Abstract
In previous work, we introduced an elegant approach for bromide recovery from water by the introduction of a hybrid physical adsorption and capacitive deionization processes for selective removal and recovery of boron from water. In this paper, we show that the harsh environment of water contaminated with bromine-moieties adversely affects the longevity of relevant electrodes, with close to 100 consecutive work hours of bromides removal without noticeable degradation. To extend the lifespan of electrodes, we used an asymmetric CDI cell with a 1:5 positive/negative electrodes ratio in which a polarity switch between electrodes is applied every six adsorption-desorption cycles in a way that in each adsorption-desorption cycle, a different electrode of the six electrodes, functions as the positive electrode. We deduce that the polarity switch reduces oxidation and subsequent degradation of the positive electrodes, resulting in an extended lifecycle. After examining nine different carbonaceous materials, carbon cloth was chosen to be incorporated in the bromide- recovery cells because of its favorable kinetics and its physical and mechanical properties. We show that with a combination between endurance of the electrodes and asymmetric mode of operation, it is possible to overcome the main barrier that holds the technology from being practical.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The bromine industry is large and diverse, covering a vast array of applications including water treatment, pharmaceutical related processes, food, energy, rubber, agriculture, and fire safety. 1,2 Removal of bromide ions released as waste 3–5 is crucial for environmental and health reasons. Trihalomethanes (THMs), haloacetic acids, and bromate ions are among hazardous by-products that form when potable water contains traces of bromide ions, 6 which are forbidden above certain levels (the World Health Organization has been judged bromate as a potential carcinogen, even at the low level as μg/L 7 ). THMs have adverse effects on the kidneys and central nervous system, 8 haloacetic acids damage the skin, 9 and bromate is a category 2B carcinogen. 10,11
Removal of bromide ions is challenging. Reverse osmosis requires high pressure,
12
while direct distillation requires a large amount of heat;
13
both thereby demand a significant expense of energy. However, specific removal of bromide cannot be attained by these methods. Regular electro-oxidation
14
requires an additional compound (KI) and releases  as a side product, which needs its own separation process, while ion-exchange resins
15
are less feasible in a large-scale setting due to their poor regeneration.
as a side product, which needs its own separation process, while ion-exchange resins
15
are less feasible in a large-scale setting due to their poor regeneration.
Capacitive deionization (CDI) is an eco-friendly and energy efficient desalination method: 16–18 Water containing salts flows through a cell on which a potential difference is applied. The positive electrode attracts the anions, while the negative electrode attracts the cations, and diluted water exits from the other side. Upon discharge, the counter-ions are returned to the stream, concentrating the outgoing solution. This two-steps capacitive process occurs at the electrical double layer. The known methods that are used to enhance the CDI charge efficiency and long-term stability are:
- 1.
- 2.Asymmetric CDI (A-CDI) which, employs an asymmetric distribution of polarities. 21,22 for example: When polarizing electrodes in a ratio of 1 to 10 between the areas of the positive and negative electrodes respectively, the negative electrodes therefore becomes less prone to degradation, as less potential falls on it, while the smaller positive electrode suffers from oxidation and degradation. 23
In order to avoid excessive gas evolution as a cause of water splitting, when the voltage difference between the electrodes is higher than 1.5 V, and to mitigate deterioration in the negative electrodes, the cells, in the reactor, were assembled in an asymmetric configuration, (i.e., different electrodes' mass ratio (cathode vs anode)), in favor of the cathode) as demonstrated in previous work. 24
This allows the avoidance of water discharge along the bromine recovery process and higher endurance of electrodes and enables larger working voltage domains.
It is worth mentioning that previous CDI studies showed high performance for selective removal of ions 25 and heavy metals, 26 and also the aspect of endurance of the electrodes was excessively studied. 27 For selective electro-oxidation of bromide ions (Eq. 1), we take under consideration the electro-oxidation potentials of chloride ions and water (Eqs. 2 and 3), which are quite close (the equations were taken from handbook of chemistry and physics).
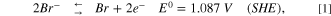
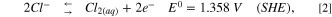
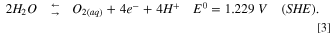
Based on a previous work, 24 specific electro-oxidation of bromide to bromine was achieved using a solution containing same concentrations of chloride and bromide ions. The new cell name was hybrid physical adsorption and capacitive deionization (HPA-CDI), due to the combination of bromine physical adsorption inside the porous structure of the electrodes, with the capacitive electrical double layer adsorption of bromide/polybromide ions.
We should clarify the "hybridization" between faradaic and electrostatic interactions. In fact, two processes take place simultaneously when a voltage difference is applied between the negative and positive electrodes. In the negative electrode, the negative charge is compensated by the double layer that is formed in the carbon/electrolyte interface whereas an electro-oxidation process take place on the positive electrode (electron transfer from bromide to the electrode). The positive electrode also experiences electrostatic interactions with oxidation byproducts like polybromides. The study showed that when using the electrochemical cell in its new asymmetric design a specific removal of bromide ions from chloride ions containing solutions, is possible by two simple steps of charge and discharge. Applying a potential, which is lower than the potential of chloride ions and water oxidation as shown in Eqs. 2 & 3 allows to work in a potential window where only bromide is oxidized. Previous results showed a separation factor of about 70.
The removal capability of bromide ions using HPA-CDI cells was about 3.5 mmole bromide ions to gr of activated carbon electrodes. To understand how much the removal capacity was high, we could compare it to capacitive mode (=CDI). In CDI the theoretical capacitance of a 1 gr AC electrode is about 100 F gr−1 (only per WE weight), which when translated into salt, it amounts to about 1 mmol per 1 gram. In previous study, the maximal theoretical desalination capacity based on electric double layer capacity was multiplied by 3.5. It was suggested that when the AC electrodes are polarized to 1 V, the bromide ions are electro-oxidized to bromine and physically adsorbed to the high surface area of the AC. Bromine and AC have a physical affinity to one another, 28 for example 1.25 mmol g−1 of bromine are adsorbed on carbon black (surface area of ∼100 m2 g−1). Additionally, the bromine molecules are formed inside the micro-porous structure of the AC, which impairs the molecule movement back into the solution; meanwhile, other bromide ions, which are electrostatically adsorbed into the pores and are electro-oxidized, interact with the bromine molecules to give tribromide and pentabromide ions, as described by Eqs. 4–6:
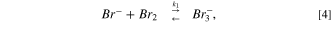
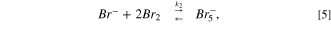
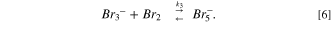
The physical adsorption of bromine molecules and the electrical double layer adsorption of polybromide ions and bromide ions as listed above allows a removal of high quantity of bromide ions from the solution, 3.5 times higher than the theoretical capacitive removal.
Aside of the excellent separation demonstrated, the high specific removal and recovery capability achieved in the above previous study, the electrodes in the cells degraded quickly (after about 5 workhours) and eventually stopped desalinating due to oxidation of the carbon electrodes because of the continuous application of potential on them. Improving cells durability in such operation is critically important for practical uses.
This study focuses on the stability of the electrodes over time using different electrodes candidates and using new cells' configuration and method of operation. In the first case, a porous carbon may be applied with a good balance of cost effectiveness, stability, adsorption capacity and kinetics. Nine different carbons as well as a combination of them were evaluated. In second case, the cell configurations with different electrode shape, flow regime (through/by), and extent of asymmetry between the negative and positive electrodes were tested.
