Abstract
The ratio of Ce3+/Ce4+ on the surface of ceria CMP slurry abrasives was maximized by altering the slurries' chemical environment. Maximizing this ratio increases the proportion of active Ce3+ sites which participate in removal reactions, leading to increased removal rates. Small amounts of peroxide and surfactant were added to three ceria slurries at varying pH values to determine how these chemicals affected the different particle surfaces. The effects of these chemicals on the oxidation ratio were determined through XPS analysis of the Ce 3d spectrum. The addition of surfactants to ceria slurry, while necessary to increase particle dispersibility, decreased the Ce3+/Ce4+ ratio and therefore limited chemical removal. Of the conditions tested, pH had no effect, surfactants decreased the ratio, and peroxide could either increase or decrease the ratio based on concentration. Understanding these interactions will help to inform the design of future ceria CMP slurries to maximize their effectiveness.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Ceria nanoparticles have been used for polishing glass lenses since the 1930's.1 Since the introduction of the 65 nm node in 2006, ceria has been embraced by the semiconductor industry as the abrasive of choice for chemical mechanical polishing of thermal silicon oxides after shallow trench isolation.2 This choice was driven by ceria's high removal rate of oxides and its high selectivity for polishing oxides over nitrides.3 The origin of these properties was detailed by Cook in 1990,4 where he described how ceria participates in both chemical and mechanical removal of silica. In this model, the normal bulk cation state Ce4+ may be replaced, at the particle surface, by a certain percentage of Ce3+ cations which can bind oxygen. This oxygen undergoes a condensation reaction with oxygen on the substrate surface (Reactions 1 and 2) leading to removal of silicates as the particle leaves.
![Equation ([1])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0001.gif)
![Equation ([2])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0002.gif)
If the ratio of Ce3+/Ce4+ (Ce3+%) can be increased there will be more active sites on the particles, leading to a greater removal rate of oxides. The removal of nitrides by ceria will also be affected by this change. This removal occurs through a two-step process where repeated abrasive impacts on the nitride surface along with water incorporation result in hydrolysis of the nitride to form a sub-oxide layer. Subsequent removal will proceed through the standard oxide removal mechanism (Reactions 1 and 2).5,6 Since the limiting step is the hydrolysis, increasing the Ce3+% on the particle surface will not significantly increase the removal rate of nitrides and the selectivity should be maintained. Most studies to date have focused on altering physical properties of the ceria to increase the Ce3+% such as decreasing particle size7–9 and altering synthesis conditions.10,11 Studies on the chemical environment of the slurry have also determined that removal of silica by ceria increased with pH to a maximum at the isoelectric point of ceria (8–9).12,13 Spectroscopic studies of the slurry post-polishing using UV-Vis determined the Ce3+% present in the slurry as a whole is dependent on pH,3 but could not determine what was present on the surface of the ceria particles specifically.14,15 To address this question, this study measures the Ce3+/Ce4+ ratio using X-ray photoelectron spectroscopy (XPS) which is both surface and chemically sensitive. In the case of ceria, orbital splitting and a series of energy transfer processes between electrons creates a total of ten photoelectron peaks in the Ce3d spectrum.16 Six of these peaks correspond to Ce4+ while the other four are associated with Ce3+. These spectra can be deconvolved to determine the percentage of Ce3+ on the particle surface and track how the slurry chemical environment (pH, oxidizing agent, surfactant binding) affects Ce3+%.
Experimental
Materials
The ceria nanoparticles were obtained from Sigma Aldrich (St. Louis, MO) and Sky Spring Nanomaterials (Houston, TX). All other chemicals used were obtained from Sigma Aldrich as well, including hydrogen peroxide (H2O2), nitric acid (HNO3), sodium hydroxide (NaOH) sodium dodecyl sulfate (SDS), polyvinylpyrrolidone (PVP), Glycine (C2H5NO2) and Triton X-100. All chemicals were used without further purification.
