Abstract
A series electrolytes for the operation of a LiFePO4 cathode over a wide temperature range were designed by employing a lithium tetrafluoroborate (LiBF4)and lithium bis(oxalato)borate (LiBOB) salt mixture and an ester co-solvent methyl butanoate (MB) in a solvent mixture of 1:1:3 (v/v) propylene carbonate (PC) /ethylene carbonate (EC) /ethylmethyl carbonate (EMC). And prepared electrolytes were compared to the reference mixture EC/PC/3EMC (LiPF6, 1 M). Cells temperature performance was systematically investigated by ionic conductivity test and various electrochemical tests, such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and charge-discharge test. It was found that the temperature performance of Li/LiFePO4 cells could be optimized by using a LiBF4-LiBOB salt mixture and MB co-solvent. At −40°C, for example, a Li/LiFePO4 cell using 1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:1:2 v/v) electrolyte could provide up to 60% of capacity, while still had a voltage platform of 3 V. And it still retained 80% of capacity after 100 cycles at 65°C, 1 C.
Export citation and abstract BibTeX RIS
In recent years, lithium batteries have been widely expanded into novel fields, such as electric vehicle, aviation and spaceflight.1 However, the narrow operating temperature range of the state of art systems has been identified by the Department of Energy (DoE) as one of the technical barriers associated with their use in plug-in-hybrid-vehicles (PHEVs), along with, limited cycle and calendar life, and poor abuse tolerance. The state of art lithium-ion systems have been demonstrated to operate over a reasonably wide range of temperatures (−40°C to +40°C),2 however, the high temperature resilience is generally very poor and as well, the discharge capacity at low temperatures isn't satisfactory. There is a continued desire to develop improved lithium-ion batteries that can operate efficiently over a wide temperature range (i.e., −40°C to + 70°C), while still providing long life characteristics.
To improve the operating temperature range of lithium-ion cells, many research groups have attempted to modify the electrolyte formulation, through (a) optimized solvents blends, (b) use of novel co-solvents, (c) use of novel electrolyte salts, and so on.
As to solvents, many researchers were attracted in finding novel co-solvents which possess low freezing temperature and high electrochemical stability to extend the low temperature operating rang. Thus, a series of esters including methyl propanoate (MP), ethyl propanoate (EP), methyl butanoate (MB), ethyl butanoate (EB), and propyl butanoate (PB) have been identified as suitable co-solvents in multi component electrolytes by the Smart group.3–6 A number of MCMB/LixNiyCo1−yO2 lithium-ion cells with methyl butyrate-containing electrolytes designed for operation over a wide temperature range were characterized by Smart,2 which were able to keep ∼50% capacity retention at −40°C, C/4, and retain ∼65% capacity after 20 cycle at 60°C, C/8. Co-solvents also gave a good low temperature performance in gel polymer systems. Smart7 have obtained excellent performance at low temperatures with ester-based electrolytes, including the demonstration of >80% of the room temperature capacity at−60°C using a C/20 discharge rate with cells containing 1.0 M LiPF6 in EC + EMC + MB (1:1:8%, v/v/v) (MB, methyl butyrate) and 1.0 M LiPF6 in EC + EMC + EB (1:1:8%, v/v/v) (EB, ethyl butyrate) electrolytes.
On the other hand, great efforts had been promoted to find replacements for LiPF6, because the LiPF6-based electrolytes have a poor thermal stability due to its decomposition in LiF and PF5 even at moderate temperatures (>55°C), leading to detrimental reactions with the electrolyte components.8 And significant efforts have been made in the past to develop alternative salts, such as LiAsF6,9 LiClO4,10 LiBF4,11 LiN(SO2CF3)2,12 LiN(SO2F)2,13 LiODFB,14 etc. Li15 investigated 1 M LiODFB EC/PC/DMC (1:1:3, v/v) as an electrolyte for LiFePO4/AG cells, results showed that the capacity retention of the cells with LiODFB-based electrolyte was 88%, much higher than that of LiPF6-based electrolyte (50%), after 100 cycles at high temperature (65°C).
