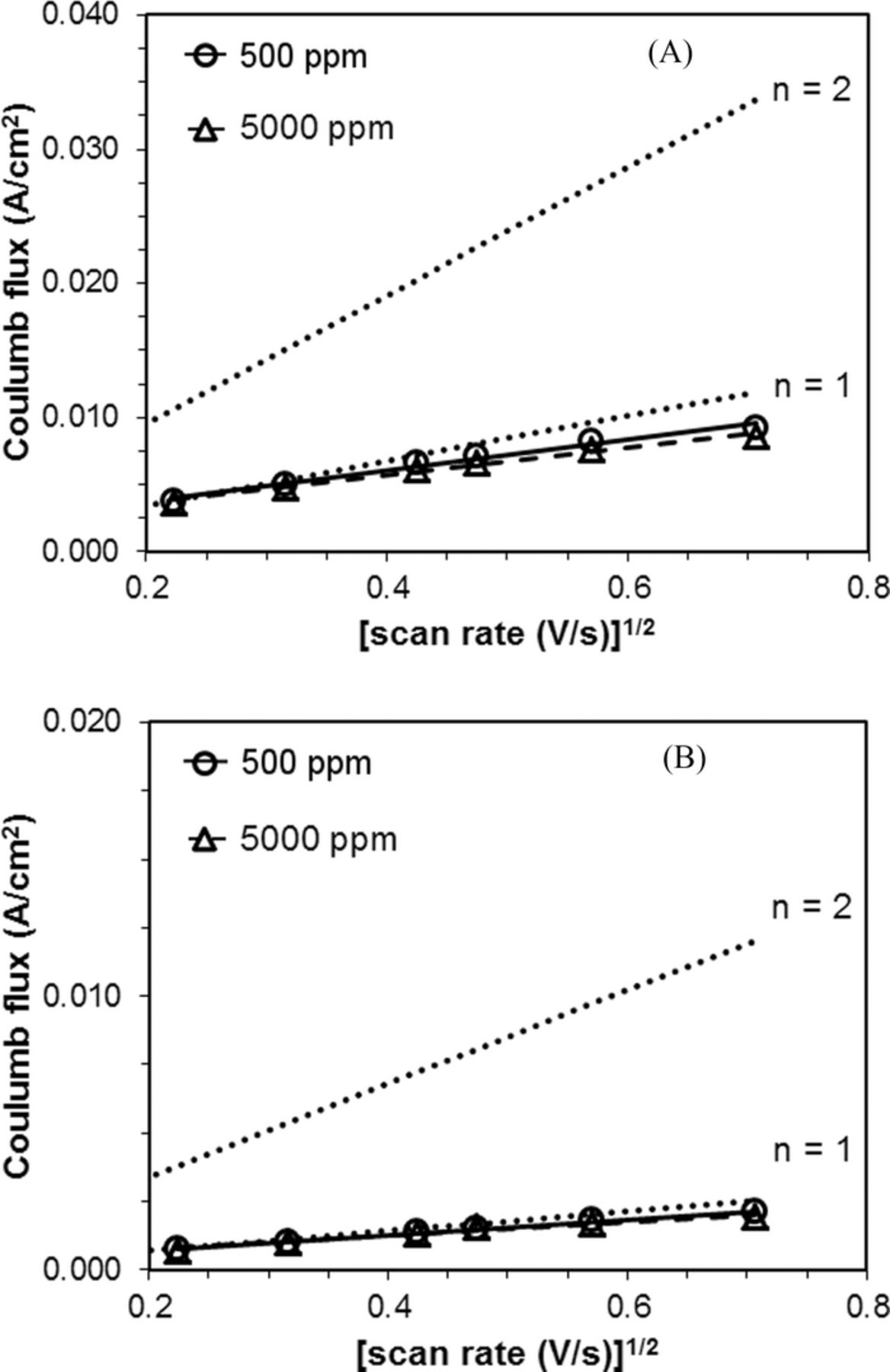
Both of the Randles-Sevcik plots were linear and corresponded well with the theoretical plot of n = 1, implying that the ORR remained a one-electron process at the glassy carbon working electrode under all scan rates and both water contents tested (Figure 5A).
