Abstract
When 3,5-di-t-butyl-1,2-benzoquinone (DBBQ) is electrochemically reduced in an unbuffered solution, the addition of small amounts of acid to the solution is found to give rise to a new peak (termed the prepeak) at a more positive potential than the original reduction potential of DBBQ. In addition, the prepeak current heights of DBBQ are proportional to the added acid concentration. From these findings, we utilized the voltammetric behaviors of DBBQ to develop an analytical method for the determination of titratable acidity in food and beverage samples. Moreover, a portable voltammetric sensor (weight, 100 g; power, two AAA batteries), that implements this analytical method, was developed in order to provide an on-site analytical device. To show the applicability of this portable sensor, the determination of titratable acidity in fruit juice, wine, Japanese sake, and shochu was performed. The results obtained by the portable sensor showed a good correlation with those from official potentiometric titration using 0.1 mol/L NaOH. In conclusion, the present sensor required a reduced sample volume and measurement time for an assay compared with conventional potentiometric titration, consequently showing potential for quality assessment in a wide range of food and beverage analyses.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Various acid compounds are widely distributed in foods and play an important role because they affect organoleptic properties (aroma, color, and flavor), stability, and the microbiological control of food products.1 As such, they are well established as chemical markers of quality, ripeness, authenticity, and sometimes used as distinguishable characteristics of the geographical origin of foods.1–3 The total acid content in foods and beverages is called titratable acidity. In the case of wine, organic acid and total acidity are generally acknowledged because too much acidity will lead to an excessively sour and sharp taste, while wines with too little acidity will taste flat and present a less-defined flavor profile.4 Additionally, alcoholic fermentation changes the concentrations of organic acids in wine, and may result in a higher or lower titratable acidity of the wines.5,6 Similarly, titratable acidity in Japanese sake has been shown to affect its taste and flavor.7–10 Meanwhile, in the case of oils and fats, the total free fatty acid content, called acid values, are used as a marker of quality, because fats and oils tend to decompose slowly upon storage in contact with the atmosphere and to release their free fatty acid constituents, resulting in the lowering of their quality.11 Thus, acid value is regarded as a key parameter routinely used to classify and assess quality, freshness, and economic value of the final commercial product of fats and oils.11–14
In order to determine total acid content, neutralization titration using an appropriate indicator and titrant15–18 is commonly and officially used. However, in this method there is the possibility of error due to an inability to detect subtle color changes of the indicator in the transition range, especially in the case of colored samples. In addition, this titration method is insufficiently sensitive to detect small acid content, and accordingly it requires large sample amounts and much time to conduct analyses.19,20 Potentiometric titration21,22 is thus commonly recommended as an alternative method. Commonly used pH measurement is not adequate for this purpose because the obtained pH value does not correspond to the titratable acidities but instead to the activity value of only the protons dissociated from the acids.
As an electroanalytical method for determining titratable acidity, potentiometric determinations have been recently proposed, and their methods are coupled with flow injection analysis and automated titration systems and have been shown to be useful for the application to real sample analysis of wine and oils.23–29 In contrast, amperometric and/or voltammetric determinations are less commonly used. Since most acids are less active electrochemically, it is hard to measure current signals due to the discharge of acids by conventional voltammetric techniques. In general, derivatization using electroactive reagents could be performed for determining electro-inactive acid compounds by high performances liquid chromatography with electrochemical detection.30 However, derivatization is less attractive for determining titratable acidity because complex procedures are sometimes required to separate unreacted electroactive reagents from analytes in a test solution. Recently, a voltammetric reduction method using an ionic liquid has been provided to detect free fatty acids and applied to the determination of acid values in olive oils,11,19 but this method has not been applied to the detection of inorganic or organic acids except in the case of fatty acids in foods and beverages. In some cases, to be able to detect titratable acidity is desired for on-site measurements for the assessment of foods and beverages, but present-day electroanalytical methods using FIA and titration are unable to do this.
We have developed a new voltammetric method for determining titratable acidity in foods and beverages, based on the fact that organic and inorganic acids in an unbuffered solution containing 3,5-di-t-butyl-1,2-benzoquinone (DBBQ) give rise to a new reduction peak (termed the prepeak) on a voltammogram at more positive potentials than those corresponding to the original reduction potential of DBBQ.31,32 Based on the voltammetry of DBBQ in the presence of acids, we assembled a portable electrochemical sensor for determining titratable acidity. This article reviews the assessment of this voltammetric method and portable sensor for determining titratable acidity in foods and beverages. We will show that the present sensor is useful as an on-site analytical device and preferable as a simpler and more rapid method for acid determination in foods and beverages than existing methods.