The present work is aimed at developing electrolytes that permit operation over a wider temperature range from −40°C to + 65°C, i.e., good low temperature performance combined with an adequate tolerance to high temperature operations. In the current work, we propose to replace the thermodynamically unstable and reactive LiPF6 by a lithium salt mixture of LiBF4 and LiBOB. Meanwhile, replace EMC in ternary EC/PC/3EMC(1:1:3 v/v) mixtures by co-solvent methyl butanoate (MB), hence to extend the range of working temperature of Li-ion batteries. Prepared electrolytes are compared to the reference mixture EC/PC/3EMC (LiPF6, 1 M). Several of these electrolyte formulations were investigated in experimental LiFePO4/Li half cells, to study the charge and discharge behavior of the cells, as well as to understand the interfacial effects on the intercalation kinetics, using a number of electrochemical techniques, including electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) to understand the interfacial effects on the intercalation kinetics.
Experimental
Battery-grade LiBF4, LiBOB, EC, PC and EMC were purchased from Guangzhou Tinci Materials Technology Co., Ltd. Methyl butanoate (MB) were purchased from Energy Chemical (purity >99%). All liquid reagents were dried using 4 Å molecular sieves. In an argon-filled glove box (Universal 2440/750, Mikrouna Mech. Tech. Co., Ltd.) with both oxygen and water contents less than 10 ppm, electrolyte solutions were prepared by adding the lithium salt mixture to the PC/EC/EMC/MB (1:1:(3-x):x v/v) mixture (X = 0, 1, 2,3), electrolytes used in this article are shown as Table I. Water and free acid contents in these electrolytes were controlled below 20 ppm.
Table I. Composition of electrolytes.
| Electrolyte | Composition(slats and solvents) |
|---|---|
| A | 1 M LiBF4:LiBOB(8:2) PC:EC:EMC(1:1:3 v/v) |
| B | 1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:2:1 v/v) |
| C | 1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:1:2 v/v) |
| D | 1 M LiBF4:LiBOB(8:2) PC:EC:MB(1:1:3 v/v) |
| E | 1M LiPF6 PC:EC:EMC(1:1:3 v/v) |
The LiFePO4 cathodes consisted of 80 wt% LiFePO4, 10 wt% carbon black and 10 wt% PVDF, were fabricated by coating the slurry of LiFePO4 active material, carbon black, and PVDF on aluminum foil collector. To evaluate the electrochemical behavior of these electrolytes in LiFePO4/Li half cells, 2025-coin type cells with lithium foil as counter and reference electrode were assembled in the glove box using Celgard 2400 membrane as the separator and filled with these liquid electrolytes.
A high-low temperature test-chamber (GDH-2005C) was used to provide a constant temperature environment for test. The charge/discharge performance of LiFePO4/Li half cells were evaluated on a Land LAND CT2001A charge-discharge system. The half cells were cycled 3 times at 0.1 C (2.5–4.2 V) at room temperature (25°C) to obtain a stable state before other evaluations. Then the charge/discharge capacity, electrochemical impedance spectroscopy (EIS) and other tests at different temperatures were conducted. In order to reach equilibrium of the cell temperature and the oven preset temperature for evaluations at different temperatures, the cells were kept at the specified temperature for at least 4 h.
Solartron 1470E Multi-Channel potentiostats (London Scientific) was employed to determine ionic conductivity of the electrolytes and to measure EIS of the half cells. AC impedances of the cells were potentiostatically measured by applying a DC bias with its value equal to open circuit voltage (OCV) of the cell and an AC oscillation of 10 mV over the frequencies from 100 kHz to 0.01 Hz. The obtained EISs were fitted by using ZView software (Scribner and Associates).