Slurry analogue preparation
Slurries were prepared with one of three ceria abrasives at a concentration of 1.0 wt% in deionized water. These slurries were then modified through pH adjustment (2–12) and/or the addition of H2O2 (0.1–5 wt%) or surfactants (0.5–2 wt%).
Characterization methods
The size, shape, and surface morphology of the abrasives were imaged by scanning electron microscopy (Zeiss LEO-1550 operated at 2–3 kV). The size distribution of the ceria particles was obtained from these micrographs using ImageJ analysis. The Ce3+/Ce4+ ratio was investigated through X-ray photoelectron spectroscopy (Thermo Scientific Theta Probe ARXPS). All XPS samples were prepared by drying the slurry on a copper plate overnight before introduction into the vacuum chamber. Spectra were acquired using a 0.05 eV step size, 50 eV pass energy, 50 ms dwell time and 40 scans. CasaXPS analysis software was used to deconvolve these spectra to determine the Ce3+/Ce4+ ratio.
Results and Discussion
Effect of pH
The first parameter investigated was pH, since it has been previously reported to have an effect on Ce3+%. To ensure the results would be representative of ceria as a whole and not due to an unknown variable in the production process, the effect was measured on ceria of three different sizes from two different manufacturers. The three sizes chosen represent the main range of particle sizes for typical ceria CMP abrasives (5–70 nm). The representative SEM images of the different ceria used in this study are shown in Figure 1. The particles in Figures 1a and 1b were obtained as a powder and re-dispersed to form a slurry while Figure 1c was already dispersed in water. These samples will be referred to as Ce1, Ce2 and Ce3 respectively for the rest of the paper. Ce1 had the largest particle size average, at 58 nm; Ce2 was 15 nm; and Ce3 was 6 nm.