Voltammetric Sensing of Acids by Means of Quinone Reduction
Quinones are cyclic conjugated carbonyl compounds, and they play vital roles in biological energy conversion process such as photosynthesis and oxidative phosphorylation in many biological systems.33–37 Quinones are regarded as one of the first organic redox couples studied by voltammetry.38 In buffered aqueous solution, quinones are well-known to undergo a 2H+/2e- reduction to give the corresponding hydroquinones, and thus only one pair of peaks appear on a cyclic voltammogram of quinones.39–42 While, in aprotic solvents, quinones generally undergo two separate 1e- reduction, first to the radical anion, and then to the dianion, and thus two pairs of peaks appear on a cyclic voltammogram of quinones.39–41 Based on these unique aspects, electrochemical reduction of quinines is extensively utilized for the developments of electrochemical analyses, materials, and devices up to the present date.43–48
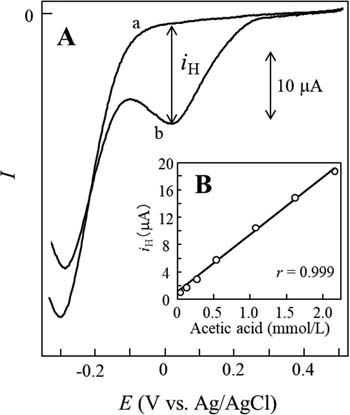
In this study, linear sweep voltammetry was conducted in an unbuffered ethanol solution containing DBBQ and NaCl. A plastic formed carbon (PFC) working electrode (3.0 mm diameter, Mitsubishi Pencil Co. Ltd, Tokyo, Japan), an Ag/AgCl reference electrode, and a platinum auxiliary electrode were used.49 As shown in Fig. 1A, the DBBQ exhibited a well-defined reduction peak on the voltammogram (curve a) at −0.3 V vs. Ag/AgCl. Following the addition of acetic acid to the solution, a prepeak appeared at a more positive potential than that corresponding to the original peak (curve b). As shown in Fig. 1B, the prepeak height (iH) was found to be proportional to the acetic acid concentration from about 0.05 mmol/L to 2.2 mmol/L with a correlation coefficient (r) of 0.999.
Figure 1. (A) Linear sweep voltammograms of DBBQ in the (a) absence and presence of (b) 1.1 mmol/L acetic acid in test solution and (B) calibration curve of acetic acid. For the preparation of the test solution, a 0.45 mL ethanol-water (9:1, v/v) containing 9 mmol/L DBBQ and 50 mmol/L NaCl was mixed with 0.55 mL acetic acid at each concentration. A PFC, Ag/AgCl, and platinum wire were used as working, reference, and auxiliary electrodes, respectively. The scan rate was set at 25 mV/s. Used and reproduced with permission from Ref. 49.
The electrochemical reduction of quinone,50–52 such as DBBQ, is generally known to involve a 2H+/2e− transfer, represented as the following reaction of Eq. 1.
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/167/3/037517/revision1/d0001.gif)
where Q and QH2 denote quinone and its corresponding hydroquinone, respectively.
The occurrence of the prepeak can be ascribed to the increased availability of protons from the added acid (HA), compared to the solvent molecules (HB). Instead of Eq. 1, the electrochemical reduction of quinone in the presence of acid is shown in Eq. 2.
