Abstract
A series of new niobate phosphors Ba3ZrNb4O15:xEu3+ (0 ≤ x ≤ 0.04) were firstly prepared, and their phase purity as well as the site occupation situation of Eu3+ ions was carefully investigated via X-ray diffraction structure refinement and series powder X-ray diffraction patterns. The morphology and microstructure were studied through scanning electron microscope and high-resolution transmission electron microscopy. Versatile luminescent properties were studied through analyzing the excitation spectra monitored at 613 nm and the emission spectra under 396 and 468 nm excitations. The contents dependent luminescent properties of Ba3ZrNb4O15:xEu3+ (0 ≤ x ≤ 0.04) under different excitation wavelengths were investigated. The optimal doping content, CIE chromaticity coordinates, color purity as well as the quantum efficiency were determined. The thermal quenching analysis showed that Ba3ZrNb4O15:Eu3+ had excellent thermal stability at 468 nm excitation and exhibited linear changed thermal quenching behavior at 396 nm excitation. The mechanism of thermal quenching behavior under different excitation wavelengths was discussed through the configurational coordinate diagram. The temperature dependent luminescence decay behavior was studied upon different excitation wavelength and the corresponding activation energy was calculated by a modified Arrhenius equation.
Export citation and abstract BibTeX RIS
Till now, trivalent lanthanide ions have been regarded as an important class of luminescent centers due to the characteristic electron transitions within their partially filled 4f shell.1 The corresponding parity forbidden transitions lead to low molar absorption coefficients and long luminescent lifetimes (up to several milliseconds). The long luminescent lifetimes are advantageous in applications such as lasers and optical amplifiers, and for excited state absorption processes, such as up-conversion.2–6 The line emissions arising from some of the trivalent lanthanide ions are also benefit for improving the color rendering of some luminescent devices. Recently, trivalent rare-earth doped materials have been widely employed in luminescence devices, lasers, optical sensors, dosimetry and micro beam radiation therapy for dose monitoring.7–9 For example, neodymium doped yttrium aluminum garnet (YAG:Nd) lasers, Er-doped optical fibers for telecommunications and Yb3+, Er3+,Tm3+ and Ho3+ single or co-doped NaYF4 for up-conversion materials.10–13 Among many lanthanide ions, trivalent europium Eu3+ has been recognized as an excellent red activator in many classic phosphors due to its 5D0→7FJ (J = 0, 1, 2, 3, 4) transitions. One of the milestones in Eu3+ doped luminescent materials is the discovery of Y2O3:Eu3+, which is key phosphors for cathode-ray tubes and fluorescent lamps, still in heavy use today.14 Moreover, Eu3+ ion is usually used as a structure probe to determine the local structure around the lanthanide ions or study structural phase transitions in many hosts.15,16
Recently, barium zirconium niobate Ba3ZrNb4O15 (BZN) with a tungsten bronze structure has got much attention attributed to their nonlinear optical behavior and ferroelectric property.17 Tungsten bronze oxide has the formula of (A1)2(A2)4C4(B1)2(B2)8O30, in which the smallest C site is generally empty. The abundant crystallographically nonequivalent cation sites guarantee artificial regulation of this structure and provide the flexibility to design the various properties by chemical substitution or ion doping.18 In this work, Eu3+ doped BZN phosphors are prepared firstly and investigated through X-ray diffraction (XRD), photoluminescence excitation and emission spectra for the fundamental scientific research and potential application in solid state lighting.
Experimental
Samples of BZN:xEu3+ (0 ≤ x ≤ 0.04) are prepared by solid state method with BaCO3 (99.9%), Zr(NO3)4·5H2O (99.9%), Nb2O5 (99.9%) and Eu2O3 (99.99%) from Sinopharm Chemical Reagent Co. Ltd. The stoichiometric starting materials are uniformly ground by adding ethanol in agate mortar, then put in an alumina crucible and calcined at 1523 K in air for 6 h. Finally, the samples are slowly cooled to room temperature with at 5 K/min.
The crystal structure and phase purity was determined by using a Rigaku Ultima IV X-ray diffractometer (XRD). The morphologies and microstructure were investigated by scanning electron microscope (SEM, S4800) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai F30, operated at 300 kV). FS5-MCS fluorescence spectrophotometer was employed to examine the photoluminescence spectra and the fluorescence decay times. The thermal quenching property was investigated by a heat booster (TCB1402C) equipped on the fluorescence spectrophotometer.