The cells which were used in this study are HPA-CDI combined with a mode of operation of polarity switching after sets of 6 charge-discharge cycles, which was designed for asymmetric cells. These cells include 4 identical sets of 6 similar electrodes made of sheets of selected activated porous carbon spread on Grafoil current collectors. The porous carbon selected exhibits both high capacitive capability and excellent redox activity with bromide/bromine species. Since the specific capacity of these electrodes in redox activity with bromide/bromine species is 5 times higher than the capacity that can be reached by capacitive/adsorption interactions, always one of the six electrodes (in each of the 4 sets of s electrodes that the cells comprise) serves as an anode (the positive electrode), while the other 5 electrodes serve together as the cathode (negative, capacitive pole). After every 6 charge -discharge cycles, a polarity switch was applied, one of the previously negative electrodes becomes the single anode, while all the other 5 electrodes in each of the 4 sets of 6 electrodes (including the previous anode) serve together as the cathode. Since the detrimental processes here are only the redox—oxidative ones, the 5 electrodes (in each of the 4 sets) which serve during every 6 cycles together as a common cathode, are in an apparent pseudo rest mode. Hence, the "rest" time for each electrode = the anode processes per 6 cycles times 5. As a result, each electrode in the cell benefits from a long period of resetting time. By this mode of operation, the degradation of the electrodes during the oxidation processes they periodically undergo, is largely mitigated and hence the electrodes' stability and the cells' durability are high. It is important to note that in this work and related paper we do not discuss the excellent selectivity achieved in separating bromide moieties from solutions containing chloride anions by CDI type processes in cells based on activated porous carbon electrodes, since this was already well demonstrated before. 27 The focus herein is on a revolutionary increase in the durability and sustainability of these electrochemical separation processes, which pose a great challenge.
Experimental
Activated carbons
The tested activated carbons (AC)s is described in Table I. Kynol 1500/2000 and PAN are carbon fabrics, E-carbon is made from a solution on top of a graphite foil, and carbon Aerogel is a thin sheet. Commercial powders of Y200, Energy2, rGO (synthesized in our lab) and YP50 were used to assemble stand-alone electrodes.
Table I. Brand name, structure, source, official name, specific area, and form of acquisition of examined activated carbons.
| Brand name | Structure | Source (company) | Official name/article | Specific surface area (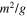 ) ) | Acquisition |
|---|---|---|---|---|---|
| Kynol 1500 | Fabric | Nippon Kynol, Japan | ACC-5092–15 | ∼1500 | Commercial ready-to-use electrodes |
| Kynol 2000 | Nippon Kynol, Japan | ACC-5092–20 | ∼2000 | ||
| PAN | Taiwan carbon technology (TCT), Taiwan | PAN | ∼1000 | ||
| E-carbon | Solution on graphene foil | Siontech, S. Korea | Anode-SCDI | ∼1500 | |
| Carbon Aerogel | Sheet | Marketech International, USA | Carbon paper grade 1 | 400–700 | |
| Energy-2 | Powder | Energy China, China | Energy-2 | ∼1500 | Powder made into stand-alone electrodes |
| Y-200 | Refael, Israel | Y-200 | ∼3000 | ||
| YP-50 | Kuraray, Japan | YP-50F | ∼1690 | ||
| PE-rGO | Lonza, Switzerland | Reduced graphene oxide | ∼443 |
Electrode preparations
PE (partially exfoliated) rGO was prepared in our lab by oxidizing graphite powder (via several oxidation reagents) followed by a scalable low-temperature thermal exfoliation by reduction under air atmosphere. 29 PE-rGO and the powdered materials: E-carbon, Y200, Energy2 and YP50, were made into stand-alone electrodes by kneading 90% active substance and 10% PTFE binder with 3–10 ml of 2-propanol (Sigma Aldrich, USA) as volatile mixing medium, flattening (SEW-Eurodrive, USA) and drying at 100 °C for 30 min.
Three electrodes' systems
Cyclic voltammetry (CV) measurements were performed to determine capacitance and faradaic reaction kinetics, and chronopotentiometry (CP) was applied to determine charge accumulation and coulombic efficiency. Simple three-electrodes' cells were constructed, consisting of an AC working electrode and Kynol 1500 counter electrode in a mass ratio of 1:10, respectively, and an SCE reference electrode. Nova (version 1.8 or 1.11) workstations were used for CV and CP with AutoLab PGSTAT302N (Eco Chemie, The Netherlands) potentiostat. CV was applied at a potential range of 0–1 V and 1 mV s−1 scan rate at 12–16 crossings. The solution consisted of 0.05 M NaBr and 0.5 M Na2SO4 (Sigma-Aldrich, USA). CP was applied with 4–10 charge-discharge cycles at a fixed narrow range of 0.7–0.8 V with several current densities (Table II). As bromine reduces back to bromide ions significantly more slowly than bromide is oxidized, the discharge performance was optimized by lowering its current density with respect to the charge to extend the discharge duration. A long-term (90-cycle) CP run of each AC was conducted at the optimal current densities, and the results were compared.
Table II. Charge-discharge current densities of examined activated carbons obtained by chronopotentiometry. Due to the known slower reduction rate of bromine to bromide, lower current density was applied at discharge to optimize performance. Bold figures represent the optimal densities based on analysis of charge capacity, accumulation, and coulombic efficiency.
| Current densities (mA/g) | |
|---|---|
| Kynol 1500 | +50/-40, +50/-30 , +50/-20 |
| Kynol 2000 | +50/-20, +50/-10 , +40/-10 |
| PAN | +50/-20, +50/-10, +40/-10 |
| E-carbon | +50/-40, +50/-30, +50/-20 , +50/-10, +40/-10 |
| Aerogel | +50/-40, +50/-30 , +50/-20, +50/-10, +40/-10 |
| Energy-2 | +50/-30, +50/-20, +50/-10, +40/-20 , +40/-10, +30/-10 |
| Y-200 | +50/-30, +50/-20, +50/-10, +40/-20 , +40/-10, +30/-10, +30/-20, +20/-10 |
| YP-50 | +50/-30, +50/-20, +50/-10, +40/-20 |
| PE-rGO | +50/-20 , +50/-10, +40/-10 |
HPA-CDI cells structure with 6 different poles
The flow-by cell is described in Fig. 1. It contains 4 sets of 6 identical activated carbon electrodes in discs shapes with a hole in their middle. The electrodes are separated from each other by disc shaped Grafoil current collector, porous polyethylene cloth separator, and PTFE spacer. Each Grafoil current collector bears on both sides two sheets of activated carbon which serve as the active mass of the electrodes. These parts are pressed together to form a hermetically sealed cylindrical cell that allows a flow-by operation, forcing the solution to flow between the electrodes through the porous separators from their perimeters to the holes in their middle, while undergoing the electrochemical processes. The solution entering the cell is distributed at its bottom by a plastic disc with appropriate holes near its perimeter, that direct the solution to flow from the outside of the cylindrical structure through the porous separators to the hollow inside of the cell, between the electrodes (a flow-by operation mode). In the middle of the cell, a reference site was placed using the same current collector and separator with an AgCl round silver mesh SCE reference electrode, previously anodized in 0.1 M HCl for 2 min at a current density of 10 mA cm−2. Samples were taken for pH measurements. However, a significant change in the pH was not observed throughout cycling. The cells are connected to a computerized operation system that polarizes the electrodes according to the special and innovative operational mode developed and adopted for the present study. The electrical contacts of the 24 electrodes (which are organized in 4 sets of 6 electrodes each) are organized in 6 poles (Fig. 1, the right image), marked by numbers. Each pole is electrically connected to 4 electrodes (distributed in the 4 electrodes sets).
Figure 1. Left: HPA-CDI cell's components: PVC top and bottom covers, PTFE outlet mask, flow distribution mask and spacers, PE separator, flexible graphite foil current collector, and ACC-5092–15 (Kynol) activated carbon electrodes. A reference electrode (SCE, not shown) is situated at the center of the cell. Right: Illustration of HPA-CDI multi-level cell with positive to negative electrodes ratio of 1:5 (six polarities, each with four levels). During a run, in each of the six charge-discharge cycles, the positive polarity switch clockwise (from electrode 1 to 2, etc.).
Download figure:
Standard image High-resolution imageHPA-CDI system setup
The layout that was built and used for the bromide ions removal system is presented in Fig. 2. Water flow: the tube system was made of Viton (Cole-Parmer, Vernon Hills, IL, USA). A 21-liter feed solution containing NaBr and NaCl (both 1,000 pp m/ 21 g, Sigma Aldrich, USA) was connected to solenoids 3 and 4, and the "Diluted" and "Conc." tanks where the diluted and concentrated bromide ion solutions flow were connected to solenoids 2 and 1, respectively. To measure the conductivity of the solution online, the CDI reactor was connected to a conductivity probe (Metrohm, 712 model) chambered in a custom-made tube and split into three pathways: solenoids 2, 4 and 1, leading to the "Diluted," "Feed" and "product" (Conc.) tanks, respectively. Below the reactor is a peristaltic pump (MRC, BT100–1 F) connected to solenoid 3. Data transfer and electric power: data acquisition controls the pump; Two relay boxes control the solenoids (four relays) and polarity switch mechanism (six relays) with an auto-range Multicorp Pro power supply and PGSTAT302N (Autolab) potentiostat. The conductometer was connected to the conductivity probe via RS232 port. The custom-made "Valve & pump control hub" Labview computer program allows, in a single user interface, to monitor and control the work potential, conductivity, current, solenoid states (on/off), pump flow rates, polarity sequence and work time of each stage. During runs the program updated two graphs online: current vs time and conductivity vs time.