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/167/3/037517/revision1/d0002.gif)
An increase in proton supply from the added acid results in a lowering of the free energy for the reduction process. Consequently, depending on the acid strength, the reduction potential corresponding to Eq. 2 shifts to a more positive direction than that of Eq. 1.50,53 In fact, in linear sweep voltammetry using 2-methyl-1,4-naphthoquinone (vitamin K3) as a quinone reagent, the half-peak potential of the prepeak shifted to a more positive value accompanied by a decrease in the pKa of the acid. Furthermore, the present voltammetric behaviours have been previously applied to determine the pKa values of organocatalysts.54–56
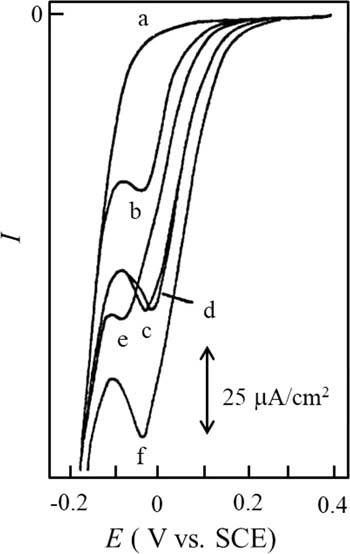
Similar to acetic acid, the addition of various kinds of inorganic and organic acids to an ethanol-water mixture containing DBBQ gave rise to a single prepeak.57,58 In all cases, the iH was found to be linearly related to each acid concentration. Moreover, the voltammetric behaviours of the DBBQ prepeaks were examined in the presence of mono- (acetic acid), di- (malic, tartaric, and succinic acids), and triprotic- (citric acid) acids. As shown in Fig. 2, the ratios of iH caused by acetic, malic, tartaric, succinic, or citric acid (1.2 mmol/L each) are approximately 1:2:2:2:3, indicating the present method has provided the determination of an equivalent concentration of acids. Based on this, total acid determination can be made by measuring the iH.
Figure 2. Linear sweep voltammograms of 3.0 mmol/L DBBQ with (a) no acid and in the presence of (b) 1.2 mmol/L acetic acid, (c) 1.2 mmol/L malic acid, (d) 1.2 mmol/L tartaric acid, (e) 1.2 mmol/L succinic acid, and (f) 1.2 mmol/L citric acid, obtained in ethanol containing 0.1 M LiClO4. A PFC, saturated calomel electrode (SCE), and platinum wire were used as working, reference, and auxiliary electrodes, respectively. Used and reproduced with permission from Ref. 57.
Choice of a quinone reagent should be made by considering its solubility, stability, and reduction potential in a test solution. DBBQ is preferably used since this compound facilitates acid determination due to its stability and solubility in aqueous and alcoholic solutions. Additionally, as seen in Fig. 1A, DBBQ exhibited a well-defined prepeak at about 0 V vs. Ag/AgCl, indicating that the dissolved oxygen causes no interference in measuring the iH. Because the removal of the dissolved oxygen from an electrolytic solution is usually required by bubbling nitrogen gas into the solution in an electrochemical cell, the use of DBBQ provided a simple procedure for voltammetric sensor analysis. The stability of DBBQ in ethanol was demonstrated by time-course measurements of their voltammograms and absorption spectra. Neither showed any appreciable change upon storage at room temperature for more than one month, indicating DBBQ to be a favourable quinone reagent for acid determination.57,59 Furthermore, DBBQ is commercially available at a reasonable cost. From these considerations, DBBQ was found to be most suitable for the development of a voltammetric method and sensor for determining titratable acidity in food and beverage samples.
Comparison of Titratable Acidity Obtained by the Present Voltammetry and Neutralization Titration
In the presence of mixed acids in the solution, stepwise prepeaks on a voltammogram obtained by the present method may appear when their pKa values differ from one another. On the other hand, when the acid strengths and diffusion coefficients were nearly the same for all of the acids, only one prepeak could be observed at an identical potential with a height proportional to the sum of the total acid concentration for all the acids. In the field of food analysis, total acid concentration is called titratable acidity, and the determination of titratable acidity can be made by measuring the iH.
In this section, the results of real sample analyses using foods and beverages are shared to show the practicality of the present voltammetric method for determining titratable acidity. Furthermore, quantitative results by the present method are compared with those by titration to show the suitability of the values obtained by the present method.