Results and Discussion
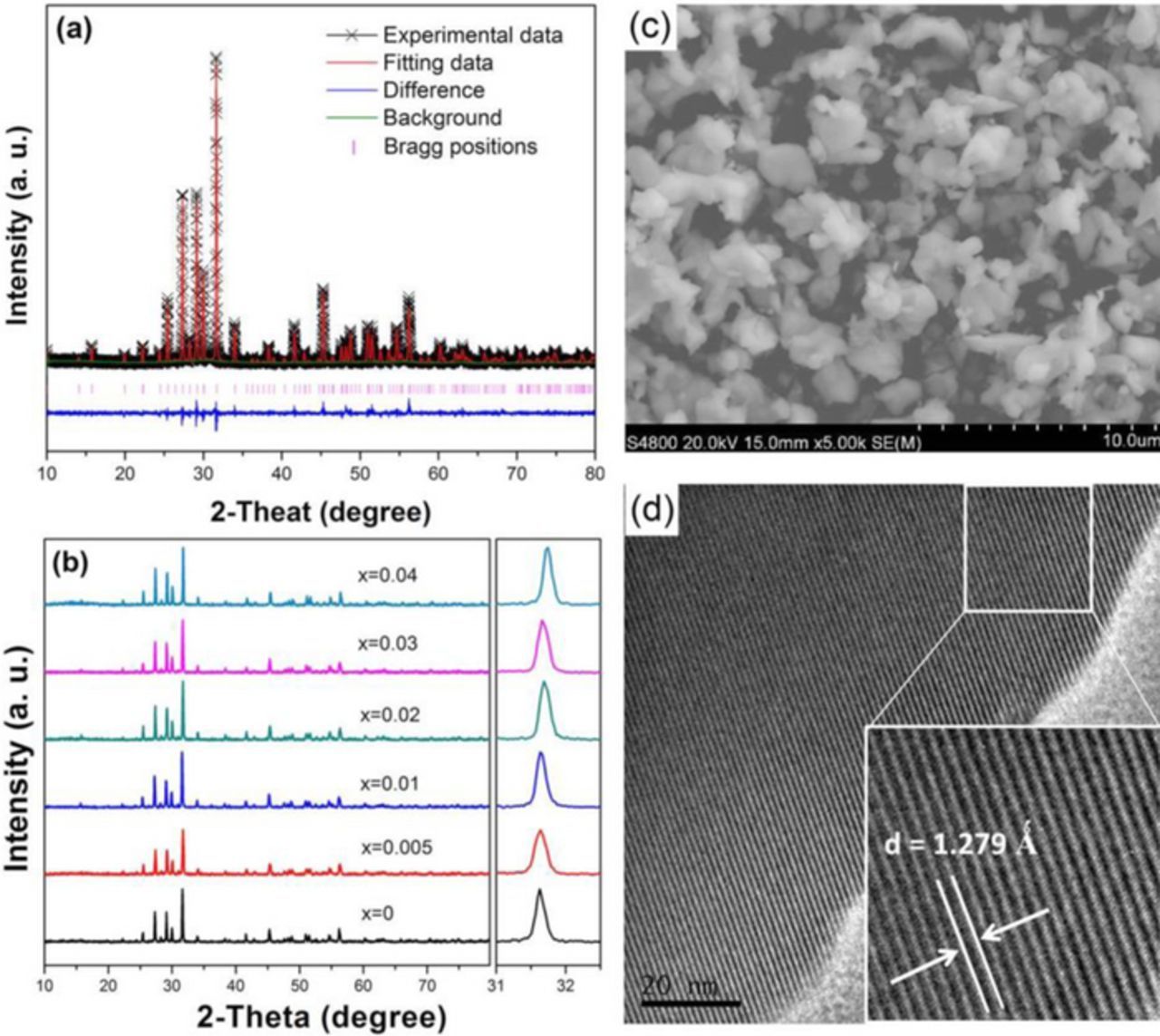
Figure 1a illustrates the XRD Rietveld refinement results of BZN host, which shows that the prepared BZN power crystallizes in a noncentrosymmetric crystal structure with a space group of P4bm and the cell parameters are calculated to be a = b = 12.6815 Å, c = 4.0135 Å. The goodness-of-fit parameters (Rwp = 11.85%, Rp = 9.16%) guarantee the pure phase of BZN host. In each BZN unit cell, there are four kinds of available cationic sites, including Ba1, Ba2, Zr/Nb1 and Zr/Nb2, of which Zr/Nb atoms adopt octahedron groups with six coordination, while the larger Ba2+ cations embed rigidity to the host framework to form the tetragonal structure. The series XRD patterns of BZN:xEu3+ (0 ≤ x ≤ 0.04) is plotted in Fig. 1b. No detectable secondary phases can be found in the series XRD patterns, showing the addition of Eu3+ has no significant change in the BZN structure. The main peak is found to slightly shift toward the larger angle direction, which is in virtue of the smaller radius of Eu3+ ions compared with that of Ba2+ ions in BZN host lattice and indicates that Eu3+ ions has successfully entered BZN host to occupy Ba2+ sites.19 Due to the low doping contents of Eu3+ ions, the charge can be balanced by generating some defects during solid state reaction process. Thus, the charge compensator is not introduced in the following discussion. Figs. 1c and 1d illustrate the SEM and HR-TEM images of BZN host. It could be seen that the obtained BZN presents irregular form with particle sizes around 2–5 μm. HRTEM image clearly reveals that the as-prepared BZN sample is well crystallized. The interplanar distance d of the selective area is measured to be 1.279 Ǻ, which is assigned to the (223) crystal plane of BZN.
Figure 1. (a) XRD Rietveld structure analysis of BZN by using Materials Studio program; (b) XRD patterns of BZN:xEu3+ (0 ≤ x ≤ 0.04); the inset shows the selected XRD patterns ranging from 31 to 32.5 degree; (c) SEM image of as-prepared BZN host; (d) HR-TEM image of as-prepared BZN host.