Figure 2. Illustration of the bromide removal setup: a CDI reactor with three solution tanks, four solenoids, a conductivity probe, and a peristaltic pump—interconnected via water flow pumps, data transfer ports and electric power cables.
Download figure:
Standard image High-resolution imageHPA-CDI work procedure
The solution was circulated through the cell for 60 min, followed by a four-step procedure: (a) 5 min open circuit voltage (OCV), 50 ml min−1, (b) 10 min 1 V, 20 ml min−1, (c) 5 min OCV, 50 ml min−1, (d) 30 min 0.5 V, 5 ml min−1. After six charge-discharge cycles, the positive charge was shifted (Fig. 1, the overall cell illustration, right side), thus each electrode underwent six "working" cycles (where it is electro-oxidized and discharged periodically), followed by thirty "resting" cycles (where it is electro-reduced). In Fig. 1, right image, there are 6 contacts' poles enabling (each) 4 electrodes (one from each set of 6 identical electrodes) to have the same voltage and charge. As an example, in Fig. 1 right, pole no. 1 applies a positive potential to the 4 electrodes which are connected to it, which are being oxidized for six charge-discharge cycles. During these 6 cycles, the other poles (2–6) apply negative potential to the electrodes connected to them. In this operation mode, during 36 cycles each electrode undergoes 6 consecutive cycles under positive polarization (serving as one of parallel 4 anodes) and 30 consecutive cycles under negative polarization (serving as one of parallel 20 cathodes).
Analysis methods
E-SEM and EDAX (Quanta FEG, FEI) were used after the electrochemical runs to qualitatively examine possible morphological changes on the surface (such as electrodes destruction) and to determine possible relevant changes to the carbon (such as bonded or present oxygen or bromine), respectively.
Ion chromatography (IC) was carried out with 9 × 10–3 M Na2CO3 (Dionex ICS-2100, Thermo Scientific, UK) to measure the weight of the different ions inside each sample. Ion weights were also measured by titration with AgNO3 at a concentration of 0.01 M (848 Titrino Plus, Metrohm, Switzerland) to confirm and evaluate ions that were difficult to evaluate by IC.
Results and Discussion
Optimal activated carbons
Any industrial process, and separation process, in particular, is eventually inspected in terms of levelized cost or the levelized cost for recovery of 1 Kg of bromide in this case. Breakdown of the levelized cost includes capacitance of the electrodes, separation factor, removal rates, life span of the electrodes and more. All these factors, which are intercorrelated, contribute directly and indirectly to capital and operational costs of the whole process.
The capacitance of the electrodes, in terms of mg removed bromide to 1 g of electrodes alongside the separation factor are critical factors for any given industrial separation process. However, although capacitance and adsorption rate are intercorrelated, the rate at which bromide moieties are accumulated in the electrodes is an important factor as well, because the dimensions of a separation plant is dictated also by the kinetics of the processes and so the overall capital costs. Sluggish kinetics is usually translated to higher working voltages (and hence higher energy consumption) things that contribute to higher operational expenses.
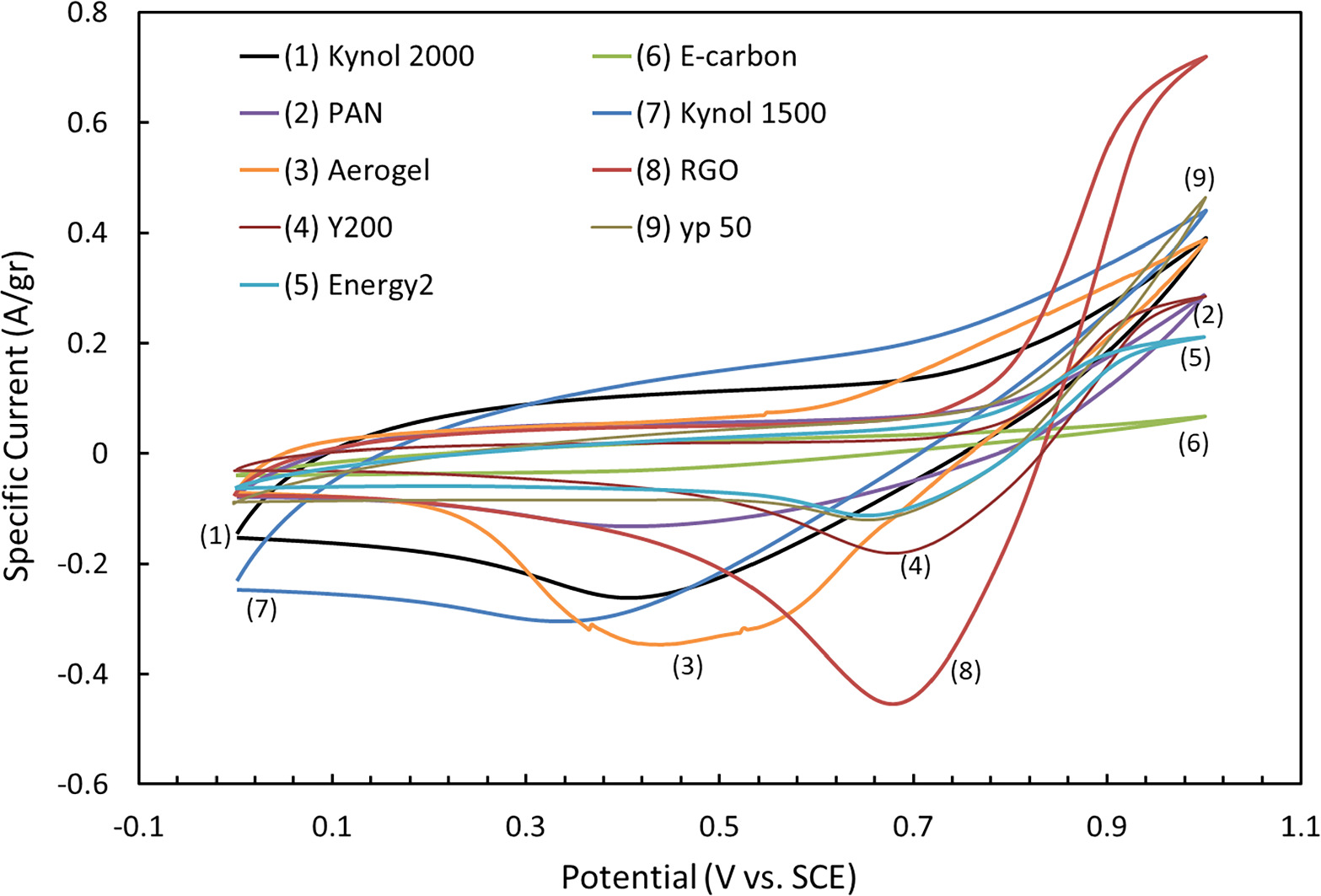
To assess 9 different carbonaceous materials, we used different electrochemical analyses. Since the electrodes in the process undergo both faradaic and electrostatic reactions, the inspection of the characteristic of the i-V curves, especially at the onset of the bromide electro-oxidation reaction, can help us to set a benchmark of charge transfer kinetics (or removal rates) from the electrodes to bromide species in the solution. Figure 3 shows cyclic voltammetry curves (current density vs potential, SCE as the reference electrodes) of electrodes comprising the 9 carbonaceous materials examined for this study, in the relevant aqueous bromides containing solution. These CV curves indicate the onset of the bromide's oxidation reactions and the corresponding reduction reactions. The most intensive oxidation and the corresponding reduction reactions response, presented in Fig. 3 belongs to the rGO electrodes demonstrating the highest current densities; Hence, qualitatively, the PE-rGO stand-alone electrodes displayed by far the fastest bromide ion redox reactions, in contrast with the carbon cloths/sheets electrodes. We speculate that this is due to the internal structure of the rGO –'graphene-like sheets in principle, which offers short pathways for adsorption sites and facilitate the diffusion of ionic species inside the micropores. 29 Among the cloths/sheets, Kynol 1500 displayed the fastest bromide ion redox reactions (largest slope), and Aerogel seems to have the second-best kinetics. E-carbon displayed by far the least impressive kinetics.