Wine
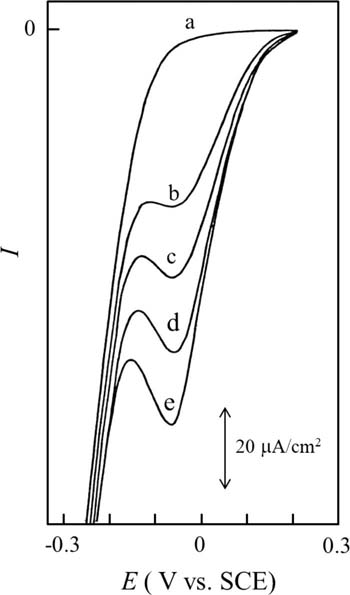
Titratable acidity in wine serves as a parameter of taste and aroma and it depends on the particular origin and growth conditions of the grapes and changes during the fermentation process. Thus, titratable acidity in wine is essential for its quality and process controls. Although tartaric, citric, malic, lactic, and succinic acids are present in wine, one well-defined prepeak was observed by the addition of 0.5 mL of wine to 40 mL of ethanol containing 3.0 mmol/L DBBQ and 0.1 mol/L LiClO4 as shown in Fig. 3 (curve b). From this method of using a standard tartaric acid solution (cf., curves c-e), the titratable acidity in wine was able to be calculated.57 In addition, the color and turbidity of the juice caused no interference with the analytical results. Furthermore, sugars contained in fruit juice, such as sucrose, dextrose, levulose, and saccharose, have been found to have no effect on the total acid values determined by a voltammetric method.59
Figure 3. Linear sweep voltammograms for determining titratable acidity in rosé wine by a standard addition method. Added amount of rosé wine: (a) 0 mL, (b--e) 0.5 mL. Final concentration of added tartaric acid standard to test solution: (a and b) 0 mmol/L, (c) 0.246 mmol/L, (d) 0.489 mmol/L, (e) 0.730 mmol/L. A PFC, saturated calomel electrode (SCE), and platinum wire were used as working, reference, and auxiliary electrodes, respectively. Used and reproduced with permission from Ref. 57.
The determination of titratable acidity in nine red, eleven white, and three rosé wines were performed by the present voltammetry, and the titratable acidities are shown as tartaric acid concentration (w/v%) according to the definition of the titratable acidity of wine according to official analytical methods.60–62 The titratable acidity in red, white, and rosé wines studied were in the range of 0.462–0.620 w/v%, 0.457–0.851 w/v%, and 0.571–0.687 w/v%, respectively, as shown in Table S1. To compare the quantitative results by potentiometric titration, 10 mL of a wine sample were neutralized with 0.100 mol/L NaOH up to pH 8.2. The titratable acidities in the wine samples are also listed in Table S1. Results of the titratable acidity obtained by the present voltammetry showed a correlation with those by potentiometric titration: y = 0.989x − 0.54 (r = 0.966), where y is the titratable acidity obtained by the present voltammetry, and x is that by the potentiometric titration. From these results, the present voltammetry was shown to be practical and useful for determining titratable acidity in wine.
Coffee
The main acid components in coffee are: chlorogenic, caffeic, quinic, acetic, formic, malic, fumaric, and citric acids, etc. Slight changes in their content readily lead to differences in aroma and the quality of the coffee beans, and content changes easily through processing, roasting, and extraction. Thus, the determination of titratable acidity in coffee is important for its quality control. Having said this, conventional methods for determining titratable acidity involving alkali titration and pH measurement are not sufficiently sensitive to track slight changes in titratable acidity in coffee.
Using another electrolyte cocktail, an ethanol-water (4:1, v/v) mixture containing 9 mmo/L DBBQ and 50 mmol/L NaCl, the present voltammetry was applied to determine titratable acidity in coffee samples. Although coffee contains various organic acids, one prepeak was caused by the addition of 0.14 mL of coffee to 0.6 mL of the electrolyte cocktail as shown in Fig. S1 (curve b). From the standard addition method using a citric acid solution (cf., curves c and d), titratable acidity in coffee was determined, and the titratable acidity is shown as a monoprotic acid concentration (mmol/L).
The determination of titratable acidity in six kinds of coffee blends: Kona, Brazil Santos, Colombian, Mocha, Kilimanjaro, and Mandheling, were performed by the present voltammetry. For a comparison of the quantitative results by potentiometric titration, 50 mL of a coffee sample were neutralized with 0.100 mol/L NaOH up to pH 8.2. The titratable acidities in coffee by both methods are also listed in Table S2. The results by the present voltammetry were in good agreement with those by titration (r = 0.995): y = 1.052x − 0.887, where y is the titratable acidity obtained by the present voltammetry, and x is that by the potentiometric titration. From these results, the present voltammetry was shown to be practical and useful for determining titratable acidity in coffee.
Oil and fat
Since fats and oils tend to slowly decompose upon storage in contact with the atmosphere and release their fatty acid constituents, quality and freshness assessments of fats and oils can be made based on the determination of free fatty acid content.11–14 In the assessment of fats and oils, total free fatty acid content is called an acid value.