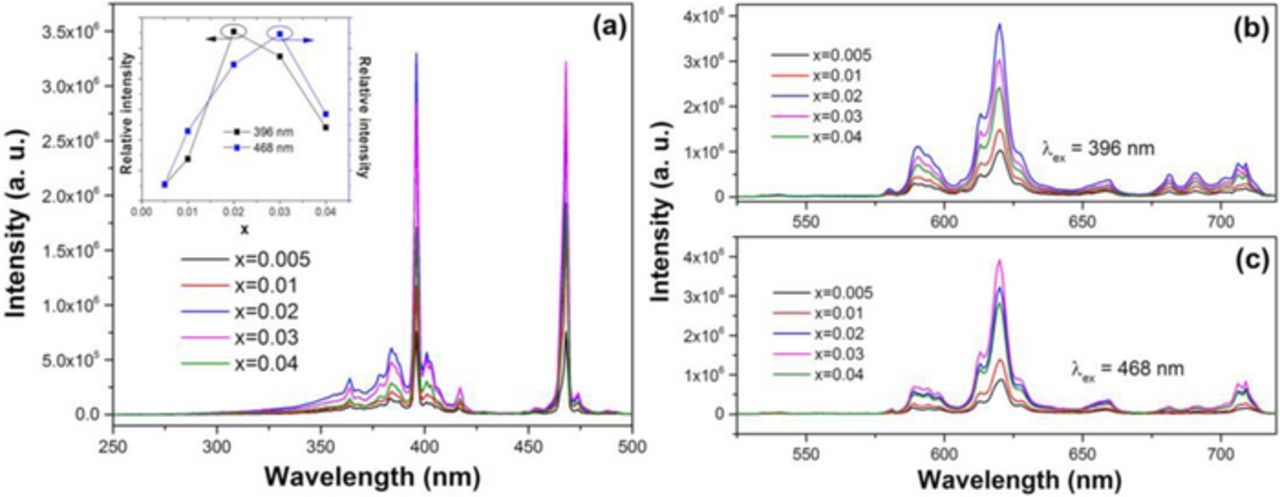
Figure 2a illustrates the excitation spectra of BZN:xEu3+ (0.005 ≤ x ≤ 0.04) monitored at 613 nm. Generally, the excitation spectrum of Eu3+ presents sharp line absorptions due to the intra-4f forbidden transition excitations and is mainly dominated by line absorptions from either 7F0→5L6 transition around 396 nm or 7F0→5D2 transition around 468 nm.20,21 However, most Eu3+ doped phosphors could be effectively excited by only one of the above two excitation wavelengths. It is interesting that in BZN:Eu3+, the 7F0→5L6 transition excitation at 396 nm and the 7F0→5D2 transition excitation at 468 nm are both dominant and have similar excitation intensity, indicating BZN:Eu3+ has versatile luminescence properties and could be pumped by either UV or blue light excitation. With increasing Eu3+ contents, the excitation intensity of 7F0→5L6 transition reaches maximum when x is 0.02. However, for the 7F0→5D2 transition, it reaches maximum when x increases to 0.03. The different contents quenching may be attributed to the different cross relaxation processes under different excitations.22 To further investigate it, versatile emission spectra under different excitations are investigated as shown in Figs. 2b and 2c. It can be seen that the emission spectra mainly consist of several sharp lines ranging from 500 to 750 nm, which are associated with the transitions from the excited state 5D0 to 7FJ (J = 0, 1, 2, 3, 4) levels of Eu3+. In particular, the highest red emission at 613 nm is due to the 5D0→7F2 electric dipole transition, indicating that Eu3+ ions occupy an anti-inversion symmetry site in BZN host based on the Judd-Ofelt theory, which is consistent with the previous analysis.23 Conversely, a reddish orange emission will be dominated ascribed to the magnetic transition 5D0→7F1 if Eu3+ occupies an inversion symmetry site. With increasing Eu3+ contents, the emission intensity under 396 and 468 nm excitation are found to be gradually increased