Figure 5. A. Randles-Sevcik plot of pure oxygen at a glassy carbon working electrode. Dotted lines represent theoretical n = 1 and n = 2 plots. For water content of 500 ppm, R2 = 0.98. For water content of 5000 ppm, R2 = 0.98. B. Randles-Sevcik plot of air at a glassy carbon working electrode. Dotted lines represent theoretical n = 1 and n = 2 plots. For water content of 500 ppm, R2 = 0.99. For water content of 5000 ppm, R2 = 1.00.
The Randles-Sevcik plots suggested in air-saturated TBABF4/MeCN containing both 500 ppm and 5000 ppm of water, oxygen was reduced under a one-electron process at the glassy carbon working electrode, with R2 of 0.99 and 1.00, respectively (Figure 5B). One-electron reductions were observed consistent with those in the oxygen-saturated solutions. Thus, no noticeable effect of oxygen concentration was observed on the reaction mechanism of oxygen reduction.
Glassy Carbon-Polypyrrole (C-cp) electrode
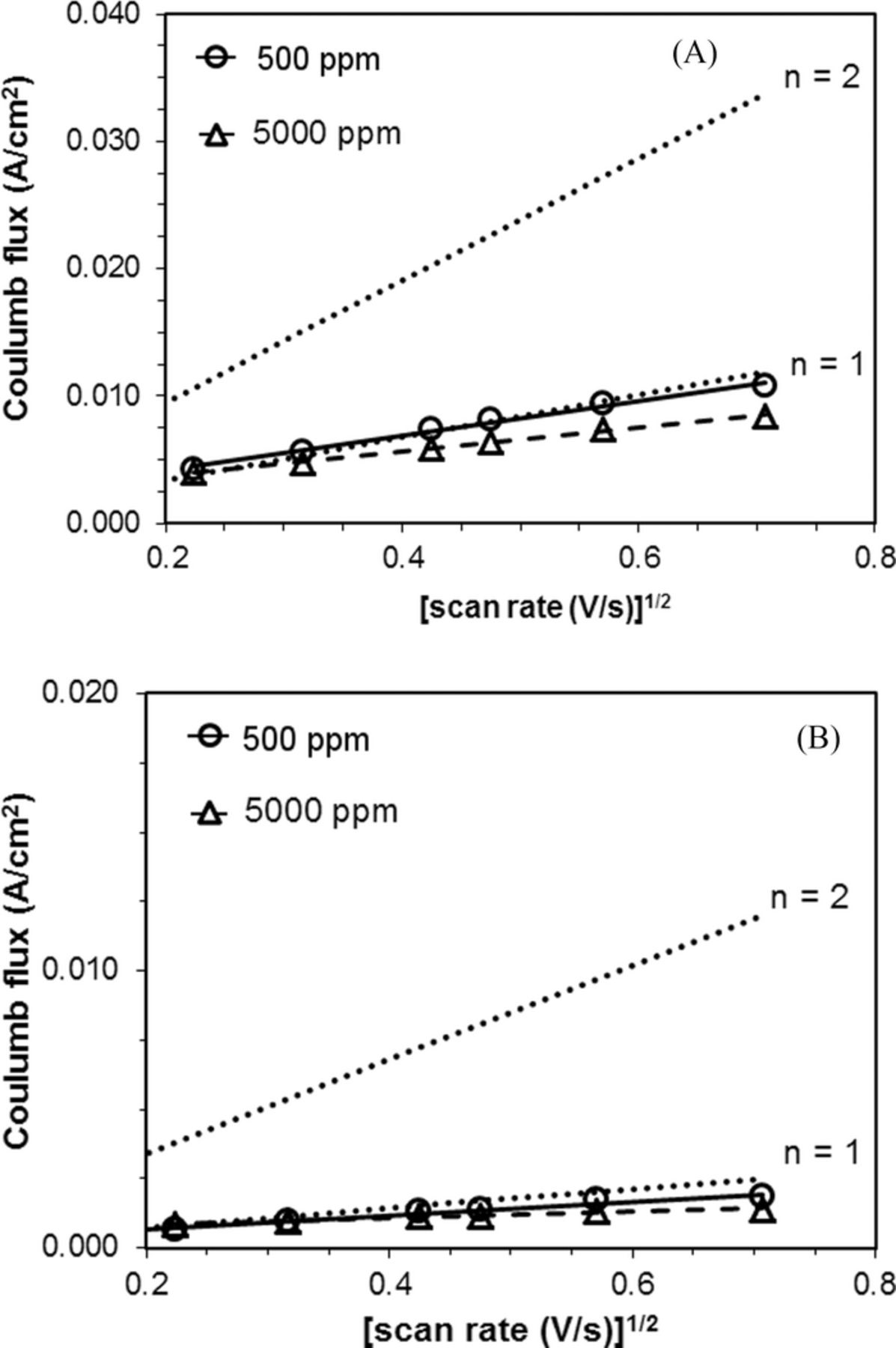
Under oxygen, the Randles-Sevcik plots were linear and showed correspondence to a one-electron reduction process under all scan rates and both water concentrations tested for the carbon-conductive polymer (C-cp) composite electrodes (Figure 6A).
Figure 6. A. Randles-Sevcik plot of pure oxygen at a glassy carbon-polypyrrole (C-cp) composite working electrode. Dotted lines represent theoretical n = 1 and n = 2 plots. For water content of 500 ppm, R2 = 0.99. For water content of 