As shown in a previous study,63 ethanol was used as a solvent to prepare electrolyte cocktail containing DBBQ. To dissolve various oils and fats into ethanol, they were dissolved once in diethylether before mixing them with the electrolyte cocktail. To avoid this complicated procedure, a new electrolyte cocktail in which fats and oils can be directly dissolved, was provided. Considering the solubility of fats, oils, and DBBQ, 1-butanol was selected as a solvent, and thus LiCl was used as a supporting electrolyte. As a new electrolyte cocktail, a 1-butanol solution containing 9 mmo/L DBBQ, 48 mmol/L LiCl, and 1.2 mmol/L palmitic acid was proposed for determining acid values in fats and oils.64 In order to obtain a well-defined reduction prepeak as shown in Fig. S2 (curve a), 1.2 mmol/L palmitic acid, as a bias, was added into the cocktail, and the voltammogram was set as blank to determine acid value by a standard addition method (curves b-d).
The determination of acid values in commercial sesame, corn, grape seed, safflower, salad, and cottonseed oils were performed by the present voltammetry, and the acid values are shown as the mass (mg) of KOH that is required to neutralize 1 g of oil or fat according to the definition of the acid value of fats and oils in official analytical methods.17,18 By the present voltammetry, acid values in samples of commercial sesame, olive, corn, grape seed, safflower, cotton seed and salad oils were determined as 0.957, 0.212, 0.141, 0.106, 0.056, 0.053, and 0.038 mg KOH/g, respectively.
To compare the results by potentiometric titration, 20 g of a fat or oil sample were dissolved in an ethanol and diethyl ether (1:1, v/v) mixture and then neutralized with a 0.100 mol/L KOH ethanol solution. The end point was determined by potentiometry, and then acid values in the samples of commercial sesame, olive, corn, grape seed, safflower, cotton seed and salad oils were determined as 0.991, 0.214, 0.159, 0.067, 0.035, 0.023, and 0.037 mg KOH/g, respectively. The results by the present voltammetry were in good agreement with those by titration (r = 0.999): y = 0.943x − 0.018, where y is the acid value obtained by the present voltammetry, and x is that by the potentiometric titration. From these results, the present voltammetry was shown to be practical and useful for determining acid values in fats and oils. In short, a voltammetric method with this simple procedure would be attractive for the development of a sensor for the analysis of fats and foods on site i.e. a restaurant.
Development of a Portable Sensor Based on the Reduction of Prepeak Measurements of DBBQ
A portable sensor, which means it is small and battery powered, is an attractive device for on-site analysis. We developed such a sensor, which has the ability to detect electrochemicals based on the present voltammetric method for determining titratable acidity. In this section, we describe the construction and procedures of use of the present sensor to perform the analyses of beverage samples.
Construction of a voltammetric sensor
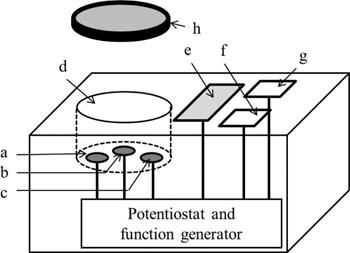
The portable voltammetric sensor consists of a potentiostat with a function generator, an electrochemical cell having a capacity of 1 mL, an integrated circuit (IC), and a monitor as shown in Fig. 4. Three PFCs (3.0 mm diameter, Mitsubishi Pencil Co. Ltd) were used for a working electrode, a pseudo-reference electrode, and an auxiliary electrode, respectively. Ag/AgCl electrode, palladium-hydrogen electrode (Pd/H2), and saturated calomel electrode (SCE) are typically used as a reference electrode in electroanalytical measurements.65–68 In the present sensor, carbon electrode, which is inexpensive compared with the above mentioned metal electrodes, is used as a pseudo-reference electrode to save the production costs of the main body. When a voltammogram of DBBQ in the presence of acetic acid was measured using graphite reinforcement carbon (GRC) as a pseudo-reference electrode, pre and original peaks of DBBQ were appeared at −0.333 and −0.593 V vs. GRC, respectively, and standard deviation (SD) of their potentials (n = 10) were 0.008 and 0.011, respectively. While, in the voltammetry of DBBQ using Ag/AgCl reference electrode, pre and original peaks of DBBQ were appeared at −0.087 and −0.350 V vs. Ag/AgCl, respectively, and SD of their potentials (n = 10) were 0.001 and 0.005, respectively.31 The repeatability of prepeak potential on a voltammogram using GRC reference electrode was slightly worse than that using Ag/AgCl reference electrode. However, it is not essential issues in the present sensor because both prepeak identification and current measurement from the voltammogram can be performed to determine titratable acidity. Thus, we showed that GRC was useful as a pseudo-reference electrode in the present voltammetry for determining acids.31 On the bottom of the electrochemical cell, each PFC electrode was embedded in the polytetrafluoroethylene (PTFE) of the bottom of the electrochemical cell. A sensor with the following dimensions and weight: 55 (W) × 109 (D) × 31 (H) mm and 100 g needs two AAA batteries to work.49,69
Figure 4. Schematic diagram of sensor a, working electrode; b, reference electrode; c, auxiliary electrode; d, electrochemical cell; e, monitor; f, button for the measurement; g, button for zero adjust; h. stainless steel lid. PFCs were used as working, reference, and auxiliary electrodes. When the sample was measured, the cell (cell volume: 1.0 mL) was covered by a stainless steel lid. Used and reproduced with permission from Ref. 69.