and reach the maximum when x is 0.02 and 0.03, respectively, as shown in Figs. 2b and 2c. The different emission quenching behaviors in BZN:Eu3+ is reasonable and should be ascribed to the different selective excitation intensities around 396 and 468 nm under different Eu3+ contents, as illustrated in Fig. 2a and the inset. The CIE chromaticity coordinates of BZN:0.02Eu3+ and BZN:0.03Eu3+ are calculated to be (0.643,0.352) and (0.656,0.343), respectively, which approaches to those of the commercial red phosphor Y2O2S:Eu3+ (0.647, 0.343).24 The dominant wavelength and color purity as compared to the 1931 CIE Standard Source C [illuminant C = (0.3101, 0.3162)] are determined based on the emission spectrum in Fig. 2. The dominant wavelength of a color is the single monochromatic wavelength of the spectrum whose chromaticity is on the same straight line as the sample point (xs, ys) and the illuminant point (xi, yi). The color purity can be estimated using the formula,
![Equation ([1])](https://content.cld.iop.org/journals/2162-8777/7/6/R94/revision1/d0001.gif)
where (xd, yd) is the color coordinate of the dominant wavelength. Based on the spectra data in Fig. 2, the color purity of BZN:0.02Eu3+ under 396 nm excitation and BZN:0.03Eu3+ under 468 nm excitation was calculated to be 91.7% and 95%, respectively. What's more, the quantum efficiency (QE) of BZN:Eu3+ phosphors is measured by the integrating sphere method as discussed in other works.25 The results show that the QE of BZN:0.02Eu3+ under 396 nm excitation and BZN:0.03Eu3+ under 468 nm excitation are measured to be 39.8% and 42.5%, respectively, which can be further enhanced by subsequent improvement such as charge compensation or adding some fluxing agent.
Figure 2. (a) The excitation spectra of BZN:xEu3+ (0.005 ≤ x ≤ 0.04); the inset shows the relationship between the contents and the relative intensity (b) the emission spectra of BZN:xEu3+ (0.005 ≤ x ≤ 0.04) excited at 396 nm; (c) the emission spectra of BZN:xEu3+ (0.005 ≤ x ≤ 0.04) excited at 468 nm.