5000 ppm, R2 = 1.00. B. Randles-Sevcik plot of glassy carbon-polypyrrole (C-cp) composite working electrode in air. Dotted lines represent theoretical n = 1 and n = 2 plots. For water content of 500 ppm, R2 = 0.98. For water content of 5000 ppm, R2 = 0.97.
When the solution was saturated under air, the ORR electrochemical behaviors at PPy-GC were similar to those under oxygen. The ORR mechanism at a polypyrrole coated electrode maintains a one-electron reduction mechanism in the air-saturated TBABF4/MeCN electrolyte solution (Figure 6B). The one-electron reduction reactions observed are consistent to those in the oxygen-saturated solutions with water content of 5000 ppm.
Silver disk (Ag) electrode
From the LSV data discussed above, the value of the product of the electrons associated with the reduction process and the transfer coefficient (nα) for the main ORR peak could be approximated according to equation 3,
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn3.jpg)
where Ep is the peak potential for the peak of interest, and Ep/2 represents the potential at one-half of the peak current.32 For the silver disk electrode under oxygen, the values of nα were calculated to be 0.16 ± 0.017 and 0.23 ± 0.022 for water of 400 and 5000 ppm, respectively, which is lower than a typical value of 0.5, consistent with slow kinetics for the ORR.16