Figure 1. SEM images of a) Ce1 and b) Ce2 at a magnification of 150kx. The final image c) Ce3 was obtained at a magnification of 450kx.
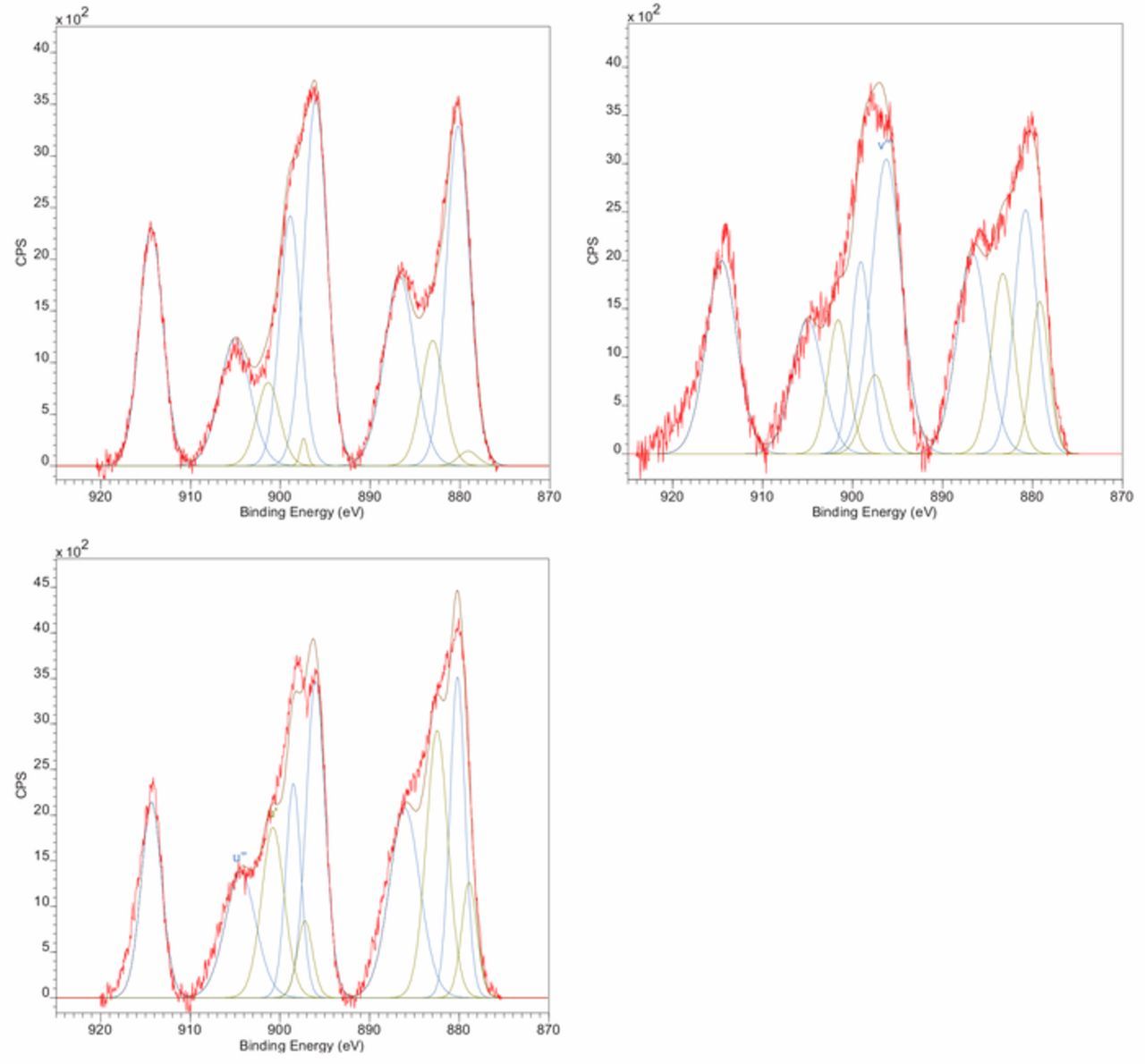
XPS spectra for the three ceria samples are shown in Figure 2. As the size of the particles decreases (from Ce1-Ce3), the size of the Ce3+ peaks in the spectrum increases, indicating a higher Ce3+% on the particle surface, consistent with other reports.7–9 The sizes and initial Ce3+% of the as-received samples are summarized in Table I.
Figure 2. XPS analysis of as-received ceria abrasives a) Ce1 b) Ce2 c) Ce3. The blue peaks correspond to Ce4+ while the green correspond to Ce3+.
Table I. Average size and Ce3+% of as-received ceria powders.
| Average | Ce3+ | |
|---|---|---|
| Particle | Size (nm) | concentration (%) |
| Sigma Powder | 58 | 12 |
| Sky Spring Nanomaterials Powder | 15 | 25 |
| Sigma Dispersion | 6 | 31 |
Ce1 and Ce2 were dispersed in water and the pH of all three samples were measured and found to be ∼8 without any modification. Model slurries were then made with pH ranging from 2–12. When samples were dried from these models, a pH strip was dipped in DI H2O and then pressed against the dried powder. The pH values measured indicated that no changes in pH had occurred during the drying process. Thus, the XPS measurements, despite being conducted on dry powders (which are not normally referred to in terms of pH) will still be representative of the pH of the liquid slurry.