Results and Discussion
Conductivity
Low ionic conductivity is one of the main reasons for poor low temperature performance of LIBs. In order to investigate the relationship between ionic conductivity and addition of co-solvent, the ionic conductivities of electrolytes were measured as a function of temperature. Fig. 1 is plotted the variation in conductivity of 1 M LiBF4:LiBOB (8:2) PC:EC:EMC:MB (1:1:(3-x):x) (x = 0,1,2,3) (A, B, C, D, respectively) solutions and reference electrolyte 1M LiPF6 PC:EC:EMC(1:1:3) (E) against the temperature in the range: −40°C to 60°C. The specific conductivities at various temperatures are listed in Table II. It is generally found in the plot that ionic conductivity increases with temperature. And LiPF6 is the more conducting salt comparing with LiBF4 and LiBOB mixture slat, but the differences in conductivity for these two salts are not large at low temperature probably due to the higher mobility of the BF4− ions relative to PF6−.16–18 With adding low temperature co-solvent MB, the conductivity of LiBF4 and LiBOB solutions increase, but unfortunately, electrolyte D (1 M LiBF4:LiBOB (8:2) PC:EC:MB (1:1:3 v/v)) has the lowest conductivity, which indicates EMC is an important part of electrolyte. The highest conductivities exist at electrolyte C (1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:1:2 v/v)), 2.16 mS/cm and 4.34 mS/cm at −40°C and −20°C respectively.
Table II. Ionic conductivity of five electrolytes at various temperature.
| Temperature (°C) | X = 0 | X = 1 | X = 2 | X = 3 | LiPF6 |
|---|---|---|---|---|---|
| −40 | 1.02 | 1.28 | 2.16 | 1.14 | 1.24 |
| −20 | 3.14 | 3.78 | 4.34 | 3.03 | 3.59 |
| 25 | 10.65 | 11.58 | 14.83 | 8.74 | 13.67 |
| 60 | 18.24 | 17.28 | 22.8 | 15.43 | 21.12 |
Figure 1. Conductivity vs. temperature of five electrolytes tested.