For an irreversible reaction, the magnitude of peak current Ip is a function of the number of electron transferred n, transfer coefficient α, electrode area A, the analyte concentration C, the analyte diffusion coefficient DO, and potential scan rate V, as described by Nicholson and Shain.33,34
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn4.jpg)
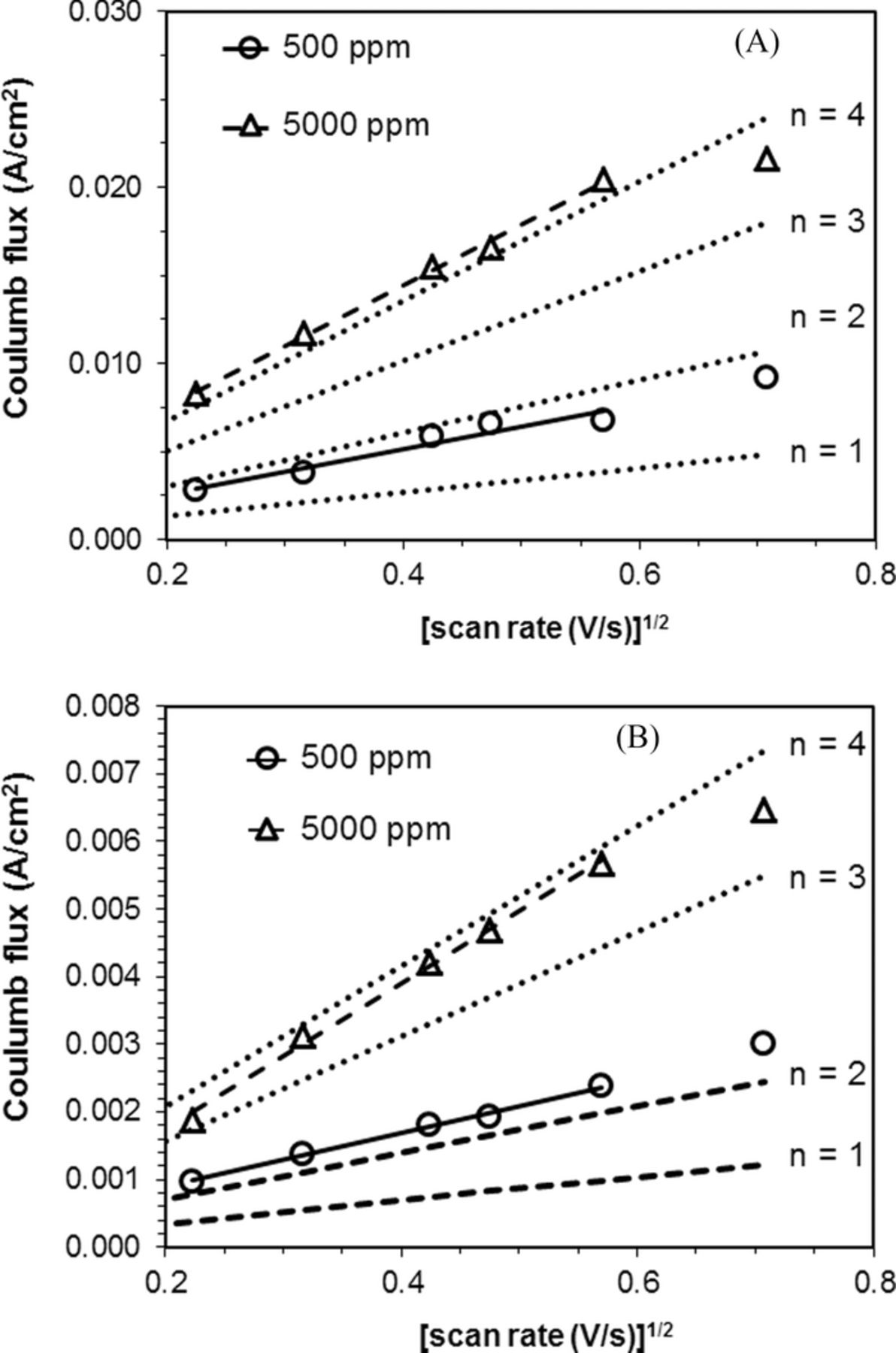
The peak currents of the experimental data were determined by fitting the LSVs with multiple peaks, and the results were compared with theoretical values (Figure 7A). For the lower water content (500 ppm) data, the experimental data were consistent with the predicted values for a two-electron reduction process (n = 2). For the higher water content (5000 ppm) data, the experimental results were consistent with the predicted values for a four-electron reduction process (n = 4) reaction. Under the highest scan rate tested (500 mV s−1), the experimental data showed lower peak current than predicted by theory, suggesting that there is a kinetic limitation to the ORR process, particularly in the higher water content electrolyte.
Figure 7. A. Nicholson-Shain plot of silver (Ag) electrode in pure oxygen. For water content of 500 ppm, R2 = 0.94. For water content of 5000 ppm, R2 = 0.99 (nα = 0.16 for n = 1 and n = 2; nα = 0.23 for n = 3 and n = 4). B. Nicholson-Shain plot of silver (Ag) electrode in air. For water content of 500 ppm, R2 = 0.99. For water content of 5000 ppm, R2 = 0.99. (nα = 0.17 for n = 1 and n = 2; nα = 0.41 for n = 3 and n = 4).