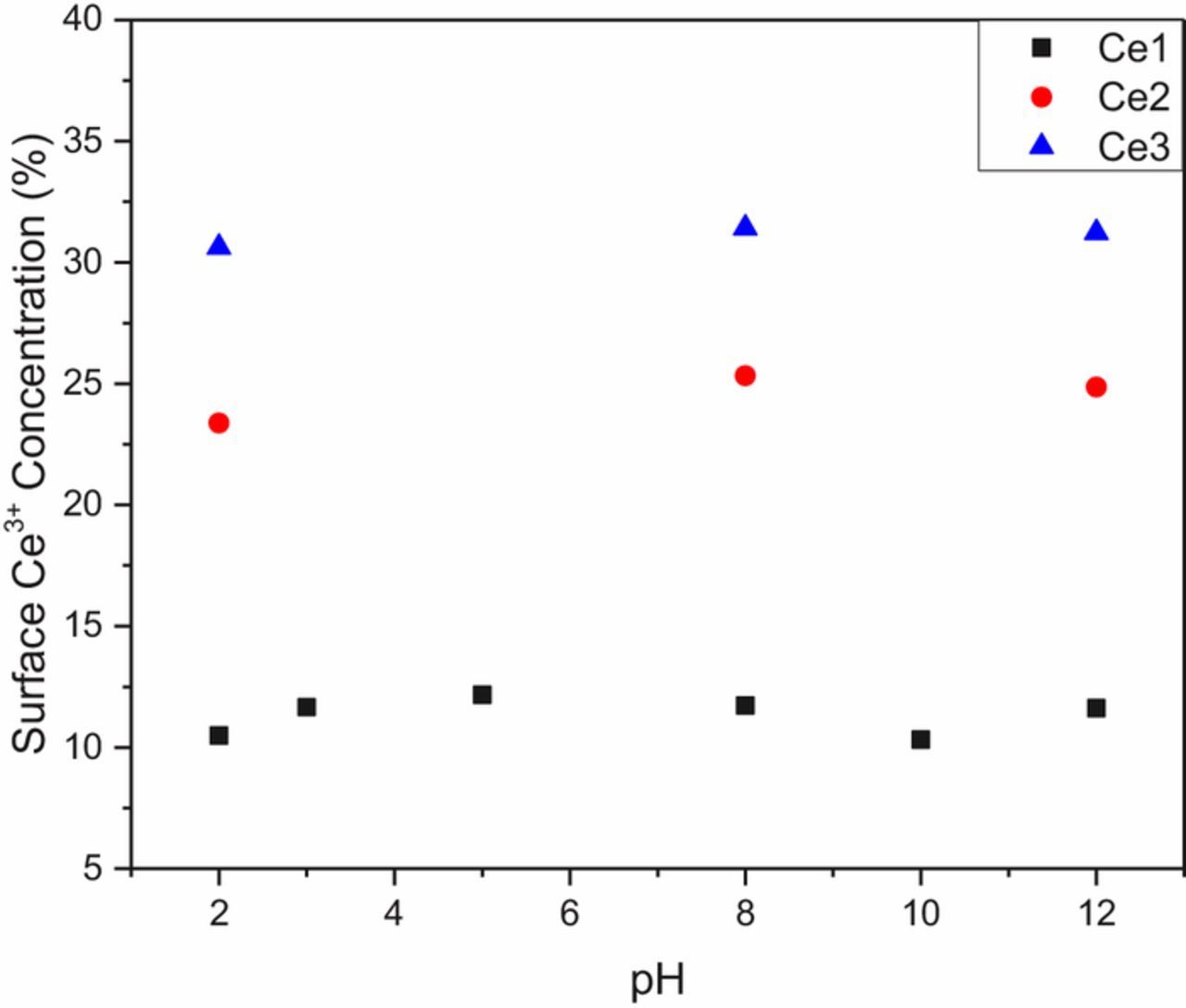
Figure 3 shows the calculated concentration of Ce3+ on the surface of each of the powders changes negligibly across a wide range of pH. While the particles differ from each other, each particle stayed at its own initial value across the pH range tested. These values are in disagreement with those found previously in the literature, which measured cerium ions in solution using UV-Vis spectroscopy post-CMP.
Figure 3. Effect of pH on the Ce3+ concentration of different ceria particles.
Since changing the pH did not change the proportion of available Ce3+ on the particles, the increase in removal rates as pH increased to the isoelectric point of ceria13 must be related to the interaction between ceria and the oxide surface. For chemical removal to occur the slurry should be at or near the isoelectric point of either the ceria or the oxide (Reactions 1 or 2). Cook stated in his paper that to obtain high removal rates the oxide must be able to undergo hydrolysis to incorporate H2O into its lattice.4 This incorporation breaks subsurface oxygen bonds upon subsequent particle impacts, thus increasing the probability that a silicate will be removed. The hydrolysis of silica has been determined to only occur at an appreciable rate above pH 7 (Reaction 3).17
![Equation ([3])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0003.gif)
Based on these points it can be seen that the highest removal rate occurs at pH 8–9 due to greater hydrolysis of silica and increased rate of condensation between ceria and silica through reaction mechanism 1. A final consequence of removal through condensation reactions is that the concentration of Ce3+ sites has no effect on reaction rate. Any increased removal due to increasing Ce3+% is instead due to a greater probability for the reaction to occur.