Figure 3. Cyclic voltammetry plots measured in standard the three-electrodes cells with all the 9 examined activated carbons. rGO (8, brown) displayed the fastest kinetics, while Aerogel (3, orange) and Kynol 1500 (7, blue( showed the highest specific capacity. CV was applied at a potential range of 0–1 V and 1 mV s−1 scan rate at 12–16 crossings and the solution consisted of 0.05 M NaBr and 0.5 M Na2SO4.
Download figure:
Standard image High-resolution imageInspecting the charge (in Columb units) that flows between the electrodes along the charge discharge cycling can bring meaningful insights into the capacitance, efficiency, and endurance of the electrodes, without the assembly of the electrodes in full "bromide removal" cells. Accumulative charge is defined as the sum of charge that flows between the electrodes during the charge process minus the charge that flows between electrodes during the discharge, the spontaneous process.
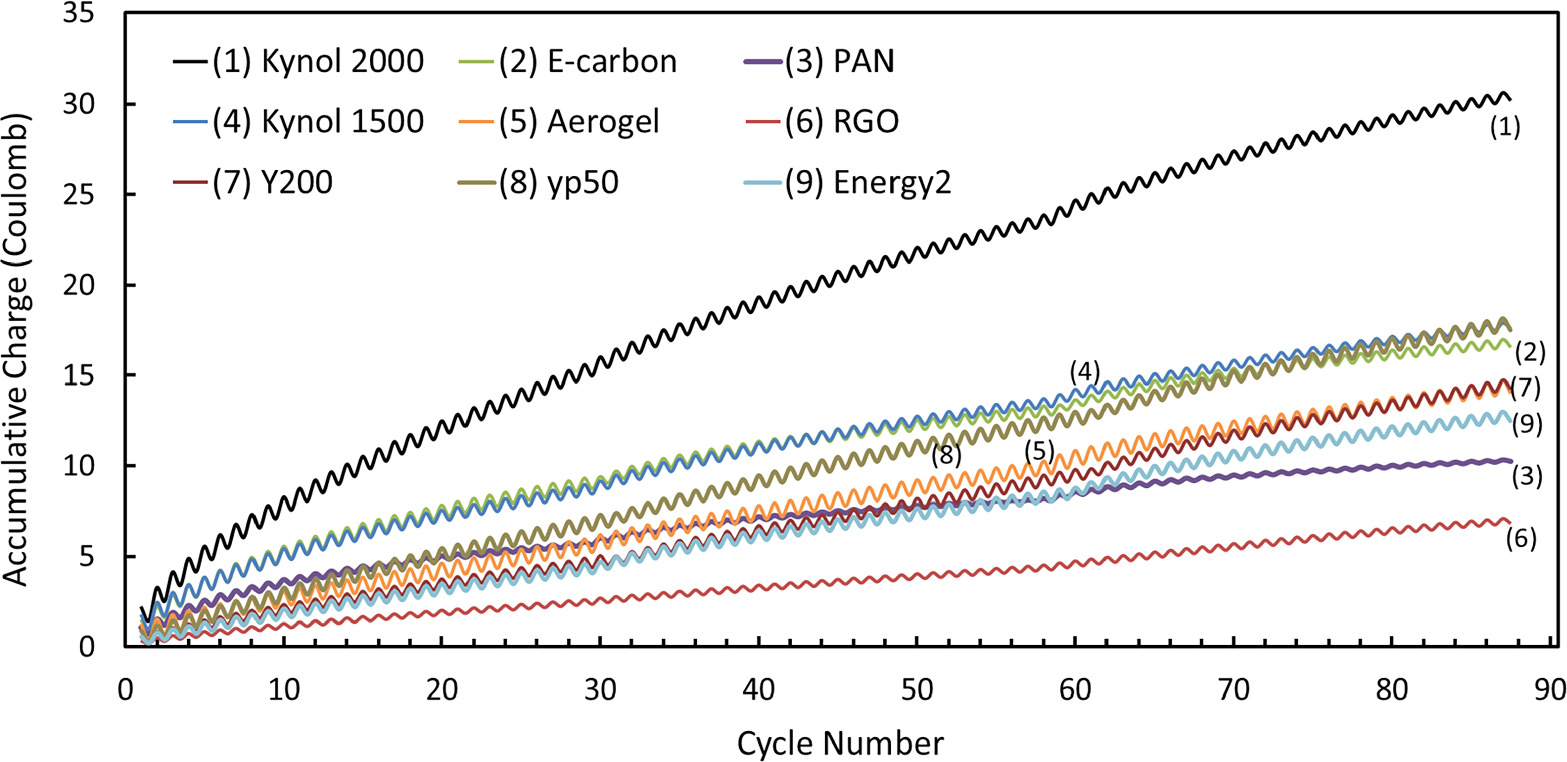
Figure 4 compares the stability of the various 9 types of carbonaceous electrodes studied herein, by demonstrating the amount of charge accumulated on the electrodes in the cells during continuous galvanostatic (constant current) operation. In ideal situations, with no side reactions, the difference in charges between the anodic (oxidation) and the cathodic (reduction) processes of the electrodes should approach zero (i.e., cycling efficiency close to 100%). In the practical systems which use high-capacity porous carbonaceous electrodes the charging process, namely, positive polarization aiming at oxidating the bromide species and the adsorption of the oxidation products within the porous electrodes, the charging/oxidation/bromides removal processes may not be reversible. It may be accompanied by side reactions related to the partial oxidation of the carbonaceous electrode's material, thus, adding irreversible charge per cycle, which reflect a slight degradation of the carbon electrode during each cycle. Hence, an important manner through which the carbonaceous electrodes can be examined and compared is the accumulative charge differences between the positive and negative polarization of each cycle during the cycling processes. During first cycles irreversible charge accumulation of these cells may be inevitable because there are always irreversible processes which are required for these systems to reach a steady state, 30 however, after a few cycles, accumulation of the irreversible charge may imply on two phenomena: Parasitic reactions that take place upon positive polarization of the anode; Irreversible bromide anions insertion to the porous carbonaceous anodes. Both two phenomena can be considered as carbon anodes' degradation. Degradation of porous carbon electrodes reduces their effective area for the required reversible bromides. As can be seen in Fig. 4, Kynol 2000 electrodes displayed by far the greatest irreversible charge accumulation after ∼90 cycles and thus the worst performance in terms of fast degradation. rGO displayed the best results (accumulation of ∼7 coulomb only), while the other electrodes accumulated 10–17 coulomb of irreversible charge during 90 cycles. For instance, Kynol 1500 and Aerogel electrodes reached accumulation of ∼16 and ∼12 coulomb, respectively.
Figure 4. Charge accumulation on the various 9 carbonaceous electrodes examinied in this study during a galvanostatic procedure (chronopotentiometry) with ∼90 charge-discharge cycles. RGO displayed the lowest accumulation (∼7 C), while Kynol 2000 showed the highest (∼32 C). The solution consisted of 0.05 M NaBr and 0.5 M Na2SO4. A 90-cycle CP run of each AC was conducted at a fixed narrow range of 0.7–0.8 V and at the optimal current densities according to Table II (the optimal current densities are underlined).
Download figure:
Standard image High-resolution imageAnother direct implication of the degradation of these anodes is the inspection of the specific charge that flows between electrodes during the charging and discharging processes upon cycling.