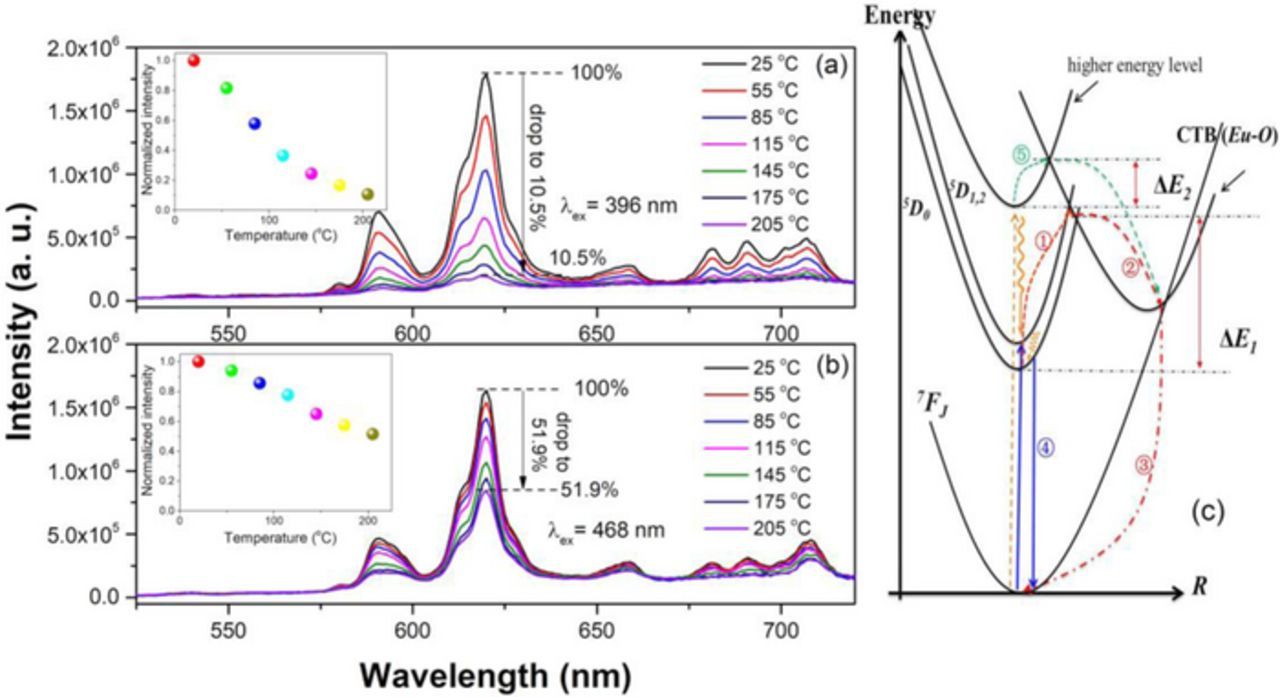
To investigate the influence of temperature on the luminescence properties, the temperature dependent emission spectra of BZN:0.02Eu3+ and BZN:0.03Eu3+ (the optimal doping contents under different excitation wavelength) are measured ranging from room temperature to 205°C under 396 and 468 nm, respectively. Interestingly, the temperature dependent emission spectra exhibit totally different emission quenching properties. As shown in Fig. 3a, when excites it at 396 nm, the emission intensity of BZN:0.02Eu3+ drops rapidly as the temperature arises. A linear changed thermal quenching behavior can be observed as illustrated in the inset of Fig. 3a, indicating that the phosphor may be suitable to be applied in some temperature sensitive luminescent devices such as optical temperature sensor.26 In Fig. 3b, when excites it at 468 nm excitation, BZN:0.03Eu3+ shows excellent thermal stability. When the temperature is increased to 205°C, the emission intensity of BZN:0.03Eu3+ still keeps 51.9% of its initial intensity. Much attention has been focused on the thermal quenching mechanism of 5D0-7F2 emission of Eu3+ ions, which demonstrates that the crossover process, in which the electrons on the 5D0 level could overcome the energy barrier and migrate to the Eu-O charge transition band (CTB) and finally relax to 7FJ levels, is responsible for the temperature quenching of 5D0 emission,27,28 as illustrated by the configurational coordinate diagram in Fig. 3c. In order to try to explain the interesting thermal quenching behavior under different excitation wavelength, a possible quenching mechanism based on the configurational coordinate diagram is inferred and illustrated. When excites it through 396 nm excitation, the electrons are excited to higher energy level. On one hand, some of them at higher energy level are relaxed by cross-relaxation to the 5D0 state (through wave line) and overcome the activation energy ΔE1 assisted by phonons as the temperature increases and feeds to the 7FJ state through Eu-O CTB, which provides the non-raditative process and results in the emission quenching (via ① to ③).29 The remaining electrons are contributed to the 5D0→7F2 emission at 613 nm through path ④. On the other hand, some of the electrons at higher energy level might overcome the activation energy ΔE2 under thermal activation effect and also provide the non-raditative process through pathway ⑤. In other words, there are two pathways of non-raditative process when excited the phosphors at 396 nm. However, when excites it by 468 nm, most of the electrons may be excited to the 5D0 level (only few or small part of electrons can reach higher energy level), thus the non-raditative transitions pathways are reduced (pathway ⑤ is blocked). More electrons return to the ground state through raditative transitions pathway ④ and finally leads to the better thermal stability than that excited by 396 nm. Anyway, the thermal stability is satisfactory and here we only discuss one of the possible causations for the observed thermal quenching behavior.