Optimizations of electrolyte cocktail containing DBBQ and measurement conditions
Because the sensor determines titratable acidity based on the measurement of iH of DBBQ caused by acid, liquid samples should be dissolved in an electrolyte cocktail containing DBBQ to perform linear sweep voltammetry. The content and composition of acids, foods, and beverages have variations. Thus, the optimization of an electrolyte cocktail and measurement conditions are required with respect to each sample and analyte. In previous studies, the present sensor had been applied to determine titratable acidities in fruit juice, Japanese sake, and shochu.49,69,70 To perform the above-mentioned sample analyses, the optimization of an electrolyte cocktail and measurement conditions were determined as follows: (1) DBBQ and supporting electrolyte (NaCl) are easily dissolved, (2) DBBQ, NaCl, and components including acids in samples are not precipitated during voltammetric measurement, and (3) the above-mentioned components and electrolyte products are not adsorbed on the surface of the electrode or electrochemical cell. For the analysis of fruit juice, Japanese sake, and shochu, we have provided the electrolyte cocktail and measurement conditions in Table S3.
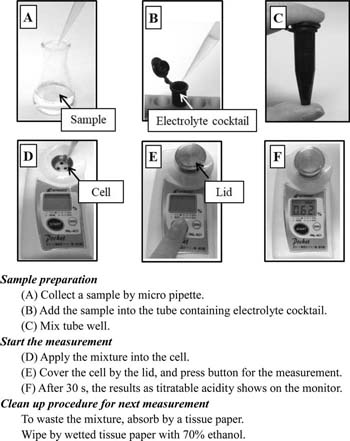
Procedure for determining titratable acidity by the sensor
Figure 5 shows a scheme of the procedure to determine titratable acidity by the sensor. The procedures for zero adjust and calibration using a standard acid are required once a month before real sample analysis. Measurement by the sensor is started when a "start button" is pushed. The sensor can automatically run a potential sweep for voltammetry to obtain reduction current and calculate titratable acidity using a calibration curve. After a series of measurements by the sensor, the titratable acidity in the sample that was used is digitally displayed on the monitor.
Figure 5. Procedure to determine titratable acidity in a sample by the sensor. Used and reproduced with permission from Ref. 69.
Real Sample Analysis for the Application of the Portable Sensor
To show that the proposed sensor is practical and useful, real sample analyses for determining titratable acidity were performed using fruit juice, Japanese sake, and shochu. To carry out the validation method, the repeatability and accuracy of the present sensor were assessed by repetitive measurements and the comparison of the results obtained by official titration methods, respectively.
Fruit juice
In the cultivation of fruits, the control of the water supply is important to harvest fruits with suitable sweet and sour tastes.71 Some farmers adjust the water supply by referring to the titratable acidity in fruits as a criterion. In order to show the usefulness of the present sensor as an on-site analytical method and to determine the titratable acidity in fruits on-site, such as in a fruit garden, the present sensor was applied to the analyses of fruit juices.
The determination of titratable acidity in orange, grape, and grapefruit juices was performed to examine whether the present sensor is applicable for the analysis of fruit samples. The titratable acidities in orange and grapefruit juices are shown as a citric acid concentration (w/v%), while those in grape juice are shown as a tartaric acid concentration (w/v%) according to the definition of titratable acidity of orange juice in official analytical methods.72,73 The sensor can be used to determine the titratable acidity of fruit juice ranging from 0.10 to 4.00 w/v%.
