Abstract
This paper investigated the corrosion behavior of three nickel-based alloys (230, 617 and 601) at 750 °C and 850 °C in a carbon dioxide environment for up to 500 h. All three alloys showed good oxidation resistance by forming mainly a protective chromia layer with low weight gains. Internal Al2O3 was precipitated beneath a thin chromia layer in all cases. For 230 and 617 alloys, NiO and Cr-rich spinel outer layers were formed, but for 601 less iron and nickel outward diffusion was observed at both temperatures. Furthermore, some minor alloy elements (Mn, Ti, and Co) were also observed in the chromia layers. Very limited carburization due to the CO2 reaction was revealed in the matrix underneath the oxide scale. Wagner's theory was applied to examine the critical chromium concentration for forming a protective chromia scale. This prediction indicated that alloy concentrations were marginal for chromia formation at both temperatures and the critical chromium concentration decreased with increasing the oxidation temperature. The presence of other alloying elements, e.g. Al, Mn, Ti, Si etc could increase corrosion resistance of these alloys by forming either additional protective oxide barriers or integrating with chromium oxide to enhance its protection.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Coal is the world's largest energy source, with coal-fired power generation accounting for 38% of global primary energy generation. 1 It is well known that the combustion of large quantities of coal in power stations and metal production plants produces a large amount of carbon dioxide. Between 1990 and 2014, global carbon dioxide emissions are reported to have increased by 58%, contributing largely to the greenhouse effect. 2
As a country that relies on burning coal to generate electricity, Australia has been committed to solving the problem of carbon dioxide emissions for many years. 3 The capture and storage of the reaction product is one of the solutions. Oxyfuel combustion is a newly developed technology for capturing carbon dioxide. 4 In this process, coal is burned in a mixture of oxygen and recycled exhaust gas to produce a flue gas consisting mainly of CO2 and H2O, making CO2 sequestration feasible. However, CO2 gas at high temperature leads to corrosion problems to boiler materials, producing not only oxidation but also carburization. The rapidly growing oxide layer will reduce the heat transfer of the alloy, therefore energy production efficiency. 5 Long-term corrosion will lead to materials failure and as a result significantly reduce the service lifetime of the equipment. Corrosion resistance is therefore one of the main issues in designing alloys especially when a higher temperature is used for increasing energy production efficiency in advanced energy production. As a result, conventional iron-based alloys could not survive, and nickel-based alloys might be a solution for CO2 corrosion at high temperatures.
High chromium content nickel-base alloys have high creep fracture strength and excellent oxidation resistance at high temperatures. Due to the selective oxidation of chromium, a thin protective chromia layer can form in oxygen and air, which contributes to the corrosion resistance of these alloys. 6 However, this corrosion resistance can be reduced in CO2-rich gas atmospheres for the Ni-Cr model alloys, 7–11 forming both oxidation and carburization. The addition of alloying elements of Al, Si, Ti and Mn was reported to improve the corrosion resistance of these alloys. 8 The corrosion resistance of some Ni-based chromia forming commercial alloys in CO2-rich gases was also investigated and found to be varied with the alloys and impurities in the gas. 12,13
This work investigated corrosion behaviour of three high Cr containing (≥22 wt%) Ni-base alloys (230, 617, 601) in CO2 gas at 750 and 850 °C for up to 500 h by examining weight gain kinetics, characterizing the reaction products and understanding the corrosion mechanisms. It is expected that these results can provide a better understanding of the effects of alloy compositions and temperatures on CO2 corrosion, providing a better materials selection to resist CO2 corrosion at high temperatures.
Experimental
Total three chromia-forming commercial alloys, 230, 617, and 601, were selected for test in this work. All commercial alloys were supplied by an alloy company and had no further treatment before the reaction. Their compositions are listed in Table I which were provided by the alloy company. These alloys are with similar Cr concentration (≥22 wt%) but with different alloy additions to investigate the alloying effects. All three alloys perform well in oxygen/air condition by forming a protective chromia scale at high temperatures.