Figure 3. (a) The temperature dependent emission spectra of BZN:0.02Eu3+ excited at 396 nm; the inset shows the relationship between the normalized emission intensity under 396 nm excitation and the temperature; (b) the temperature dependent emission spectra of BZN:0.03Eu3+ excited at 468 nm; the inset shows the relationship between the normalized emission intensity under 468 nm excitation and the temperature; (c) the configurational coordinate diagram of the thermal quenching process of Eu3+ in BZN.
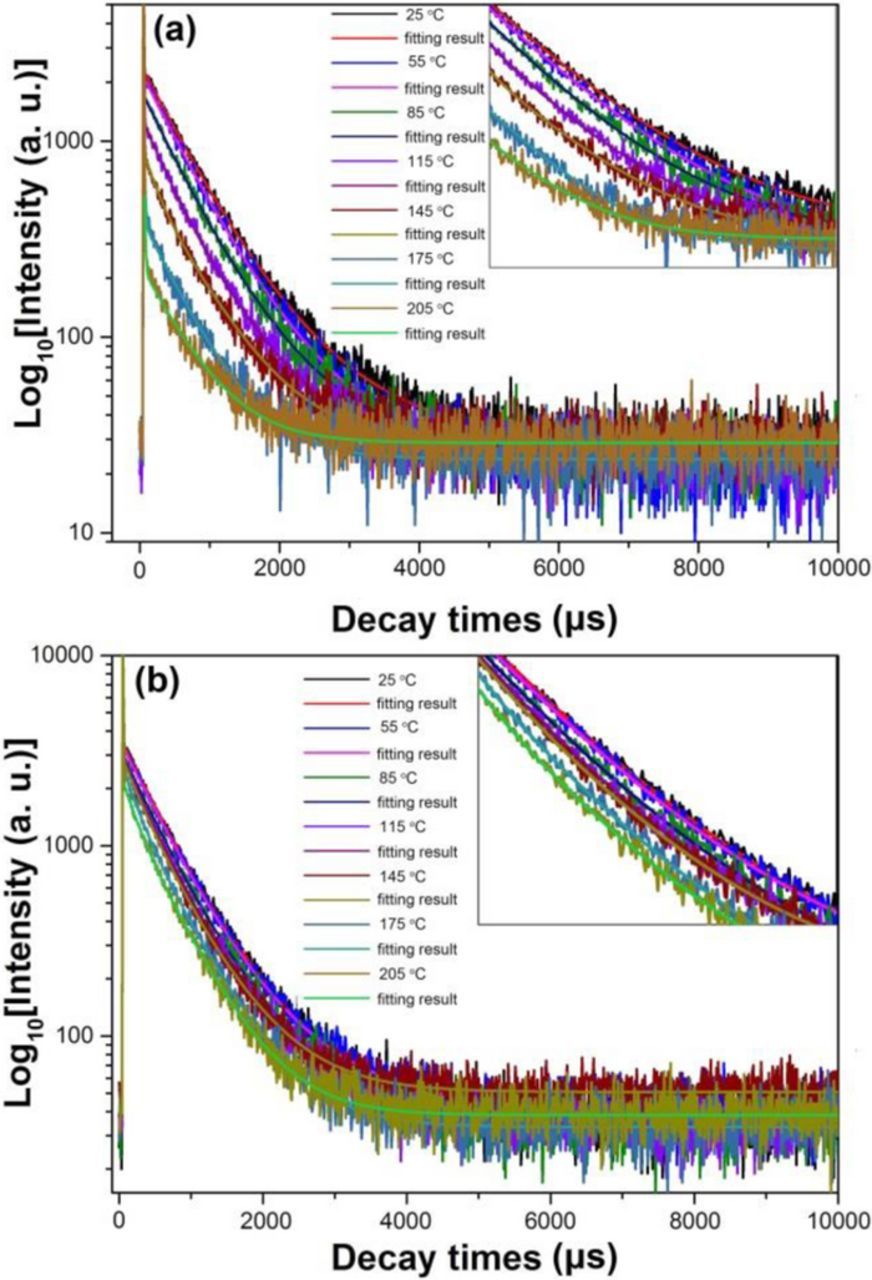
Additionally, in order to further investigate the temperature quenching process, the temperature dependent luminescence decay curves of BZN:Eu3+ under 396 and 468 nm excitations are measured from room temperature to 205°C, as illustrated in Fig. 4. Upon 396 nm excitation, it can be seen that the decay behavior of BZN:Eu3+ strongly depends on the temperature, indicating that the decrease of the Eu3+ lifetime is also caused by the thermal vibration. All the decay curves can be well fitted to a double exponential model and the average decay time τ can be determined by the formula,30
![Equation ([2])](https://content.cld.iop.org/journals/2162-8777/7/6/R94/revision1/d0002.gif)
where I(t) is the luminescence intensity; A1 and A2 are constants; t is the time; and τ1 and τ2 are rapid and slow lifetimes for exponential components. Using these parameters, the average decay times (τ) can be determined by the formula given in the following,30
![Equation ([3])](https://content.cld.iop.org/journals/2162-8777/7/6/R94/revision1/d0003.gif)
Figure 4. The temperature dependent decay curves of BZN: Eu3+ under 396 nm (a) and 468 nm (b) excitation. The partial enlarged drawings are shown in the insets.