The determination of titratable acidity in the orange juice sample (#1) listed in Table I was performed by the sensor under the listed conditions in Table S3. After a series of procedures for determining titratable acidity by the sensor, a value of 0.72 was displayed as the titratable acidity of orange juice (#1) on the monitor.70 The repeatability of the titratable acidity obtained by repeated measurements was 1.0% RSD (n = 6). To compare the titratable acidity by potentiometric titration, 5 mL of the orange juice sample (#1) were neutralized with 0.100 mol/L NaOH up to pH 8.2. The titratable acidity in orange juice (#1) by the potentiometric titration was 0.71 with a repeatability of 1.2% RSD (n = 6). These results indicate that the titratable acidity in the orange juice (#1) by both the sensor and titration were essentially the same.
Table I. Titratable acidity in fruits juices and grapefruits obtained by the present sensor and titration.a
| Sensor | Titration | ||||||
|---|---|---|---|---|---|---|---|
| Titratable acidity | RSD c | Titratable acidity | RSD c | ||||
| Sample | # | (w/v%)b | (%) | (w/v%)b | (%) | ||
| Juice | Orange | 1 | 0.72 | 1.04 | 0.71 | 1.18 | |
| 2 | 0.77 | 0.66 | 0.78 | 1.35 | |||
| 3 | 0.72 | 1.27 | 0.69 | 1.50 | |||
| 4 | 0.69 | 1.79 | 0.75 | 1.12 | |||
| Grapefruit | 5 | 0.93 | 1.73 | 0.98 | 0.79 | ||
| 6 | 1.07 | 1.12 | 1.10 | 0.82 | |||
| Grape | 7 | 0.36 | 2.48 | 0.32 | 0.50 | ||
| 8 | 0.21 | 2.38 | 0.28 | 0.63 | |||
| Fruit | Grapefruit | 9 | 1.11 | 1.61 | 1.11 | 0.76 | |
| 10 | 1.21 | 1.09 | 1.22 | 0.97 | |||
aUsed and reproduced with permission from Ref. 70. bTitratable acidities for orange and grapefruit juices are shown as concentration of citric acid (w/v%), while that for grape juices are shown as concentration of tartaric acid (w/v%). cn = 6.
Next, the titratable acidity of four orange, two grape, and two grapefruit juices was determined by our sensor, and these results were compared with those by titration as shown in Table I. The results by the present sensor were in good agreement with those by titration (r = 0.989): y = 0.973x − 0.002, where y is the titratable acidity obtained by the present sensor, and x is that by the potentiometric titration.70 Moreover, the sensor was applied to determine titratable acidity in grapefruit as shown in Table I. From these results, the present sensor was shown to be practical and useful for determining acid values in both fruits and their juices.
Japanese sake
Titratable acidity in Japanese sake serves as an indicator of taste and it continues to change during the brewing process. In order to produce Japanese sake with a favorable taste, titratable acidity in Japanese sake is monitored during brewing. Under optimal voltammetric conditions as shown in Table S3, the iH was found to be proportional to the concentration of succinic acid in a test solution from about 0.05 to 3.1 mmol/L with a correlation coefficient (r) of 0.999. The titratable acidity of Japanese sake is shown as the volume (mL) of 0.1 mol/L NaOH that is required to neutralize 10 mL of Japanese sake according to the definition of the titratable acidity of Japanese sake in official analytical methods.62 Considering the results of the above linear range, the present sensor can be used to determine titratable acidity of Japanese sake ranging from 0.10 to 6.8.69
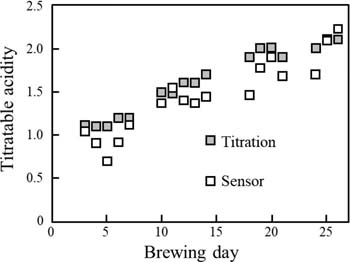
The determination of titratable acidity in a Japanese sake sample (#1), listed in Table S4, was performed by the sensor. The quantitative result of titratable acidity in the Japanese sake sample (#1) by the sensor was 1.41 with a repeatability of 2.3% RSD (n = 6), while that by titration was 1.42 with a repeatability of 1.7% RSD (n = 6). The pH at 7.2 was defined as an end point in this titration. These results indicate that the titratable acidity of the Japanese sake (#1) determined by both the sensor and titration were essentially the same. Moreover, titratable acidity in 18 kinds of commercial Japanese sakes were determined by the sensor, and these results were compared with those by titration as shown in Table S4. The results by the present sensor were in good agreement with those by titration (r = 0.977): y = 1.040x − 0.097, where y is the titratable acidity obtained by the present sensor, and x is that by the potentiometric titration. In addition, the present sensor was applied to determine titratable acidity in unfiltered Japanese sake (moromi) during the Japanese sake brewing process. In Fig. 6, the titratable acidities obtained by the sensor and titration are plotted against the brewing days. With the sensor, we were able to monitor increases of the titratable acidities after brewing. From these results, the present sensor was shown to be practical and useful for quality control of Japanese sake in the brewing process.