Cell performance at low temperature
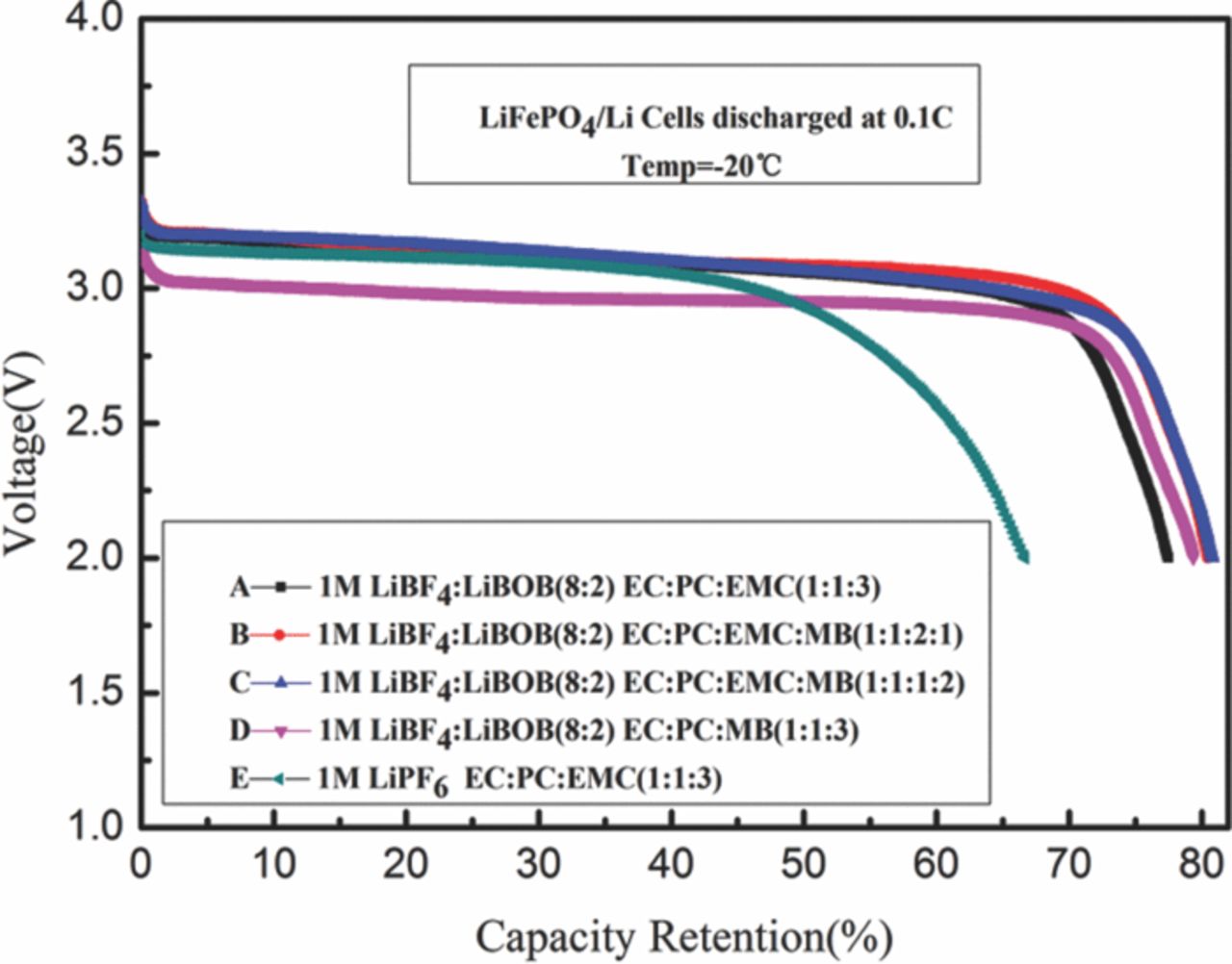
Fig. 2 and Fig. 3 compare the capacity retention of the Li/LiFePO4 cells with different electrolytes when discharged at 0.1C, at −20°C and at −40°C respectively, in which the capacity retention is expressed as the ratio of discharge capacity at a specific temperature to that at 25°C. As shown on the discharge curves, cells with LiPF6 based electrolyte provide ∼70% of the room temperature capacity at −20°C, and ∼50% at −40°C, similar to the research by Smart7 and a little better than the work of Liao.19 LiBF4 and LiBOB mixture salt containing cells have a good performance at low temperature than that of LiPF6 salt, which provide approximately 80% of the room temperature capacity at −20°C and 60% at −40°C. Moreover, LiPF6 based cells show a lower discharge plateau at −40°C. Among the electrolyte with LiBF4 and LiBOB mixture salt, electrolyte C (1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:1:2 v/v)) exhibits the best low temperature performance, which can be attributable to the fact that this electrolyte is expected to have the highest conductivity of the group studied. Although the differences in capacity retention are not lager, the discharge plateau of cell with electrolyte D (1 M LiBF4:LiBOB(8:2) PC:EC:MB(1:1:3 v/v)) is obvious lower than others at −20°C. The results suggest that adding MB is beneficial to improve low temperature performance, but EMC also is an important part of electrolyte duo to its high dielectric constant and low viscosity.
Figure 2. Discharge capacity retention of experimental lithium-ion cells at −20°C (0.1 C rate) containing different electrolytes.
Figure 3. Discharge capacity retention of experimental lithium-ion cells at −40°C (0.1 C rate) containing different electrolytes.