Table I. Chemical composition of test commercial alloys (wt%).
| Alloys | Fe | Ni | Cr | Mn | Si | Al | C | Ti | Mo | Co | W | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 230 | 3 | 57 | 22 | 0.5 | 0.4 | 0.3 | 0.1 | 0.1 | 2 | 0 | 14 | Nb:0.5, B:0.015 |
| 617 | 1.32 | 54.22 | 22.01 | 0.04 | 0.11 | 1.18 | 0.06 | 0.52 | 8.85 | 11.65 | 0 | Cu:0.04 |
| 601 | 13.81 | 60.54 | 22.75 | 0.61 | 0.2 | 1.34 | 0.04 | 0.42 | 0.02 | 0.03 | 0 | Nb:0.07, Cu:0.04 |
Samples were cut to form 10 mm × 8 mm × (0.8–3) mm rectangular shaped coupons. After cutting, all sample surfaces were ground up to 1200-grit finish, and further polished down to 3 μm. Electro-polishing in 15% concentrated hydrochloric acid was used to remove the subsurface deformation zone. The grain sizes of 617 alloy are larger (100–200 μm) than those of 230 alloy (20–80 μm), and the 601 alloy (10–30 μm) has the smallest grain size. All samples were ultrasonically cleaned with ethanol and dried for the reaction. All sample coupons were hung in a sample holder which sits inside an alumina tubular reactor in a horizontal furnace. At first the reactor was flushed with argon gas to expel the remaining air and then the furnace was heated to the required temperature (750 °C and 850 °C) with a heating rate of 10 °C min−1. When the furnace temperature was stable, the gas was switched to Ar-20%CO2 with a linear gas flow rate of 2 cm s−1 at the reaction temperature. The CO2 purity was ≥99.995 %, with impurities of H2O (≤7 ppm), O2 (≤10 ppm), total hydrocarbons (≤5 ppm), and CO (≤7 ppm). The purity of Ar was ≥99.997 %, with impurities of H2O (≤10 ppm) and O2 (≤5 ppm). After that, the sample holder was inserted from the cold zone to the hot zone of the furnace for the reaction. The reaction time was set for 150 h and 500 h where new samples were used for each time set of test. After that, the furnace was switched off and the sample holder was pulled out slowly from the hot zone to the cold zone for cooling. When samples were cold down, samples were carefully removed from the sample holder for further analysis.
Weight gain kinetics were determined by measuring the weight change of samples before and after the reaction, using an analytical balance (Mettler Toledo XP205) with an accuracy of 0.01mg. Before reaction, sample dimensions were measured, and surface area of each sample was then calculated. The weight gain per unit area of each sample can be measured by the following equation:
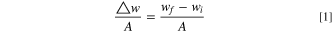
Where  and
and  are sample weights before and after reactions, respectively, and A is the total original surface area of the sample.
are sample weights before and after reactions, respectively, and A is the total original surface area of the sample.
Reacted samples were analysed by surface X-ray diffraction (PANalytical X'pert MPD with Co-Kα radiation). After that, samples were cold mounted and cut to make a cross-section. The polished samples were analysed by an optical microscope (Nikon-200) and a scanning electron microscope (FEI Nova NanoSEM 450) equipped with an energy dispersive X-ray spectroscope (Bruker) for elemental analysis. After cross-section analysis, samples were etched with Murakami's reagent (2g K3Fe(CN)6 + 2g KOH + 20ml H2O) to reveal chromium carbides. Some selected samples were further analysed by a transmission electron microscope (TEM: JEOL JEM F200). TEM specimens were prepared by using a focused ion beam (FIB: Nova Nanolab 200) milling.
Results
Weight change kinetics
Figure 1 compares the weight gains after 150 h and 500 h reaction of all three alloys at 750 (Fig. 1a) and 850 °C (Fig. 1b). In general, weight gains were small, less than 0.18 mg cm−2 at 750 °C, but increased rapidly at 850 °C. No scale spallation was observed after cooling.
Figure 1. Weight gains of Ni-base alloys after 150 h and 500 h oxidation in Ar-20CO2 at (a) 750 °C and (b) 850 °C.
Download figure:
Standard image High-resolution imageReaction product characterisation
Three Ni-based alloys after 150 h reaction were analyzed by XRD to identify phases of oxides and the results are shown in Fig. 2. For all three Ni-based alloys, corundum Cr2O3 and (Ni, Fe, Cr)3O4 spinel were detected, together with the Ni matrix. In addition, NiO was detected on the surface of alloys 230 and 617.
Figure 2. XRD patterns for Ni-base alloys after 150 h oxidation in Ar-20CO2 at (a) 750 °C and (b) 850 °C.