Effect of H2O2
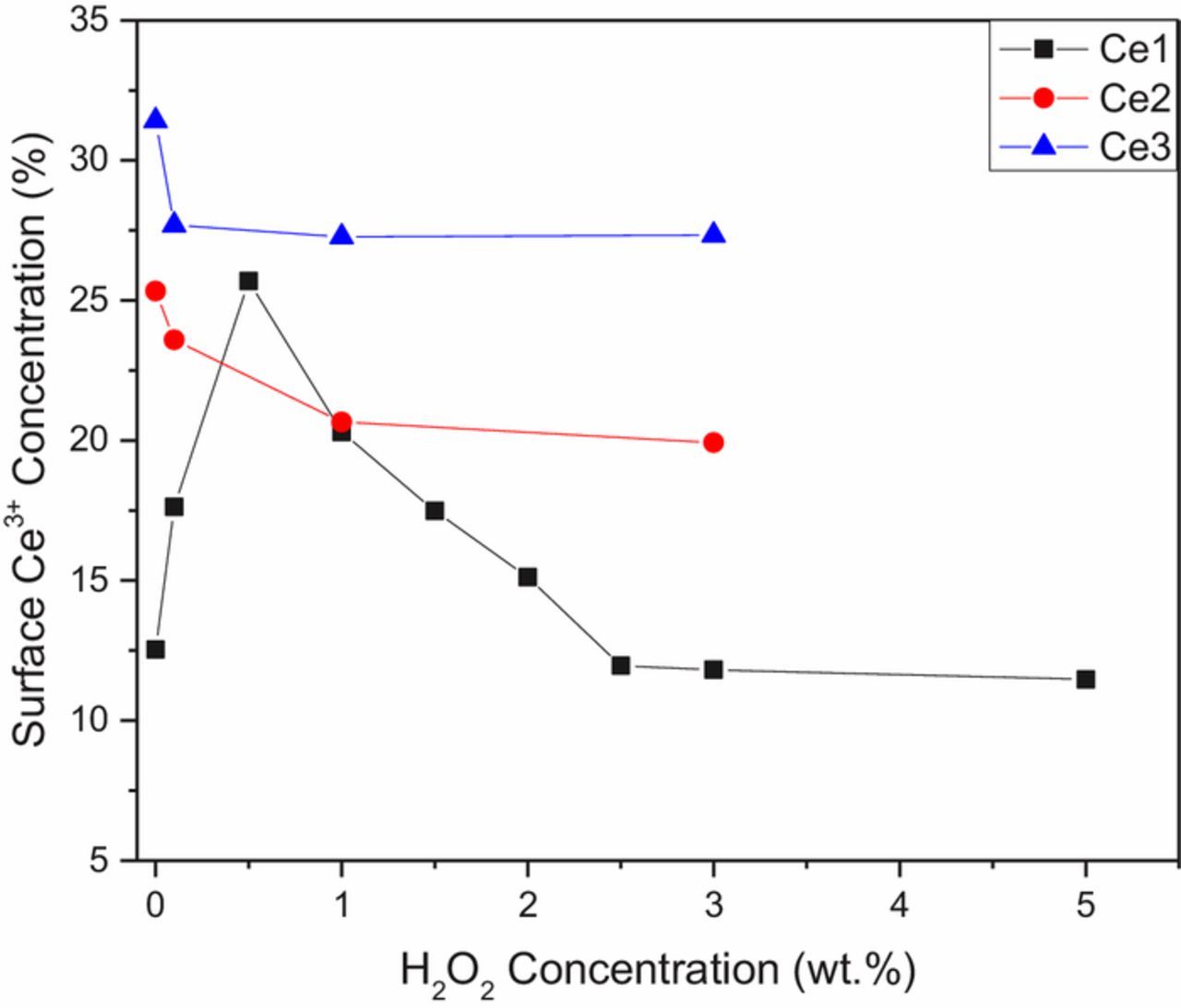
The second parameter tested was the effect of oxidizing agent on the Ce3+% of the particles, shown in Figure 4. The initial Ce3+% values given in Table I correspond to the 0% H2O2 data points in this figure. It would normally be assumed that as the oxidizing agent concentration increases, the cerium particles will be oxidized and the Ce3+% will subsequently decrease. This behavior holds true for both Ce2 and Ce3, but surprisingly not for Ce1, where a more complicated relationship was found. In particular, the Ce3+% on the surface of Ce1 doubles with the addition of small amounts of H2O2, then gradually decreases back to its initial concentration.