On the basis of Eq. 2 and the measured decay curves, the average lifetime of Eu3+ in BZN can be calculated to be 752.64, 731.15, 694.56, 669.53, 622.58, 551.16 and 542.64 μs, respectively. Such significant lifetime shortening presents evidence that there is the intrinsic severe quenching in BZN:Eu3+ upon 396 nm excitation.31 The shortening in lifetime is accompanied by the decrease of emission intensity, which can also be seen from the decay curves where t equals to 0 in Fig. 4a, corresponding to the initial emission intensity I. The suddenly shorter decay times indicate that there are more non-radiative transition pathways for the electrons to return to ground state, which is consistent with the configurational coordinate diagram as illustrated in Fig. 3c. But unlike the decay behavior of BZN:Eu3+ upon 396 nm excitation, the decay behavior upon 468 nm excitation changes slightly as shown in Fig. 4b, which can also be seen at t = 0, the initial intensity decreased tardily along with increasing the temperature. Finally, the average lifetimes of BZN:Eu3+ upon 468 nm excitation can also be well fitted by double exponential model and are calculated to be 679.89, 669.70, 644.88, 612.46, 574.32, 546.49 and 533.31 μs, respectively. Many works have focused on the calculation of activation energy through the emission intensity by employing Eq. 4,32
![Equation ([4])](https://content.cld.iop.org/journals/2162-8777/7/6/R94/revision1/d0004.gif)
where I0 is the intensity at 300 K and IT is the intensity at temperature T, c is a constant, k is the Boltzmann constant and ΔE is the activation energy. Here we evaluate the activation energy the temperature-dependent relative lifetime rather than emission intensity by a modified Arrhenius equation,33
![Equation ([5])](https://content.cld.iop.org/journals/2162-8777/7/6/R94/revision1/d0005.gif)
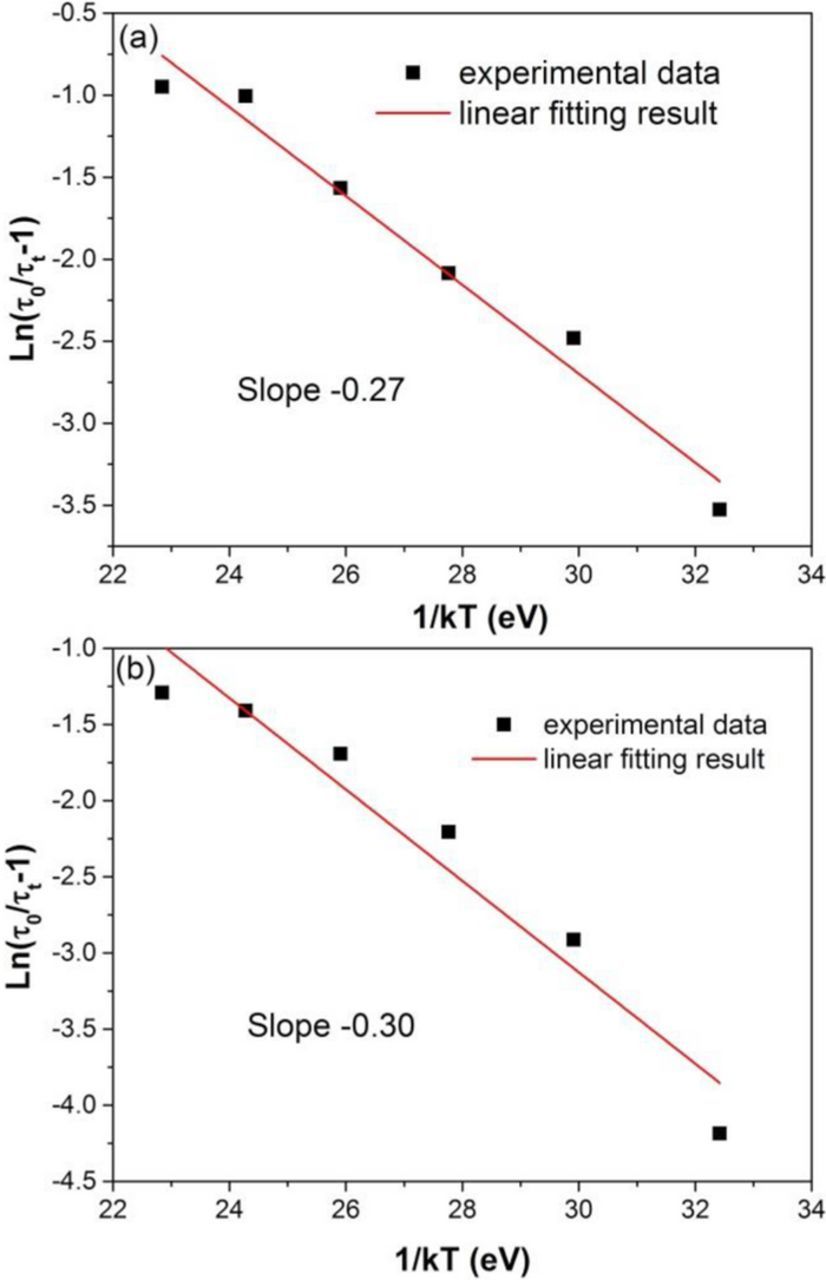