Electrochemical impedance spectroscopy (EIS)
To understand the different effects of electrolyte on low-temperature performance of LiFePO4/Li cells described above, EIS measurements of the discharged LiFePO4 electrodes in various electrolyte solutions under various temperatures were carried out and analyzed by Zview software. The impedance spectra of LiFePO4 electrodes under room temperature and −20°C are demonstrated in Fig. 4a and 4b. As we can see from Fig. 4, a typical EIS of LiFePO4/Li cell which is composed of one partially overlapped semicircle and a straight slopping line at the low frequency end is observed. The LiBF4-LiBOB based cells with MB exhibit smaller resistance than which without MB both at room temperature and at −20°C. The impedance seems to be slightly lower for the LiPF6 based cell than LiBF4-LiBOB based cell without MB at ambient temperature. As shown on the figure, the impedance values increase at low temperature, more significantly for LiPF6 based cell. LiPF6 based cell exhibits the biggest resistance than the others at −20°C. Furthermore, the impedance at both ambient temperature and −20°C is lower for the cell containing electrolyte C than the others.
Figure 4. Impedance spectra of LiFePO4 electrodes in various electrolytes.
The impedance spectra were fitted by an equivalent circuit shown in inset of Fig. 4a, in which Rb denotes the resistance of cell bulk including the electrolyte, electrode and separator, Rct is the charge-transfer resistance, and Cdl is the double-layer capacitance, and a Warburg impedance (wo) to represent a slow solid state diffusion of lithium ions inside the cathode. As our previous search found,20 Rct is the most important factor affecting low temperature performance, so we listed the Rct values of the LiFePO4/Li half cells containing different electrolyte at room temperature and at −20°C in Table III. As illustrated in Table III, Rct of all cells increase at low temperature, and electrolyte C exhibits the lowest Rct value whatever the temperature is, which is 115.50 Ω, and 308.90 Ω, at 25°C, and −20°C, respectively. This result is in accordance with the low temperature performance.
Table III. Rct values of the LiFePO4/Li half cells containing different electrolytes at room temperature and −20°C.
| Rct (Ω) | |||||
|---|---|---|---|---|---|
| Temperature (°C) | A | B | C | D | E |
| 25 | 406.80 | 336.10 | 115.54 | 229.10 | 289.00 |
| −20 | 587.90 | 550.71 | 308.90 | 479.00 | 752.84 |
Cell performance at high temperature