Figure 4. Effect of H2O2 on the Ce3+ concentration of different ceria particles (lines included as a guide for the eye only).
Traditionally, the effect of H2O2 on ceria has been explained using reduction-oxidation reactions due to ceria's use as a catalyst. Reactions 4 and 5 correspond to the reduction of ceria, whereas Reactions 6 and 7 detail ceria oxidation.18
![Equation ([4])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0004.gif)
![Equation ([5])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0005.gif)
![Equation ([6])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0006.gif)
![Equation ([7])](https://content.cld.iop.org/journals/2162-8777/8/10/P629/revision1/d0007.gif)
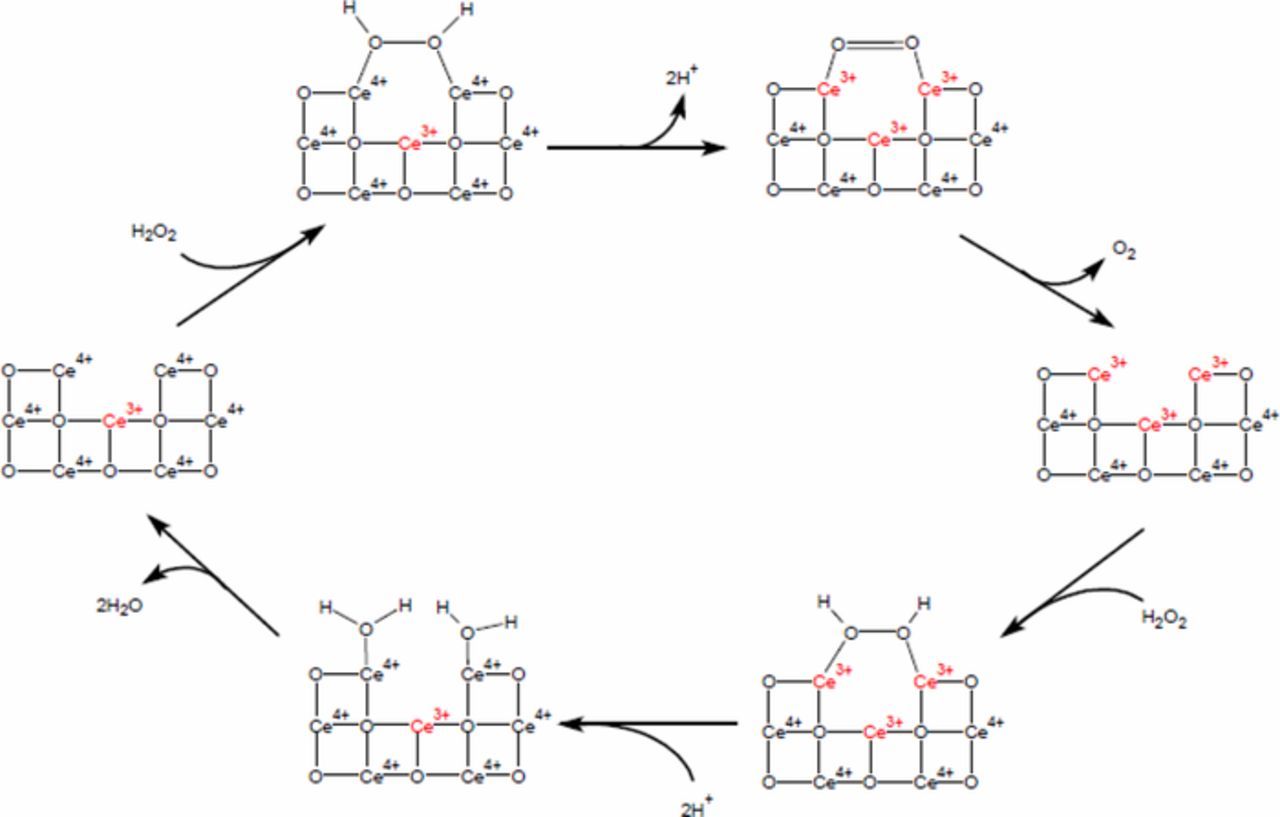
Based on these reactions the reduction of ceria is favored, yet only Ce1 exhibits reduction, and even then, it is only upon small additions of H2O2. To understand this seemingly counterintuitive result requires a deeper investigation into how ceria switches oxidation states. Ceria is often used to scavenge oxygen through catalysis,19 but its more relevant use is in the scavenging of reactive oxygen species (ROS) from biological cells.20,21 For this purpose, ceria can mimic two different ROS scavengers, the enzymes catalase and superoxide dismutase (SOD). Ceria's SOD mimetic activity requires reacting with both H2O2 and oxygen radicals, whereas catalase only reacts with H2O2. Since the concentration of H2O2 in the slurry is much higher than the naturally occurring ●O−2 the ceria will react according to the catalase reaction mechanism (Figure 5 adapted from Reference 22). Through this mechanism ceria reacts with H2O2 twice. First converting from Ce4+ to Ce3+ (top of diagram) and the second time reverts the particle back to the Ce4+ state (at the bottom of the diagram).