Download figure:
Standard image High-resolution imageAlloy 230
At 750 °C, the 230 alloy showed an excellent oxidation resistance, forming a thin uniform protective layer (Fig. 3). The SEM analysis in Fig. 3 showed some bright contrast nodules scattered on top of the thin oxide scale. There were also very fine dark precipitates underneath the oxide scale. Further TEM/EDS analysis was carried out and the results are shown in Fig. 4. As revealed by the EDS mapping and the point analysis, this oxide scale consisted of several layers. The top layer had complex compositions with the mixture of Ni-rich oxides (points 2 and 3), and Mn-rich oxides which contain also Cr and Fe (point 1). The second layer (point 4) was a continuous thin Cr-rich oxide layer covering the whole alloy surface. Underneath this thin chromia layer, there was another thin oxide layer (third layer) containing both W and Cr (point 5). Discrete Al and Si-rich oxide precipitates were observed mainly at the scale-alloy interface.
Figure 3. BSE-SEM cross-section of 230 alloy reacted in Ar-20CO2 at 750 °C after 500 h.
Download figure:
Standard image High-resolution imageFigure 4. (a) TEM analysis of the thin scale formed on 230 alloy reacted for 500 h at 750 °C in Ar-20CO2 and (b)–(i) corresponding EDS mapping. (j) EDS point analyses marked in (a).
Download figure:
Standard image High-resolution imageAt 850 °C, the metallographic and SEM-BSE cross-sections of 230 alloy are shown in Fig. 5. Similar to those at 750 °C, a thin uniform protective layer with some small bright contrast protrusions was observed after 500 h reaction. However, there were some internal oxides penetrating the matrix, probably along the grain boundaries (Figs. 5a and 5b). The SEM-EDS area mapping of the oxide scale showed that a discontinuous (Ni, Fe)-rich oxides formed above a thin uniform Cr-rich oxides with a thickness of 2.7 (± 0.7) μm, andapparent Mn dissolved in this layer. Meanwhile, some W was diffusing into Cr-rich oxide layer. Apparently, Al-rich oxide precipitates were found underneath the chromia scale and penetrated locally into the matrix via the grain boundary. No clear Si enrichment was found at this interface under the limited resolution of the SEM (Fig. 5j).
Figure 5. (a) Metallographic cross section and (b) SEM-BSE-image of cross-section of 230 alloy after 500 h reaction at 850 °C in Ar-20CO2, (c)–(j) corresponding EDS mapping results.
Download figure:
Standard image High-resolution imageAlloy 617
The SEM cross-section image of alloy 617 after 500 h at 750 °C showed a continuous protective oxide layer, together with some small particulate precipitates underneath this oxide layer (Fig. 6). The thickness of this protective oxide layer was not uniform. Further analysis of this oxide was carried out by TEM and the results are shown in Fig. 7. The oxide scale is rather complex, containing different oxides. There was a continuous Cr-rich oxide containing Ti on the alloy surface. On the top of this layer, there were Ni-rich oxides containing some iron, forming almost a continuous layer located on the surface of Cr-rich oxide layer. No Mo was detected inside the Cr-rich layer. Discrete Al oxide particles were formed underneath the chromia layer. It is worth noting that a large amount of Co was found inside the whole nickel oxide and top part of chromia layer.
Figure 6. SEM-BSE cross-section of 617 alloy reacted for 500 h at 750 °C in Ar-20CO2.
Download figure:
Standard image High-resolution imageFigure 7. (a) TEM analyses of thin scales formed on 617 alloy after 500 h at 750 °C reactions in Ar-20CO2 and (b)–(h) corresponding EDS mapping results, and (j) EDS point analyses marked in (a).
Download figure:
Standard image High-resolution imageSEM-BSE cross-section of 617 alloy after 500 h reaction in Ar-20CO2 at 850 °C is shown in Fig. 8. Compared with those formed at 750 °C, scale morphologies grown on 617 alloy at 850 °C remains the same. A thin, discontinuous Ni and Fe-rich oxide layer was formed on the top of Cr-rich oxide. Co was also found to be present in this layer. Meanwhile, an enrichment of manganese was observed in the chromium oxide layer. However, the thickness of chromium oxide layer was significantly increased, in comparison with that at 750 °C. There was also a thin aluminum enriched sub-layer beneath the Cr-rich oxide, and small, scattered aluminum oxide particles tended to penetrate deeper into the matrix.