Cycling tests at high temperatures were performed on the cells to determine their high temperature resilience. After cycling 3 times at 0.1C at room temperature (25°C) to make sure solid electrolyte inter-phase (SEI) films are formed stably, LiFePO4/Li cells were cycled at 1C at 65°C for 100 times.
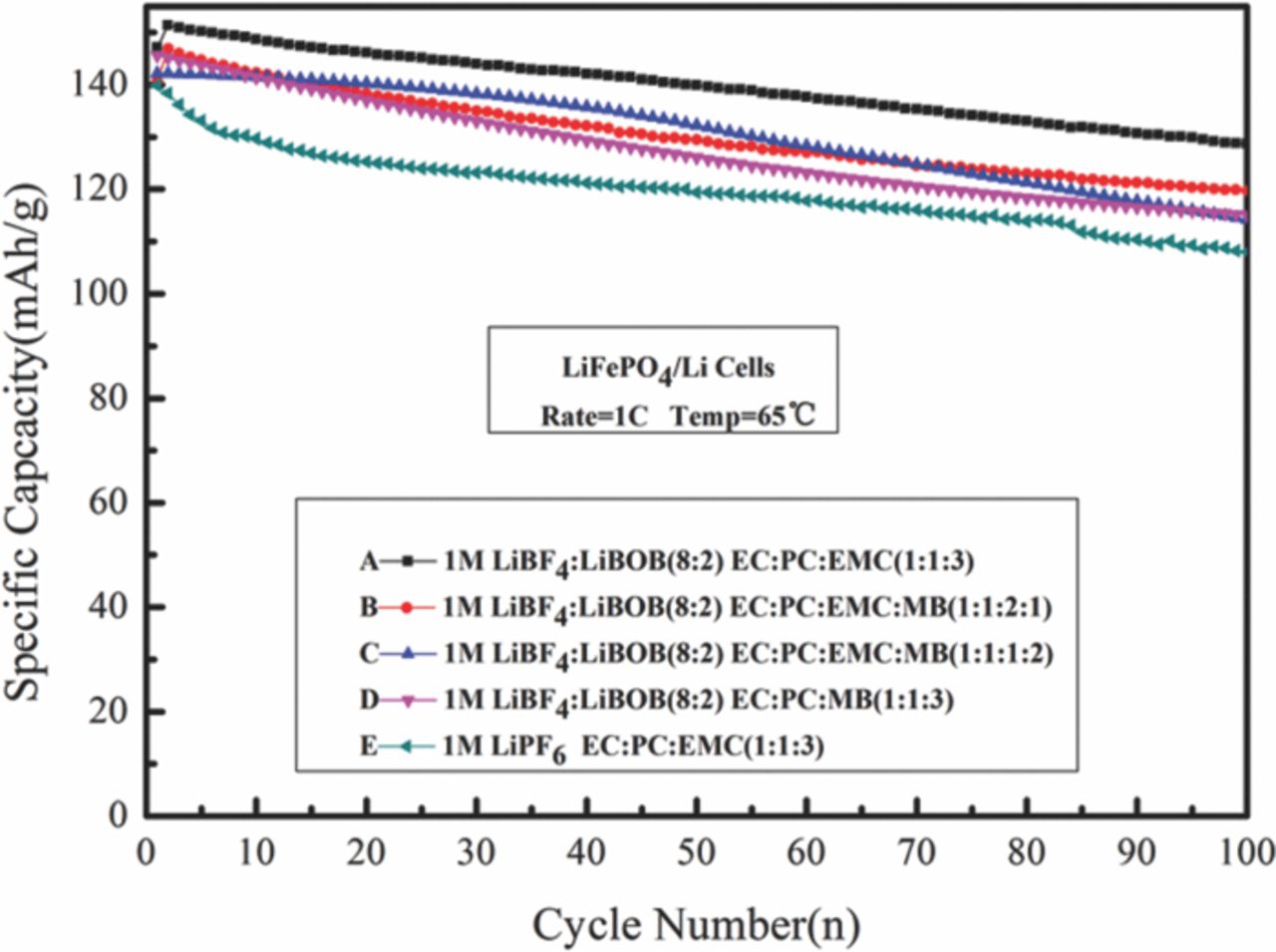
As illustrated in Fig. 5, cells with LiBF4 and LiBOB mixture salt have relatively higher capacity comparing with LiPF6 salt. Among these cells with LiBF4 and LiBOB mixture salt, the MB containing cells show lower capacity than that without MB. This reveals that addition of MB solvent into the LiBF4 and LiBOB mixture salt electrolyte has a little adverse impact on the specific capacity of LiFePO4/Li cells at high temperature.
Figure 5. Cycling performance of experimental LiFePO4/Li half cells containing different electrolytes at high temperature (65°C).
The cells with all the five electrolytes cycle well with a limited capacity fade after 100 cycles. The cells with LiPF6 based electrolyte have capacity retention of 77% after cycle, which is similar to the result of Fu21 and Zhou.22 LiFePO4/Li cells containing electrolyte A, B, C, D keep 85%, 81%, 80%, 79% of capacity retention respectively, which all better than the LiPF6 cells. The difference of the capacity loss after high temperature cycle among electrolyte B, C, D cells is no more than 2%, which is within the systematic error range. It seems that the better thermal stability of LiBF4-LiBOB mixture salt play an important role in improving the high-temperature resilience of LiFePO4 electrode.16,23–27
The 50th cycle charge-discharge curves of cells with LiBF4 and LiBOB mixture salt and with LiPF6 salt at 65°C were exhibited in Fig. 6. Obviously, it can be seen that cell with LiPF6 salt shows a lower discharge capacity, and furthermore the difference value of cell with LiPF6 salt between charge plateau and discharge plateau is much bigger than that of cell with LiBF4 and LiBOB mixture salt. This illustrates that cell with LiPF6 based electrolyte shows really a bigger polarization at high temperature, which may be caused by LiPF6 decomposition. S. E. Sloop28,29 has confirmed that LiPF6 is unstable at elevated temperature and it is in equilibrium with LiF and PF5 in battery electrolyte solutions. The product from the equilibrium, PF5, reacts with EC-containing solvents.30 The series reacts generated from LiPF6 could cause charge carriers lost, solvent viscosity increasing and cell resistance increasing. All the effects above lead to big polarization and cell resistance increasing, thus, accelerate cell capacity fade.