In the air-saturated TBABF4/MeCN solution containing water content of 500 ppm, the values of nα were calculated to be 0.17 ± 0.02 and 0.41 ± 0.04 for ORR at the Ag disk electrode for water contents of 500 and 5000 ppm, respectively, which is lower than a typical value of 0.5, suggesting sluggish kinetics for the ORR.
The Nicholson-Shain plots of ORR at Ag electrode under air showed that the water amount in the solution strongly affects the mechanisms of ORR in a trend similar to ORR under oxygen (Figure 7B). At water content of 500 ppm, the ORR occurred via a two-electron (n = 2) path, indicating the formation of peroxide as the ORR product. After the water amount in electrolyte solution was increased to 5000 ppm, the ORR current values increased as a result of four-electron (n = 4) ORR, forming hydroxide at the Ag electrode. This analysis showed that the changes of the ORR mechanism resulting from water addition were consistent both under air and oxygen.
Glassy Carbon-Polypyrrole-Silver (C-cp-Ag) electrode
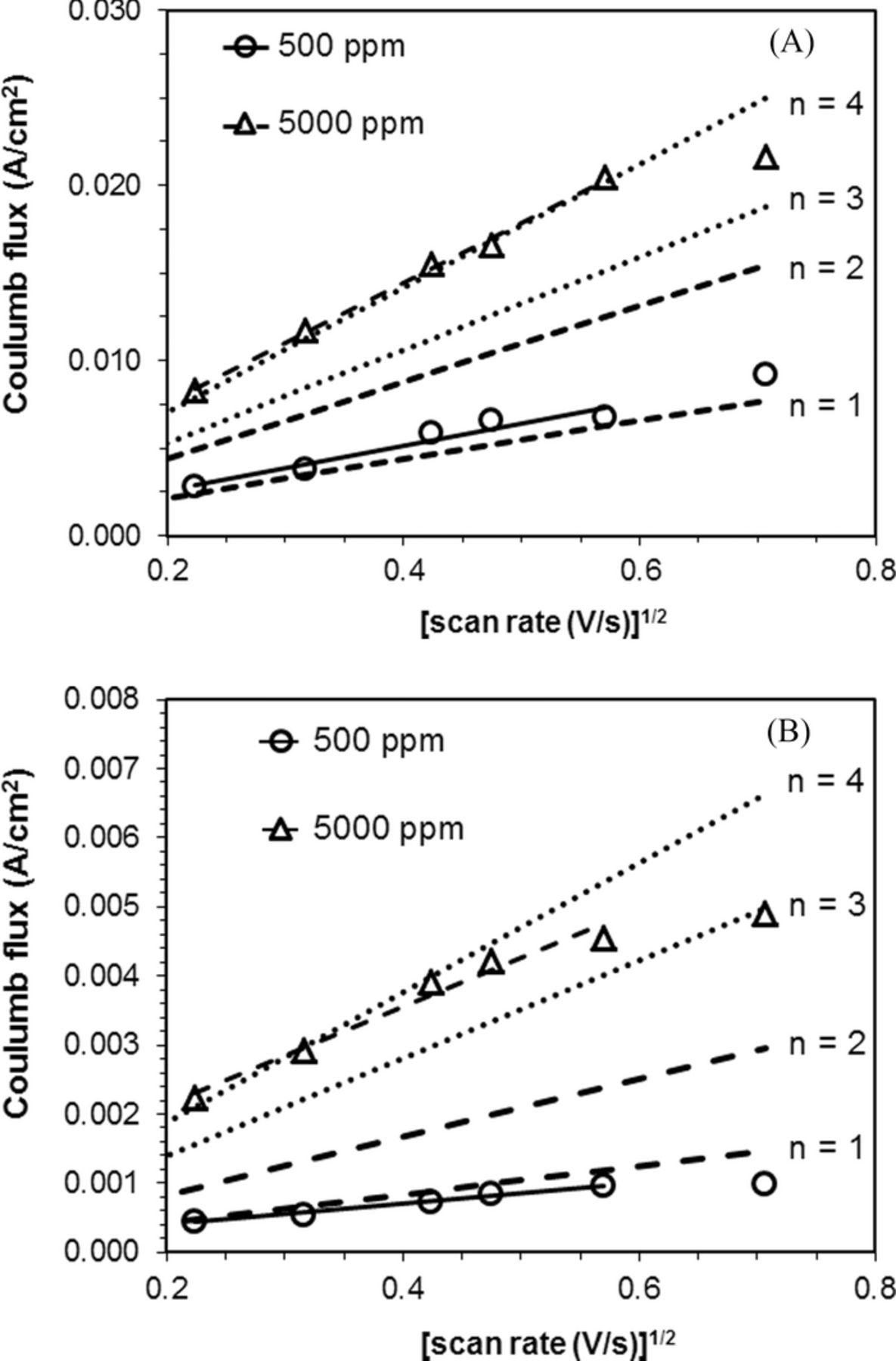
According to equation 3, the values of nα were calculated to be 0.34 ± 0.04 and 0.22 ± 0.02 for the LSVs at 500 ppm and 5000 ppm of water, respectively, for the glassy carbon-polypyrrole-silver (C-cp-Ag) composite electrode. These values were smaller than 0.5 and indicated slow kinetics for the ORR at the C-cp-Ag composite electrode. Based on the Nicholson-Shain plots, the number of electrons involved in ORR was determined to be n = 1 and n = 4 for scans at 500 and 5000 ppm, respectively (Figure 8A). Thus, the change in water amount present in the solution resulted in a substantial change in the ORR mechanism at the C-cp-Ag composite electrode.