Figure 8. (a) SEM-BSE cross-section of 617 alloy after 500 h reaction at 850 °C in Ar-20CO2 and (b)–(h) corresponding EDS mapping results.
Download figure:
Standard image High-resolution imageAlloy 601
Figure 9 shows the SEM cross-section of 601 alloy after 500 h at 750 °C. A thin protective oxide scale with some integrated metal particles was observed. There were some internal precipitates underneath this protective scale.
Figure 9. SEM-BSE cross-section of 601 alloy after 500 h at 750 °C reactions in Ar-20CO2.
Download figure:
Standard image High-resolution imageFurther analysis of this sample by TEM is shown in Fig. 10. A non-uniform Cr-rich oxide layer was formed on the alloy surface (Fig. 10a). Inside this oxide scale, Ti and Mn were also present (Figs. 10f and 10h). A thin Mn rich oxide scale was found to cover the whole Cr-rich oxide layer (Fig. 10f). Very small amounts of Fe and Ni in the form of particles were observed inside the Cr-rich oxide scale (Figs. 10d and 10e). Meanwhile a thin Al-rich oxide sub-layer formed underneath the Cr-rich oxide scale and tended to penetrate deeper into the bulk alloy.
Figure 10. (a) TEM analyses of thin scales formed on 601 alloy after 500 h at 750 °C reactions in Ar-20CO2 and (b)–(h) corresponding EDS mapping results (i) EDS point analyses marked in (a).
Download figure:
Standard image High-resolution imageSEM-BSE cross-section of 601 alloy after 500 h reaction at 850 °C in Ar-20CO2 and corresponding EDS mapping results are shown in Fig. 11. Microstructures of scales grown on 601 alloy at 750 °C and 850 °C were somewhat different. The (Cr, Mn, Ti)-rich scale was 5.4 ± 1.1 μm thick. The aluminum oxide particles were larger in size than those formed at 750 °C and penetrated deeper into the matrix. There were many fine metal particles (rich in Fe and Ni) beneath the Cr-rich oxide layer (Fig. 11a).
Figure 11. (a) SEM-BSE cross-section of 601 alloy after 500 h at 850 °C reactions in Ar-20CO2 and (b)–(h) corresponding EDS mapping results.
Download figure:
Standard image High-resolution imageCarburization
Comparison of alloy carburization in Ar gas with that in CO2 gas at 750 oC is shown in Fig. 12. There was slightly increased carbide density along the grain boundaries after reaction in CO2 for alloy 230 (Figs. 12a, 12b). For alloys 617 and 601 almost no newly formed carbides were observed (Figs. 12d, 12e, 12g, 12h). At 850 °C, carburization was clearly observed along grain boundaries of alloys 230 and 617 (Figs. 12c, 12f), and some fine carbide precipitates were found in alloy 601 (Fig. 12i). Some large black precipitates seen in alloy 230 microstructure (Figs. 3a–3c) could come from etching pits of this particular alloy.
Figure 12. Metallographic cross-sections of (a) 230, (d) 617 and (g) 601 after annealing in Ar gas for 150 h at 750 °C; (b) 230, (e) 617, (h) 601 after 500 h reaction in Ar-20CO2 at 750 °C, and (c) 230, (f) 617, (i) 601 after 500 h reaction in Ar-20CO2 at 850 °C.
Download figure:
Standard image High-resolution imageDiscussion
According to the above experimental results, corrosion products of three Ni-base alloys after 500 h reactions at 750 °C and 850 °C in Ar-20CO2 are summarized in Table II. All three Ni-base alloys showed good oxidation resistance with low weight gains and formed mainly protective chromia layers. For alloys 230 and 617, NiO and the nickel-rich spinel formed above the Cr2O3 layer together with Al2O3 underneath the Cr2O3 layer at both temperatures. The difference is that 230 formed an internal SiO2 layer, whilst Co was integrated inside the outer oxide layer on alloy 617. The other difference is that in addition to Ni and Fe, for alloy 230, W was found inside the chromium-rich oxide scale but for alloy 617 it was Co. For both alloys, as the temperature increased to 850 °C, Mn diffused into the Cr-rich oxide scale. For alloy 601, almost no Fe and Ni but Mn and Ti diffusion outside was observed, and the Cr-rich spinel and Al2O3 formed underneath at both temperatures.