Figure 6. Titratable acidity measured by the sensor and titration during the preparation of unfiltered Japanese sake (moromi). Used and reproduced with permission from Ref. 69.
Shochu
Although the main acid components in shochu are acetic and formic acids, their concentration is remarkably low since shochu is prepared via distillation. However, if the fermentation of sake mush, which contains citric, lactic, and acetic acids, is excessive, titratable acidity will increase in shochu.74,75 Thus, the determination of titratable acidity in shochu was performed for its quality and process controls.
Under optimal voltammetric conditions as shown in Table S3, the iH was found to be proportional to the concentration of acetic acid in a test solution from about 0.09 to 4.0 mmol/L with a correlation coefficient (r) of 0.999. The titratable acidity of shochu is shown as the volume (mL) of 0.01 mol/L NaOH that is required to neutralize shochu 50 mL according to the definition of the titratable acidity of shochu by official analytical methods.62 Considering the results of the above linear range, the present sensor can be used to determine the titratable acidity of shochu ranging from 0.10 to 4.0. Although the concentration of acid components in shochu is about a thirtieth of that compared to Japanese sake, the present sensor has satisfactory sensitivity for determining titratable acidity due to the use of an electrolyte cocktail for shochu and an optimized mix ratio between the electrolyte cocktail and shochu samples as shown in Table S3.
The determination of titratable acidity in the shochu sample (#1) listed in Table S5 was performed by the sensor. The quantitative results of the titratable acidity in the shochu sample (#1) by the sensor was 1.06 with a repeatability of 2.7% RSD (n = 6), while that by titration was 1.10 with a repeatability of 0.7% RSD (n = 6).49 These results indicate that the titratable acidity in the shochu (#1) by the sensor and titration were essentially the same. Furthermore, the titratable acidity in 12 kinds of commercial shochu was also determined by the sensor, and these results were compared with those by titration as shown in Table S5. The results by the present sensor were in good agreement with those by titration (r = 0.994): y = 1.005x − 0.005, where y is the titratable acidity obtained by the present sensor, and x is that by the potentiometric titration. From these results, the present voltammetric sensor was shown to be practical and useful for determining acid values in shochu.
Conclusions
In this review, a voltammetric sensor has been proposed for determining titratable acidity, and it was applied to the analyses of food and beverage samples. Our method of acid determination, based on an acid-mediated amperometric reduction of quinone, made it possible to fabricate a voltammetric sensor for detecting acid components in food and beverage samples.
The titratable acidities obtained by the present sensor show a good correlation with the titratable acidities obtained by conventional potentiometric titration. Further, the present sensor is superior in sensitivity, and rapidity, and accordingly, it requires smaller sample volumes and a shorter analysis time, compared to a conventional potentiometric titration method, which requires relatively larger sample volumes and a longer time. For example, conventional titrations require about 10–50 mL volume of sample and 5–10 min of measurement time for one assay. While in the case of the present sensor, the required sample volume and measurement time were about 0.1 mL and 30–60 s, respectively. Moreover, the fact that sample color causes no interference in the detection of acids permits the present sensor to be widely applied to colored beverages such as wine, coffee, and fruit juice. Because of the ease of handling, the method is practical and useful for checking maturity during the cultivation of fruits and the brewing processes of wine and Japanese sake. Thus, the method was shown to have potential for assessing the quality of a wide variety of foods and drinks.
Acknowledgments
The authors thank Mr. Masahiro Matsumaru and Mr. Atsushi Ohsuga, ATAGO Co., Ltd., Japan, for their technical supports to prepare the portable voltammetric sensors.
ORCID
Akira Kotani 0000-0002-0113-3353