Figure 8. A. Nicholson-Shain plot of glassy carbon-polypyrrole-silver (C-cp-Ag) composite electrode under pure oxygen. For water content of 500 ppm, R2 = 0.99. For water content of 5000 ppm, R2 = 1.00. (nα = 0.34 for n = 1 and n = 2; nα = 0.22 for n = 3 and n = 4). B. Nicholson-Shain plot of of glassy carbon-polypyrrole-silver (C-cp-Ag) composite electrode under air. For water content of 500 ppm, R2 = 0.99. For water content of 5000 ppm, R2 = 0.97. (nα = 0.34 for n = 1 and n = 2; nα = 0.22 for n = 3 and n = 4).
When the TBABF4/MeCN solution was saturated with air, according to equation 3, the values of nα were calculated to be 0.29 ± 0.01 and 0.35 ± 0.04 for the LSVs at 500 ppm and 5000 ppm of water, respectively. Based on Nicholson-Shain plots for the LSVs in air saturated solution, the number of electron involved in ORR was determined to be n = 1 ORR at C-cp-Ag composite electrode in 0.1 M air-saturated TBABF4/MeCN solutions containing 500 ppm of water (Figure 8B). For ORR in the same electrolyte solution containing 5000 ppm of water, the Nicholson-Shain plot lies closely to the n = 4 theoretical line at potential sweeping rates under 225 mV/s. A deviation from the n = 4 theoretical line was observed for the peak current at sweeping rate over 325 mV/s, consistent with a kinetic limitation to the ORR process.