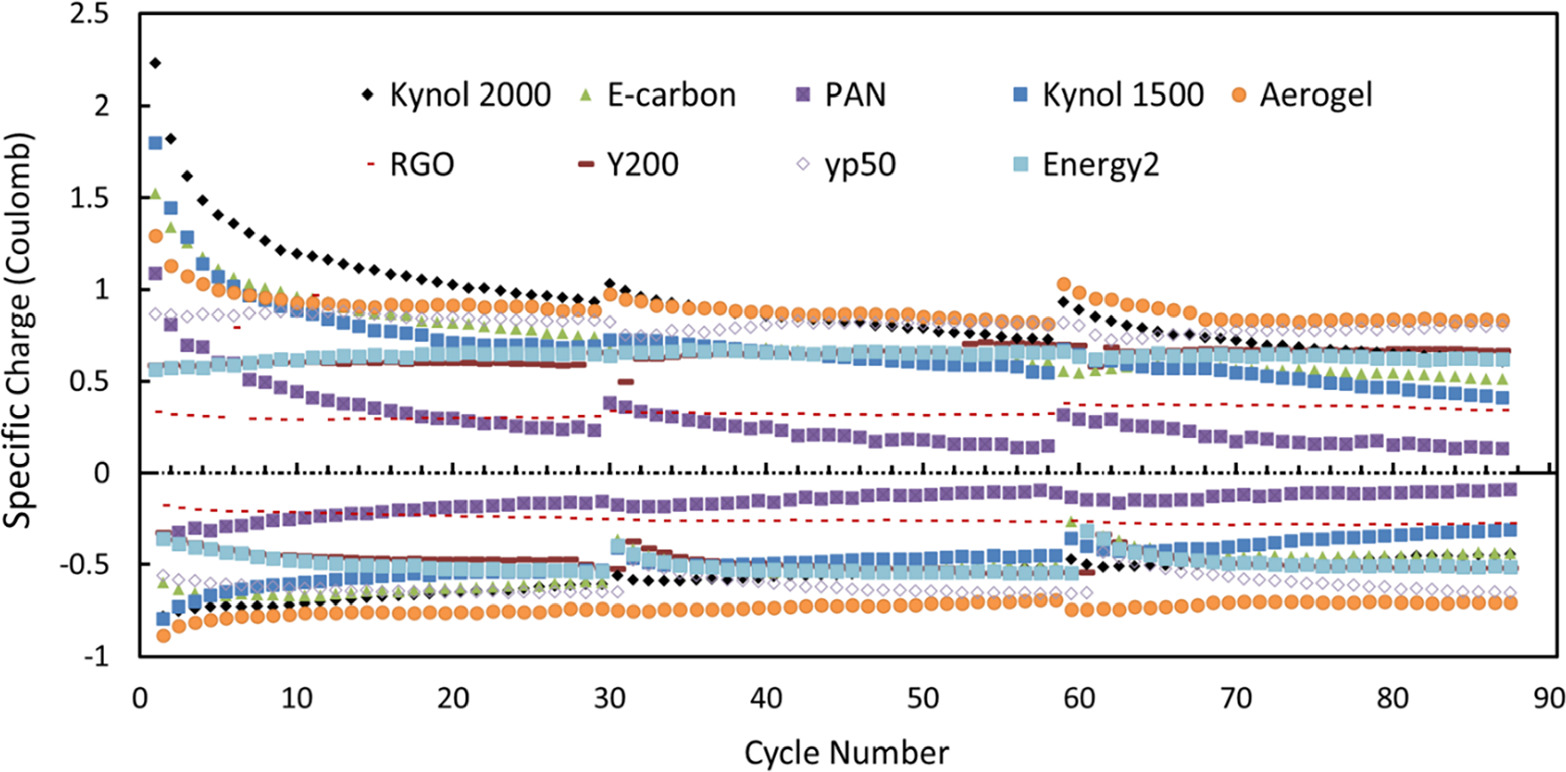
Long term comparative experiments with cells containing the 9 types of carbonaceous anodes were carried out in three phases of 30 consecutive galvanostatic cycles. After each set of 30 cycles the electrodes were relaxed by a long discharging process (before a new phase started with a charging process first). The charts in Figs. 5 and 6 reflect this mode of operation by the "jumps" observed after each set of 30 cycles.
Figure 5. Charge flows between electrodes (charge and discharge) during chronopotentiometry. Each charge (above 0) and discharge (below 0) represents the amount of bromide removed or retrieved per cycle, respectively. The experiments with the 9 types of anodes were carried out in three phases of 30 consecutive galvanostatic cycles. After each set of 30 cycles the electrodes were relaxed by a long discharging process (before a new phase started with a charging process first). This mode of operation is reflected by the "jumps" observed after each set of 30 cycles. Aerogel electrodes had the highest and most stable differentials over time, while PAN and rGO had by far the lowest values. The solution consisted of 0.05 M NaBr and 0.5 M Na2SO4. A 90-cycle CP run of each AC was conducted at a fixed narrow range of 0.7–0.8 V and at the optimal current densities according to Table II (the optimal current densities are underlined).
Download figure:
Standard image High-resolution imageFigure 6. Long-term coulombic efficiency of the 9 carbonaceous electrodes studied during chronopotentiometric measurements (prolonged cycling). The experiments with the 9 types of anodes were carried out in three phases of 30 consecutive galvanostatic cycles. After each set of 30 cycles the electrodes were relaxed by a long discharging process (before a new phase started with a charging process first). This mode of operation is reflected by the "jumps" observed after each set of 30 cycles.The general trend is a gradual improvement in each phase (cycles 1–30, 31–60, 61–90). Aerogel was the most consistent overall, while the other active carbons became more and more distinct. PAN displayed a sporadic performance throughout the run. The solution consisted of 0.05 M NaBr and 0.5 M Na2SO4. A 90-cycle CP run of each AC was conducted at a fixed narrow range of 0.7–0.8 V and at the optimal current densities according to Table II (the optimal current densities are underlined).
Download figure:
Standard image High-resolution imageSuch a comparison is shown in Fig. 5. Although Kynol 2000 electrodes start as the electrodes of the highest charge transfer level (redox activity + adsorption), the specific charge per cycle of these electrodes deteriorated after about 35 cycles. While some electrodes displayed better stability, for example those comprising Energy2, rGO and YP50, carbons, Aerogel electrodes showed the best results overall, as it had the highest charge and discharge amplitudes. The worst performers in this respect were PAN and rGO electrodes, therefore the latter ones were not used as the primary electrodes for the further studies, despite the great kinetics of the rGO electrodes. Kynol 1500 electrodes were moderately successful, dropping in amplitude only in the last phase. Long-term stability of electrodes in the cells used herein is determined by a complex interrelations between various parameters 31 such as feed conditions (oxygen levels in the feed, for instance), operational parameters (cut-off voltage) and inherent properties of the electrodes such as the crystallinity degree or defect in the electrodes' morphology. 32 However, the aim of this work was to inspect several electrodes candidates to be assembled in lab-scale "bromide removal" apparatus and to demonstrate how adopting a unique operational mode (referred herein as "polarity switch") can significantly affect the endurance of the process rather than elucidating the degradation mechanism of each individual type of electrode and then carrying out complex studies which intend to mitigating electrodes' capacity fading mechanisms.
The final comparison considers the coulombic efficiency of each type of electrode during prolonged cycling. As seen in Fig. 6, all electrodes displayed the same general trend; the coulombic efficiency was the poorest at the beginning of each phase (1st, 31st and 61st cycles) and rose to reach relatively stable values. When an electrochemical system requires a few charge-discharge cycles to reach a steady state, it is reflected by the stability at the charge efficiency value (we referred to relevant discussion therein [B]). Another trend is observed in which most of the electrode efficiencies started in a relatively close range, then began to spread out, some higher and some lower, so that by the end of phase 3 all lines became distinct. The most efficient were the Aerogel electrodes in the 83%–84% cycling efficiency range, when the electrodes reached steady state. E-carbon electrodes showed surprisingly good stability (79%–84%) yet they were excluded from the next part of the study due to their poor kinetics (as reflected by the CV measurements). The least efficient were the Kynol 2000 and the PAN electrodes mentioned above.
The combined results in Figs. 5 and 6, showed an interesting trade-off between accumulated charge and total charge that flows between electrodes (Fig. 5). Over 90 cycles, the rGO electrodes' charge flow was steady (in accordance to Fig. 4), but the total charge that these electrodes could exchange per process was one of the lowest (2nd place after PAN). Thereby the rGO electrodes were abandoned for the next step of the study.