Figure 6. The 50th cycle charge-discharge curves of LiBF4 and LiBOB mixture salt and LiPF6 at 65°C.
Cyclic voltammograms before and after cycle
In order to evaluate the electrochemical behavior of the LiFePO4 electrode in different electrolyte before and after cycle at high temperature, cyclic voltammetry was performed. Cells with LiBF4-LiBOB based electrolyte and LiPF6 based electrolyte were carried on cyclic voltammetry characterization between 2.5 V and 4.2 V vs. Li/Li+ with a scan rate of 0.1 mV/s before cycle and after 50th cycle at 65°C, as shown in Fig. 7. There is only one peak pair, consisting of one anodic peak (charge) and one cathodic peak (discharge), which corresponds to the two-phase charge/discharge reaction of the Fe3+/Fe2+ redox couple. The redox couple corresponds to the electrochemical intercalation and deintercalation reactions of lithium-ion.19 The oxidation and reduction peaks of LiFePO4 cathode with LiPF6 based electrolyte and LiBF4-LiBOB based electrolyte have a little difference before cycle. Peak potential differences for the electrodes with LiPF6 based electrolyte before cycle is nearly 0.3 V, and that of LiBF4-LiBOB based electrolyte is about 0.35 V. After cycle at high temperature the potential separation between the cathodic and the anodic peak increase, especially for the electrodes with LiPF6 based electrolyte. Furthermore, the peak intensity decreases and the peaks are broadened, which indicates that the reversible insertion of lithium ions in the LiFePO4 cathode is hindered at high temperature due to sluggish kinetics.31 The peak potential difference for the electrode in LiBF4-LiBOB based electrolyte is about 0.45 V, a little bigger than that of before cycle, while that in LiPF6 based electrolyte is as high as 0.7 V, indicating that the electrode polarization of LiFePO4 cathode at high temperature decreases by LiBF4-LiBOB salt. These results agree well with the 50th cycle charge-discharge curves.
Figure 7. CVs of LiFePO4/Li cells with LiBF4-LiBOB electrolyte and LiPF6 electrolyte before cycle and after 50th cycle at 65°C.
Conclusions
In terms of ionic conductivity, results show that the optimized performance is obtained when the low temperature co-solvent methyl butanoate (MB) is used in the ternary mixture EC/PC/3EMC containing LiBF4-LiBOB mixture salt. The electrolyte 1 M LiBF4:LiBOB(8:2) PC:EC:EMC:MB(1:1:1:2 v/v) exhibits a conductivity as high as 2.16 mS/cm and 4.34 mS/cm at −40°C and −20°C respectively, and a high discharge capacity at low temperature is thus logical. It suggests that the replacement of part EMC by MB ester could enhance cells low temperature performance. The use of LiBF4 and LiBOB mixture salt, instead of LiPF6 certainly improve cells' high temperature performance. LiFePO4/Li cells containing LiBF4-LiBOB based electrolyte keep 85% capacity retention comparing 77% that of LiPF6 based electrolyte after 100 cycles at 65°C. Furthermore, the electrode polarization of LiFePO4 cathode at high temperature decreases by LiBF4-LiBOB salt. In summary, LiBF4-LiBOB based electrolyte containing MB is a very promising electrolyte candidate for lithium batteries that operate over a wide temperature range.
Acknowledgments
The authors thank the financial support of the Teacher Research Fund of Central South University (2013JSJJ027). We also thank the support of the Engineering Research Center of Advanced Battery Materials, the Ministry of Education, China.