Discussion
The oxygen reduction reaction in nonaqueous solution at glassy carbon (C) electrodes was previously reported to be a reversible, one-electron reduction process forming superoxide.16,25 Sawyer et al. reported a ΔEp value of 170 mV for ORR in oxygen saturated 0.1 M TEAClO4/MeCN, where the large ΔEp was attributed to a combination of uncompensated resistance and possible surface reactions.25 After iR correction, Laoire and coworkers reported reversible ORR with peak separations of 62 and 67 mV at 100 mV s−1, in 0.1 M TBAClO4/MeCN and 0.1 M TBAPF6/MeCN, respectively, close to the theoretical value of 57 mV at 25°C for a reversible, one-electron system.16 Solvents with low water content of 70 ppm25 and 20 ppm16 were used in the prior studies, however, the role of water was not fully investigated. In this study, the cyclic voltammograms of oxygen reduction in 0.1 M TBABF4/MeCN containing 500 ppm water had a peak separation of 205 mV at 100 mV s−1. When the amount of water was increased to 5000 ppm, the value of ΔEp increased to 260 mV. For a reduction reaction in a reversible, one electron system, the reduction potential can be expressed as E1/2 = EA + ΔGsolv – K, where EA is the electron affinity of the neutral molecule in the gaseous state, ΔGsolv is the difference in free energy of solvation of R and the radical anion, and K is a constant.35 Notably, solvation effects for superoxide in water versus acetonitrile are significant (7.6 kcal/mol), larger than would be expected based on the differences in dielectric constants of the two solvents.36
To our knowledge the ORR activity of glassy carbon-polypyrrole (C-cp) composite electrodes has not been previously investigated. Therefore, our results are discussed in light of prior reports showing similar voltammetry, namely carbon based electrodes. As noted above, in addition to the main peak, under some low scan rates, a minor cathodic peak was observed near −1.8 V for C-cp in the electrolyte solution containing 500 ppm of water (Figure 2A). With 5000 ppm of water present in the electrolyte solution, the second peak near −1.8 V was not observed (Fig. 4B). The change in the voltammograms with increased water present can be rationalized as follows. The superoxide formed in TBABF4/MeCN can undergo a disproportionation reaction in the presence of water as previously reported (Eq. 4), which would compete with the oxidation of superoxide (Eq. 5).30,37,38 Higher amounts of water molecules would result in more superoxide disproportionation, further suppressing the superoxide/peroxide redox process.
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn5.jpg)
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/162/1/A69/revision1/jes_162_1_A69eqn6.jpg)
Comparison of the ORR data on silver (Ag disk electrode) shows a +400 mV positive shift in peak potential with increasing water content from 500 to 5000 ppm water data, accompanied by a significant increase in peak current. A positive shift in peak potential and increase in the peak current were previously reported to occur for ORR at a platinum electrode in the nonaqueous electrolyte solutions of acetonitrile (MeCN),27 and N-nitrosodimethylamine.39 This was rationalized as a shift in the reaction mechanism at the platinum electrode from a one electron reduction to O2− to a two electron reduction with the formation of peroxide HO2− in the higher water content electrolyte, similar to that observed in aqueous solution. Acetonitrile is a poor solvating agent of superoxide.35 Because acetonitrile does not hydrogen bond to water, radical anions (such as superoxide) generally have a short half-life in MeCN.40 A dominant characteristic of superoxide ion is its ability to act as a strong Bronsted base via formation of HOO, which reacts with itself or a second superoxide ion.35 Thus, the presence of even small molar quantities of water in a primarily acetonitrile based electrolyte solvent is mechanistically significant. In the presence of water, superoxide ion is rapidly converted to dioxygen and hydrogen peroxide ion, consistent with our observations at a silver disk electrode.
While the ORR at Ag in aqueous electrolyte has been a topic of scientific study for over forty years,41 the stoichiometry (n, electrons per formula unit of oxygen reduced) associated with this reaction remains a topic of ongoing investigation. In the case of supported Ag-based catalysts, n has been shown to vary from 2–4 electrons depending on specifics of the catalyst used.42 There is widespread agreement that formation of the superoxide radical anion •O2− upon addition of the first electron to O2 is the rate determining step, however, the ORR process may include several elementary steps involving different reaction intermediates, where n is dependent on the electronic properties of the electrode materials and the nature of the solution used.43 Thus, direct measurement of n at a specific electrode in a specific solution of interest is a valuable undertaking relevant to ORR, particularly in the case of multifunctional composite electrodes and hybrid electrolytes.