In summary, in terms of kinetics, differential charge and efficiency, Kynol 1500 and Aerogel electrodes displayed the most impressive results, and therefore were chosen as the electrodes tested for selective bromide ions removal, as described in the next section. Kynol carbon was finally employed as the bulk backbone of the selected anodes because of its mechanical, physical and electrochemical properties, while Aerogel served as the main active electrodes' material owing to its efficiency and high specific charge exchange performance.
Bromide ions removal via regular processes with HPA-CDI cells
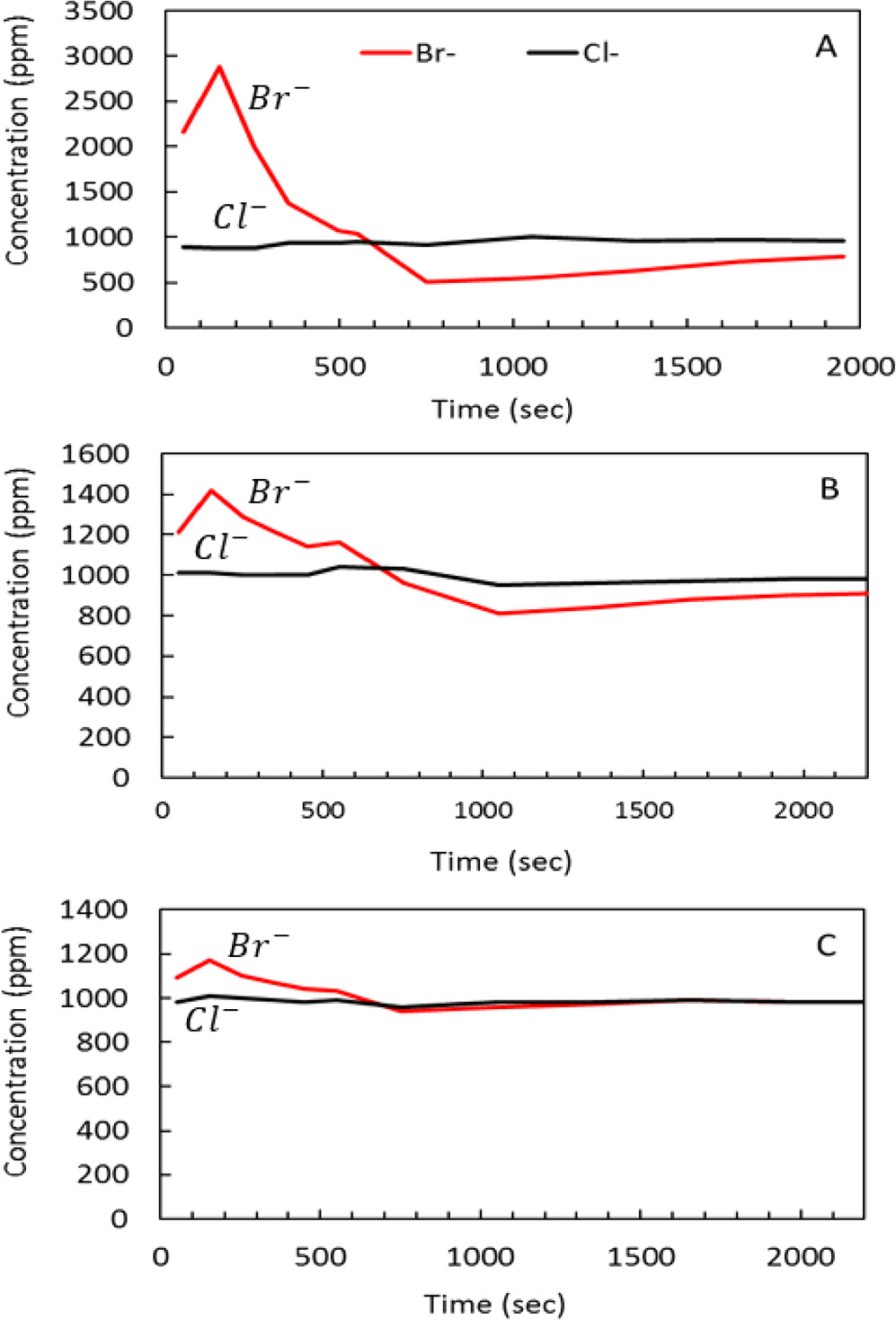
In a practical separation process separation is reached in two stages: in the first one, cells are being fed with solutions containing both bromide and chloride anions, together with many other possible contaminants, positive polarization of the cells leads to a fully specific removal of bromine species through adsorption/oxidation processes at the carbonaceous anodes (resulting in solution containing low concentration of bromide anions). In the second stage, the cells are fed with a fresh solution, into which bromide moieties are being discharged, thus forming the main product of the whole process. In this work we fed the cells with solutions containing both chloride and bromide ions, comparing the bromides concentration after cells' charging (bromides depletion) and discharging (solution is enriched by bromide ions. Throughout these cyclic processes, there was no change in the chloride's concentration. It was possible to follow the concentration changes by simple conductivity measurements (decrease upon charging, increase upon discharging processes). The regular HPA-CDI cells were operated according to the procedure described in a previous study. 24 15-liter of aqueous solution containing 1000 ppm NaBr and 1000 ppm of NaCl was circulating through the cell for 80 min. The cell was discharged for 6 min to 0.6 V and charged for 30 min to 1 V. The process was repeated over two workdays of 12 h each. On days 3 and 4, the discharge potential was 0 V. A representative performance in these experiments at days 1–3 is presented in Figs. 7a–7c. The separation was evident and was the highest at the beginning (500/3000 ppm charge/discharge difference in bromides concentration), reducing gradually with time. After a total of 3.5 workdays (∼40 h), the cells lost their separation capability (due to anode degradation), however their performance became lousy already in the middle of day 2 (Fig. 10b), showing separating capability of 800/1400 ppm, which reduced to 950/1200 ppm on day 3 (Fig. 10c). On day 4, the bromides and chlorides concentration during the electrochemical processes became indistinguishable from one another.
Figure 7. Concentration vs time measured in a regular HPA-CDI cell. A typical response in a selected discharge/charge cycle is presented: An increase in bromides concentration upon discuarge and depletion upon the charging process. A (day 1): bromine separation is clearly visible initially ∼500/3000 ppm charge/discharge difference. B (day 2): significantly decreased separation capacity, ∼800/1400 ppm initial charge/discharge. C (day 3): inefficient performance, ∼950/1200 ppm charge/discharge. This behavior reflect a pronounced electrodes degredation during 3 days of operation. The feed solution contained a 21-liter of NaBr and NaCl (both 1,000 ppm/21g).
Download figure:
Standard image High-resolution imageBromide ions removal with HPA-CDI cells with a new design version 1
Another cell was built with 24 electrodes setup according to Fig. 1, including Aerogel for its kinetic properties. Conductivity was monitored rather than concentration to test the long-term stability of the system, following the recent proof-of-concept for selective bromides separation. 5 As presented in Fig. 8, periodic low and high conductivity values reflected the status of the solution during charging (bromides removal) and discharging (enriching solution with bromides) The differences in the amplitude of the conductivity values along cycling is an indication of the degradation of electrodes or stability of the process. Furthermore, after establishing a calibration curve between conductivity and concentration, the in situ nature of continuous conductivity measurements provided a much more accurate representation of what was going-on inside the cell. Figure 8 demonstrates that the cell lifetime increased significantly to ∼83 h. The inconsistent minimum-maximum amplitudes, however, mean that each polarity site performed in its own manner, which is an indicator for detrimental processes and a problem in long-term or upscale estimations. Sites 4–6 performed worse than others in terms of kinetics and amplitude, but most acutely, after about 83 h, sites 4 and 5 show a complete shutdown of the desalination process, and the cell stopped working/desalinating completely. All sites behaved this way after that.