where τt is the emission decay life time at temperature(K), τ0 is the initial emission life time, C is a rate constant for the process. The decay lifetimes are more reliable than the emission intensity because it is not affected by the testing conditions.31 According to Eq. 5, the activation energy (ΔE) can be calculated to be 0.27 eV and 0.30 eV under 396 and 468 nm excitations, respectively, and the fitting results are illustrated in Fig. 5.
Figure 5. Linear fitting results of ln(τ0/τt-1) along with 1/kT under (a) 396 and (b) 468 nm excitations.
Conclusions
In summary, a series of novel red emission phosphor BZN:Eu3+ was synthesized for the first time. XRD Rietveld structural refinement indicated that the obtained BZN was single phased and well crystallized with space group P4bm. The site occupation situation of Eu3+ in BZN was investigated through series XRD analysis and revealed that Eu3+ was favor of occupying Ba2+ sites in BZN. The excitation spectra indicated that BZN:Eu3+ had versatile luminescence which could be efficiently excited by both UV and blue light. Upon 396 and 468 nm excitation, the phosphors could exhibit red emission with dominated peak at 613 nm due to 5D0→7F2 transitions of Eu3+, but showed different contents quenching. The interesting thermal quenching properties under different excitation wavelengths were studied and the mechanism was discussed. Finally, the temperature dependent luminescence decay curves of BZN:Eu3+ were discussed in detail to further investigate the thermal quenching process. The activation energy (ΔE) was calculated to be 0.27 eV and 0.30 eV under 396 and 468 nm excitations, respectively.
Acknowledgments
This work is supported by the Doctoral Research Fund of Liaoning Province (Nos. 201601351, 20170520277), the National Natural Science Fund (Nos. 117040435, 1702020) and the Special Foundation for theoretical physics Research Program of China (Nos. 11747113, 11747117).
ORCID
Ge Zhu 0000-0002-2109-4857