Figure 5. Catalase mimetic activity of ceria. In this cycle, ceria reacts with H2O2 twice. At the top of the cycle, it converts Ce4+ to Ce3+, while at the bottom of the cycle it converts Ce3+ to Ce4+ (adapted from 22).
When the particle starts with a low initial Ce3+ state, like Ce1, it will proceed in the forward reaction converting 4+ to 3+ until enough H2O2 is added to continue the reaction and oxidize the cerium.23 The other particles, Ce2 and Ce3, enter the cycle with a higher Ce3+/Ce4+ ratio and therefore proceed through the second half of the cycle only. With this knowledge, larger particles (which naturally have a lower Ce3+/Ce4+ ratio) and those with low Ce3+% due to other manufacturing conditions can be tailored through small additions of H2O2 to increase this value and make them more viable as polishing abrasives.
Effect of surfactants
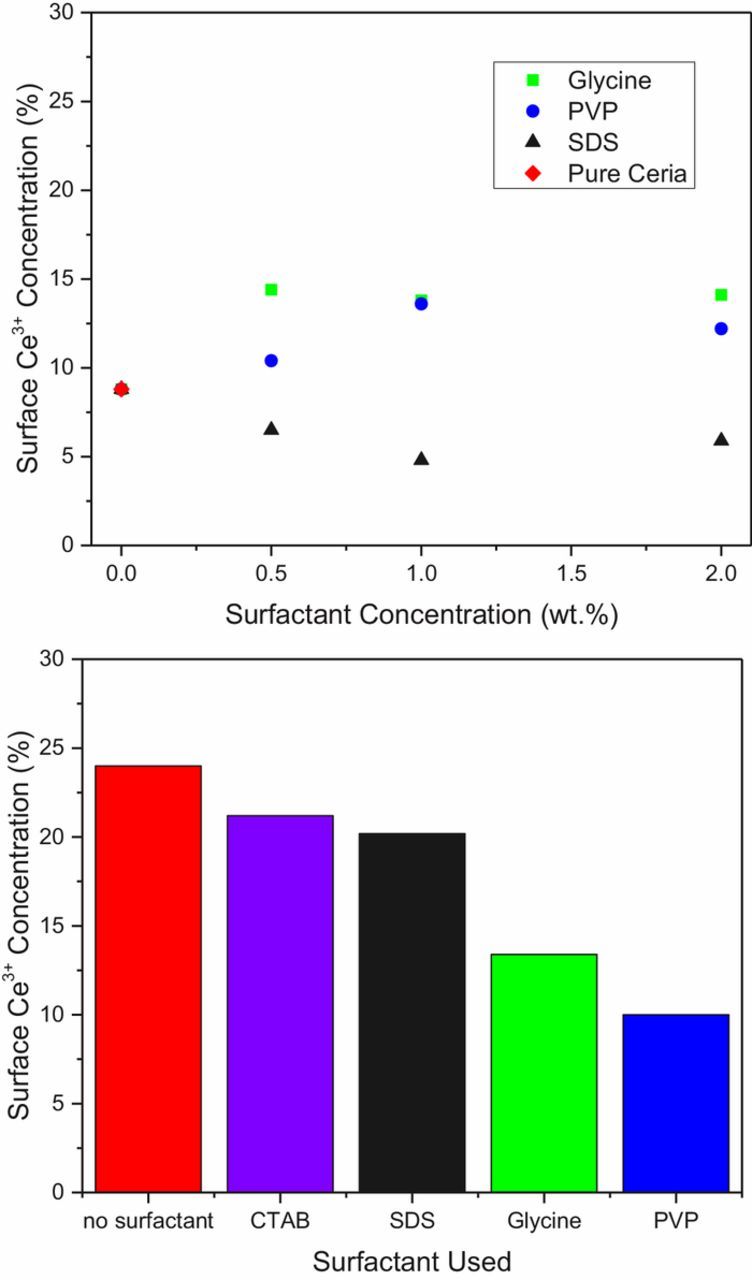
The final parameter investigated was the effect of surfactants on the peroxide's ability to increase Ce3+%. Only Ce1 was used for this set of experiments since there was no increase in Ce3+% upon H2O2 addition for either Ce2 or Ce3. As seen in Figure 6a, the addition of surfactants to Ce1 without H2O2 had no significant effect on the Ce3+% across a range of concentrations.
Figure 6. Effect of surfactants on the Ce3+ concentration both a) without H2O2 and b) with 0.5 wt% H2O2 at 0.5 wt% surfactants.