Figure 8. Conductivity vs time and current vs time in HPA-CDI cell version 1: electrodes comprising Kynol 1500 and Aerogel. The cell contains 4 sets of 6 identical electrodes each. The electrodes are connected accordingly to 6 poles through which potential is applied to the cell. Each electrode in the 4 sets, serve as the anode (4 anodes in parallel) during 6 consecutive cycles while the other 5 electrodes in each of the 4 sets serve (in parallel) as cathodes. The poles which connect electrically the electrodes are marked herein (1–6). Hence each 4 electrodes connected to the poles undergo 6 cycles as anodes (positive polarization) and 30 cycles as cathodes (negative polarization). Note that each set of 4 electrodes connected to one of the poles develop its unique performance, as reflected by the different amplitudes in current and conductivity measured along the 6 consecutive anodic cycles that the electrodes undergo. The sets of consecutive 6 cycles related to the 6 poles are reflected well in the figure (sporadic performance in terms of bromides separation capacity and kinetics. The cell's activity deteriorated after ∼83 h of continuous operation. The feed solution contained a 21-liter of NaBr and NaCl (both 1,000 ppm/21g). The solution was circulated through the cell for 60 min, followed by a four-step procedure: (a) 5 min open circuit voltage (OCV), 50 ml min−1, (b) 10 min 1 V, 20 ml min−1, (c) 5 min OCV, 50 ml min−1, (d) 30 min 0.5 V, 5 ml min−1.
Download figure:
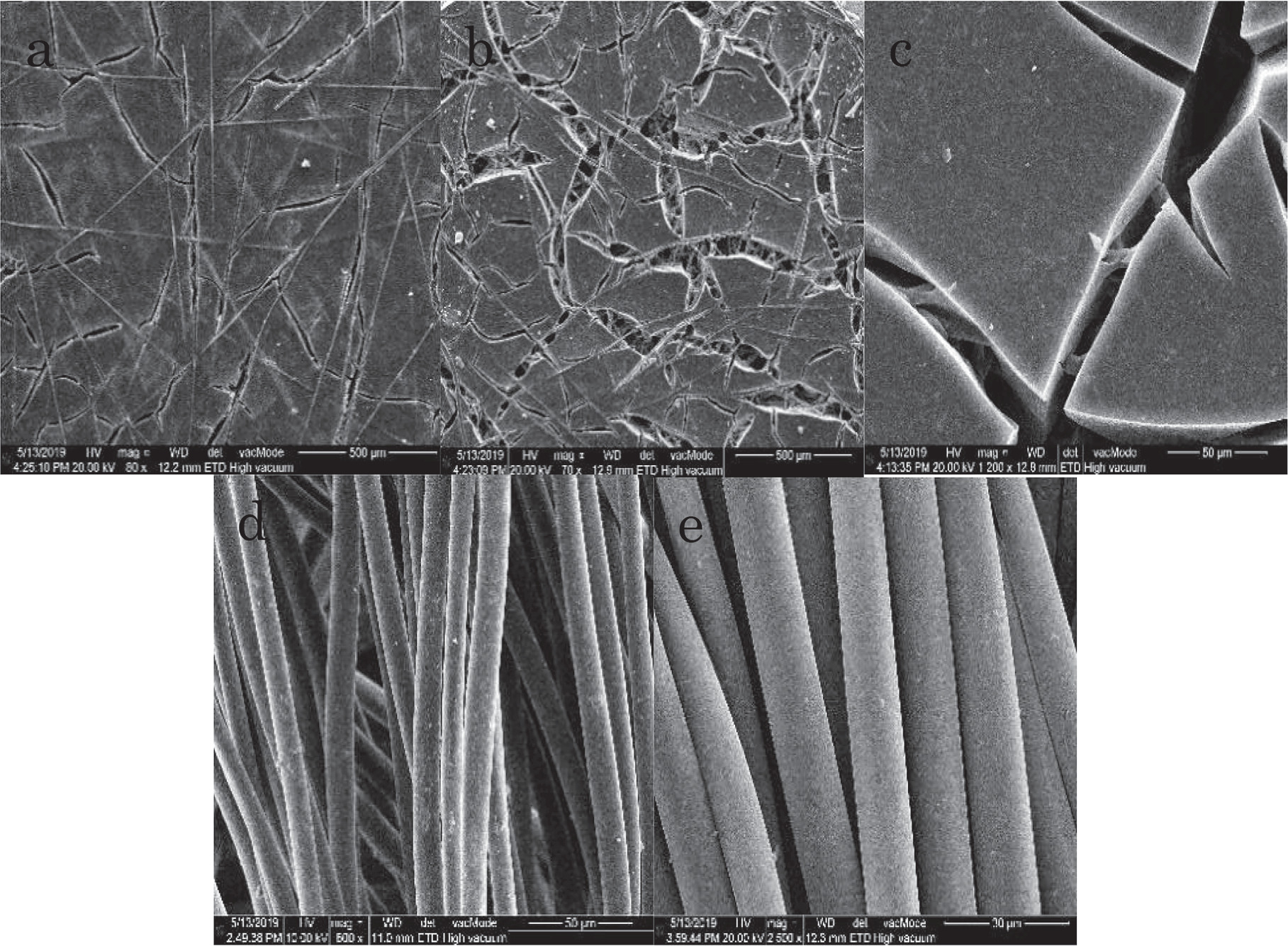
Standard image High-resolution imageWhen analyzed by E-SEM (Fig. 9), the Aerogel carbon parts displayed a clear destruction of their surface; the strongest destruction was observed on the central sheets (3 and 4). Kynol 1500 showed no morphological change due to its flexibility, electrochemical stability, and mechanical strength. 16 EDAX showed no indication of oxidation or content of bromine species, which excludes detrimental oxidation of the Keynol 1500 carbon as the reason behind the capacity fading and destruction mechanism. This finding is meaningful, and it is speculated that the destruction of the surface may be a result of charge transfer onto the cloth, as the Aerogel carbon sheets in the cells were situated between it and the current collector, creating small bubbles by minor surface electrolysis reactions that cause continuous liberation of small bubbles that eventually ruptured the aerogel structure. This observation needs, however, a further rigorous study, which is less important at this stage.
Figure 9. SEM images of activated carbons involved in the HPA-CDI bromides removal processes. Figure 8 and related caption, explain well the experimental details. The Aerogel carbon displayed a clear destruction of its surface after the run (b), (c) compared to before it (a). Kynol 1500 before (d) and after (e) the run was similar, with no indication of degradation.
Download figure:
Standard image High-resolution imageBromide ions removal with HPA-CDI cells with a new design, version 2
In the second version, the HPA-CDI consisted of only Kynol 1500 electrodes, without Aerogel. Figure 10 shows a ∼97 h run, spread out to a total of seven workdays. All sites (except for no. 1) performed the same, without any deterioration in their desalination ability, until the end of the 21-liter feed batch, which was consequently divided into two solutions—∼12 liter of ∼1.9 mS/cm ("Diluted") and ∼9 liter of ∼3.8 mS cm−1 ("Conc."). Each site displayed better performance with time as seen in the amplitude values, which reflect the kinetics of bromide ions removal and retrieval. For example, site no. 1 had the slowest kinetics in its first 6-cycle iteration and showed much better kinetics later. We concluded that the polarity switch and addition of intermediate steps in which the cells were held at OCV with a low flow rate of the solution, contributed well to the stable performance.
Figure 10. Conductivity vs time and current vs time, in HPA-CDI version 2 cell with Kynol 1500 electrodes. See the caption of Fig. 6 which explains the electrochemical operation. The numbers above represent the 6 poles of the cell when their relevant electrodes serve as anodes, undergoing 6 consecutive cycles under anodic (positive) polarization. The consistent amplitudes of conductivity show stable performance after more than ∼97 h. The feed solution contained a 21-liter of NaBr and NaCl (both 1,000 ppm/21g). The solution was circulated through the cell for 60 min, followed by a four-step procedure: (a) 5 min open circuit voltage (OCV), 50 ml min−1, (b) 10 min 1 V, 20 ml min−1, (c) 5 min OCV, 50 ml min−1, (d) 30 min 0.5 V, 5 ml min−1.