The Nicholson-Shain analysis of the data collected at the silver disk electrode indicates that the water content in the TBABF4/MeCN system significantly affects the ORR mechanism, as the number of electrons involved for ORR changed from n = 2 to n = 4 (Table III). Notably, we report here that with only 5000 ppm of water present in the nonaqueous TBABF4/MeCN electrolyte, the ORR occurs via a four-electron route. Thus, we hypothesize that the product generated at the Ag electrode via ORR changed from peroxide to hydroxide due to the increase from 500 ppm (22 mM) to 5000 ppm (220 mM) of water.
Table III. Summary of number of electrons (n) involved in the oxygen reduction reaction for the electrode types and electrolytes studied.
| electrolyte [H2O] | carbon-conductive polymer composite | carbon-conductive polymer-silver composite | |||
|---|---|---|---|---|---|
| (ppm) | O2 source | carbon (C) | (C-cp) | silver (Ag) | (C-cp-Ag) |
| 500 | O2 | 1 | 1 | 1 | 1 |
| 5000 | O2 | 1 | 1 | 4 | 4 |
| 500 | Air | 1 | 1 | 1 | 1 |
| 5000 | Air | 1 | 1 | 4 | 4 |
It appears that the LSV at C-cp-Ag is a combination of the LSVs of a C-cp and an Ag disk electrode, as the two main distinctive peaks have their characteristic potentials and comparable magnitude of current. When the TBABF4/MeCN solution contained water less than 500 ppm, the C-cp-Ag composite electrode behaved like a combination of C-cp and Ag electrodes. The n = 1 behavior suggested that oxygen was reduced to superoxide, which is usually observed at a C-cp electrode. With 5000 ppm of water present in the solution, the electrochemistry of ORR at the C-cp-Ag composite working electrode changed from a combination of partial C-cp and partial Ag behavior to behavior similar to that of an Ag disk electrode. When the water concentration was increased to 5000 ppm, the C-cp-Ag composite electrode demonstrated ORR activity very similar to that of an Ag disk electrode, characterized by the n = 4 reduction of oxygen to form hydroxide. This demonstrates the sensitivity of the ORR activity of the C-cp-Ag to the amount of water present in the nonaqueous solution. With water content as low as 500 ppm, the C-cp-Ag composite electrode exhibited ORR activity with features of both of C-cp and Ag electrodes, showing more prominent C-cp activity. When the water amount is increased to 5000 ppm, the ORR behavior similar to that of an Ag disk electrode dominated the C-cp-Ag composite electrode, demonstrating the characteristic LSV peaks of comparable current values to an Ag electrode.
Summary
The results of ORR experiments with different type of electrodes, carbon (C), carbon-polypyrrole (C-cp), silver (Ag) and carbon-polypyrrole-silver (C-cp-Ag), with water added to the electrolyte suggest several conclusions. The increase in water from 500 to 5000 ppm in the initially nonaqueous electrolyte leads to changes of the ORR, and the changes vary depending on the electrode type used. With higher levels of water present in solution the ORR behavior approaches the reactions observed in aqueous solution. For GC and Ag electrodes in aqueous systems, the reduction of oxygen was reported to occur in two-electron (n = 2) and four-electron (n = 4) pathways, respectively. Interestingly, the ORR reaction at Ag and C-cp-Ag shows a n = 4 reduction process with only 5000 ppm of water present in primarily nonaqueous solution, while ORR at C and C-cp still remained as n = 1 at the same water level. Thus, it can be observed that the presence of an Ag layer in a composite electrode, C-cp-Ag allows transfer of four electrons for reduction of one oxygen molecule. The use of a multilayer composite electrode provides an opportunity to benefit from the ORR mechanistic pathways provided by a silver electrode in conjunction with the opportunity for electrode design variation including 3-dimensional constructs.
Acknowledgments
Studies involving mechanistic interrogation in moderate water (500 ppm) containing hybrid electrolytes were supported by the Air Force Office of Scientific Research under Award No. FA9550-09-1-0334. Studies involving mechanistic interrogation in higher water (5000 ppm) containing hybrid electrolytes were supported by the Department of Energy, Office of Electricity, administered through Sandia National Laboratories, Purchase Order #1275961.