Figure 6b shows the impact of surfactant after 0.5 wt% H2O2 had been added to the Ce1 slurry, which maximized the initial Ce3+%, as determined in the previous section. In this case, a difference is observed between the surfactants, where glycine and PVP both reduced the Ce3+% significantly while CTAB and SDS had almost no effect. While all the surfactants were added at 0.5 wt%, this concentration is above the critical micelle concentration for both SDS and CTAB, to ensure stable a dispersion,24 whereas glycine and PVP were in the form of single molecules or polymer chains respectively. This structural difference means that fewer surfactant molecules were available to bind to the ceria surface for the micellular surfactants than the linear ones. Subsequently, there are more unbound Ce3+ sites in slurries that contain the former, resulting in a greater Ce3+% at the particle surface.
Conclusions
A model for the effect of chemical environment on the oxidation state of ceria abrasives for CMP is described. Each particle has a different initial Ce3+/Ce4+ ratio that is determined by their size and synthesis conditions. This ratio is not affected by the pH of the solution that the particles were dispersed in. By introducing H2O2 to the slurry, the cation ratio can be altered in a predictable way based upon the initial conditions of the particle because ceria undergoes a catalase-like reaction in the presence of peroxide. Finally, the physical structures of surfactants added to the slurry effects the number of surface sites that were blocked leading to differences in their effect on the 3+/4+ ratio. These findings can be used to inform and improve the design of future ceria slurries to maximize their chemical removal of oxides.
Acknowledgments
The authors thank SUMCO (Tokyo, Japan) for their support of Prof. Dunn's CMP laboratory.
ORCID
C. M. Netzband 0000-0003-4952-166X
K. Dunn 0000-0002-7488-7981