Download figure:
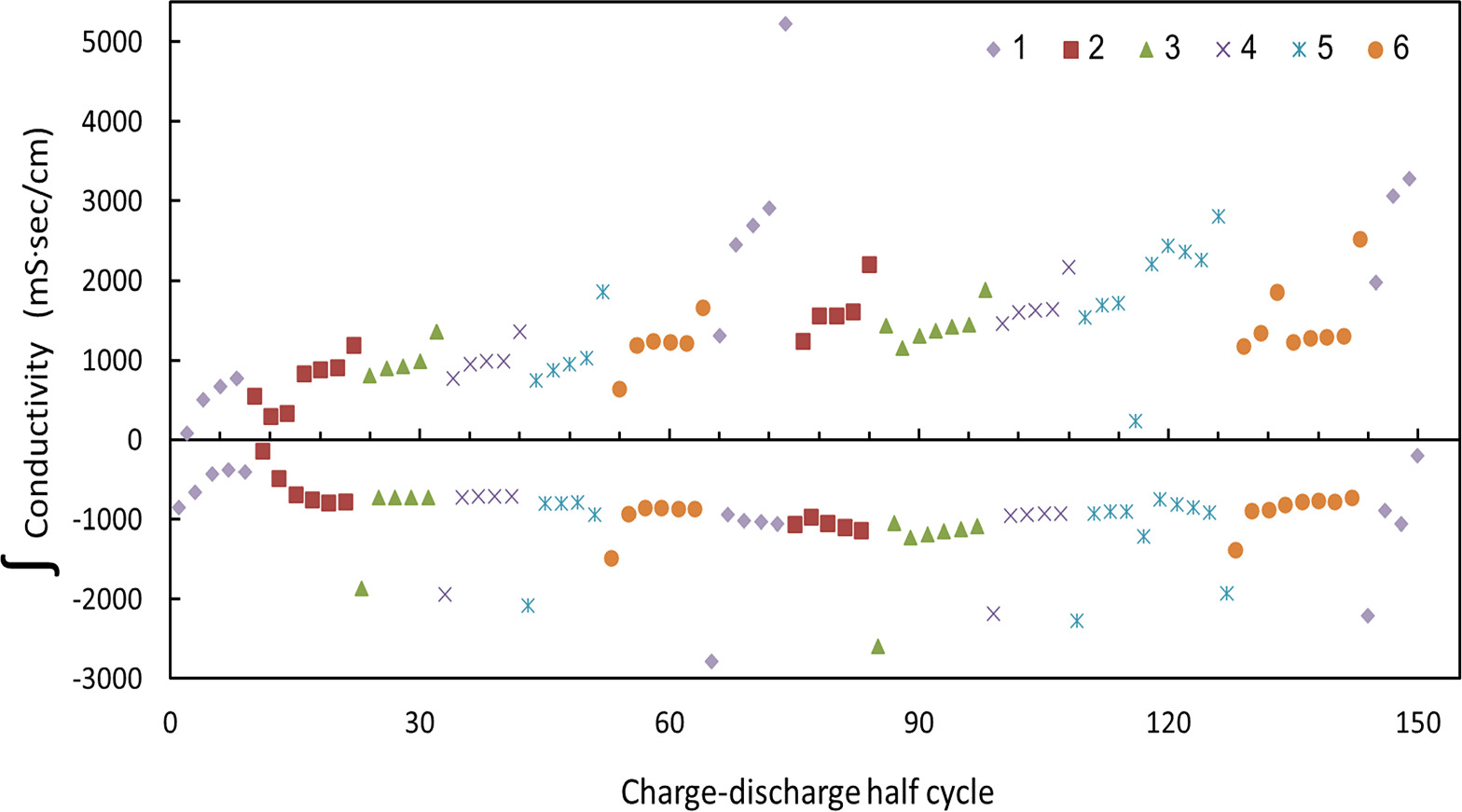
Standard image High-resolution imageFor determining raw removal and retrieval capabilities per cycle (rather than kinetics), an integrated conductivity chart from Fig. 10 was constructed (Fig. 11). The x-axis is periodic charge and discharge half cycles and the y-axis is  related of each of the half cycle processes separately. Positive and negative values in the y-axis of Fig. 11 relate to the charging and discharging processes respectively. Experiments of consecutive 75 cycles were carried out, thereby the x-axis of Fig. 11 counts 150 processes. The cutoff between charge and discharge was based on whether the conductivity was lower or higher than the conductivity of the feed solution (2.7 mS cm−1). The amount of bromides removal increased after the first cycles (at each active polarity site) and remained fairly stable. It is clearly shown that adopting this mode of operation, in which one of the electrodes changes its functionality from anode to cathode in a periodic manner, extends the lifespan of the cells and the whole system significantly. Hence, we demonstrate herein a promising approach for electrochemical separation processes in general and bromide ions extraction in particular.
related of each of the half cycle processes separately. Positive and negative values in the y-axis of Fig. 11 relate to the charging and discharging processes respectively. Experiments of consecutive 75 cycles were carried out, thereby the x-axis of Fig. 11 counts 150 processes. The cutoff between charge and discharge was based on whether the conductivity was lower or higher than the conductivity of the feed solution (2.7 mS cm−1). The amount of bromides removal increased after the first cycles (at each active polarity site) and remained fairly stable. It is clearly shown that adopting this mode of operation, in which one of the electrodes changes its functionality from anode to cathode in a periodic manner, extends the lifespan of the cells and the whole system significantly. Hence, we demonstrate herein a promising approach for electrochemical separation processes in general and bromide ions extraction in particular.
Figure 11. Integrated conductivity extracted from Fig. 10. The X axis represents half cycles: charge and discharge processes which conductivity response was integrated (positive for the charging processes and negative for the discharging processes). This chart report on 75 consecutive cycles what means a response of 150 processes as indicated. See the caption of Fig. 6 which explains the electrochemical operation. The numbers above represent the 6 poles of the cell when their relevant electrodes serve as anodes, undergoing 6 consecutive cycles under anodic (positive) polarization. The cutoff between charge and discharge was based on whether the conductivity was lower or higher, respectively, than the feed conductivity (2.7 mS cm−1). By comparing different iterations of the same polarity, the desalinated amount is seen to increase over time.
Download figure:
Standard image High-resolution imageConclusions
In this work, long-term stability enhancements were presented for electrochemical removal and retrieval of bromide ions from solutions containing both chloride and bromine ions. It should be noted that we did not need to demonstrate here again the excellent selectivity in bromide ions separation and extraction, that is achieved in CDI type processes based on cells comprising porous carbon electrodes, because this issue was discussed and exhibited previously. 27 The big challenge in this area relates to stability aspects, that now can be remarkably improved, thanks to the present work. After analysis of nine different carbonaceous materials, ACC-5092–15 (Kynol 1500) was chosen as the primary electrodes' material for the special CDI cells we developed, because of the very good kinetics, stability, affordability, and ease of use of this carbonaceous material. Kenol 1500 electrodes were integrated in novel cells' configuration, based on an asymmetric CDI scheme, which included electrodes' polarity switching every six charge-discharge cycles. The so-called HPA-CDI scheme, used herein, consisted of 96 Kynol discs with Aerogel carbon sheets (ver. 1) or without them (ver. 2), a SCE reference electrode, a 21-liter feed of NaBr and NaCl (1000 ppm each), a 1:5 positive to negative electrodes' ratio, 1/0.5 V charge/discharge cycles, and optimized charge and discharge durations.
HPA-CDI operation with polarity switch showed much better stability than a regular HPA-CDI operation (both with Kynol 1500 electrodes). While the latter operation mode showed limited stability of ∼40 h, HPA-CDI ver. 1 was stable for ∼83 h. HPA-CDI ver. 2, with no Aerogel carbon in the electrodes, showed improved stability performance of 97 consecutive workhours without any noticeable deterioration in the bromide ions removal capability. This additional improvement is attributed to elimination of the Aerogel carbon from the cells. It appears that Aerogel electrodes undergo pronounced degradation during the anodic polarization required for the bromide ions removal.
Moreover, the performance of the electrodes improved over time in terms of both (faradaic) kinetics and the amount desalinated per cycle, thanks to the periodic operation that allow each electrode in the cell (24 electrodes in the cells used herein) to undergo 30 cycles while serving as the one of the cathodes, after undergoing 6 cycles as one of the anodes. This exchange in polarity has a major contribution to the greatly increased cell stability, as it avoids continuous degradation of the carbon electrodes during the positive polarization, required to extract the bromide moieties from solution. The results warrant further investigation and optimization towards the establishment of HPA-CDI as an applicable method for selective bromide removal and retrieval.