Table II. Summary of oxidation products of Ni-base alloys reacted in Ar-20CO2 at 750 °C and 850 °C.
| 750 °C | 850 °C | |||||
|---|---|---|---|---|---|---|
| Alloy | Outer | Inner | IOZ | Outer | Inner | IOZ |
| 230 | NiO + (Mn,Cr,Fe)Ox | Cr2O3 + (Cr, W)Ox | Al2O3/SiO2 | (Ni,Fe,Mn)Ox | (Cr,Mn)2O3 + Alloy particles | Al2O3/SiO2 |
| 617 | (Ni,Co,Fe)Ox | (Cr,Ti)Oy | Al2O3 | (Ni, Co, Fe)Ox | (Cr, Mn)Oy + Alloy particles | Al2O3 |
| 601 | (Mn,Ti)Ox | (Cr,Ti,Mn)2O3 + Alloy particles | Al2O3 | (Mn, Ti)Ox | (Cr,Ti,Mn)2O3 + Alloy particles | Al2O3 |
At 750 °C, carburization was not apparently increased after 500 h reaction for both 617 and 601 Ni-based alloys but was detected for alloy 230 after 500 h reaction. At 850 °C, alloy 230 had obvious carburization, and alloy 617 was mainly carburized along grain boundaries. Some fine carbide precipitates were found in alloy 601.
The following discussion will evaluate the corrosion behavior in Ni-base alloys, and then discuss different morphologies of oxides formed. The influence of oxidation temperature on different alloys and carburization of these Ni-based alloys are also discussed.
Critical level of Cr for chromia formation
The critical concentration of Cr for the transition from internal to external chromia can be calculated according to Wagner's theory: 14
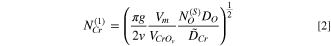
Here g is a critical volume fraction of oxide precipitate, generally approximated as 0.3,
15
 = 1.5 is the stoichiometric coefficient for CrO1.5, Vm
= 6.7 and
= 1.5 is the stoichiometric coefficient for CrO1.5, Vm
= 6.7 and  = 14.6 cm3 mol−1 are the molar volumes of alloy and oxide, respectively,
= 14.6 cm3 mol−1 are the molar volumes of alloy and oxide, respectively,  is the oxygen solubility (mole fraction) in the alloy in equilibrium with the oxygen activity at the scale–alloy interface, DO the diffusion coefficient of oxygen in the nickel alloys,
16
and
is the oxygen solubility (mole fraction) in the alloy in equilibrium with the oxygen activity at the scale–alloy interface, DO the diffusion coefficient of oxygen in the nickel alloys,
16
and  is the alloy interdiffusion coefficient of Cr.
17
is the alloy interdiffusion coefficient of Cr.
17
 was calculated from the measured tracer diffusion coefficients for Cr + 65 wt% Ni and since the three Ni-based alloys have similar Cr and Ni content, the values of
was calculated from the measured tracer diffusion coefficients for Cr + 65 wt% Ni and since the three Ni-based alloys have similar Cr and Ni content, the values of  are assumed to be the same.
are assumed to be the same.
For dissolution of oxygen in the alloy:
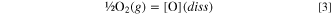
and values of  could be calculated by Sievert's equation:
could be calculated by Sievert's equation:
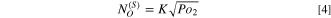
Here K is the equilibrium constant for Eq. 4 and available from,
18
and  is the partial pressure of oxygen set by the NiO–metal interfacial equilibrium
is the partial pressure of oxygen set by the NiO–metal interfacial equilibrium
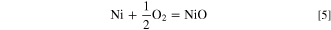
the equilibrium constant for which is obtained from standard thermodynamic data. 18
Calculated values of critical Cr contents at different temperatures are summarized in Table III. The results show that Ni-based alloys ( for alloy 230,
for alloy 230,  for alloy 617,
for alloy 617,  for alloy 601) are marginal to form a protective chromia oxide layer at 750 °C but all alloys would form the chromia at 850 °C. All three alloys developed a protective chromia oxide layer and small weight gain was observed after a long exposure even at 750 °C. This observation could be attributed to additional protection due to other element effects, e.g. the addition of Al, Mn and Si which will be discussed in the next section.
for alloy 601) are marginal to form a protective chromia oxide layer at 750 °C but all alloys would form the chromia at 850 °C. All three alloys developed a protective chromia oxide layer and small weight gain was observed after a long exposure even at 750 °C. This observation could be attributed to additional protection due to other element effects, e.g. the addition of Al, Mn and Si which will be discussed in the next section.
Table III. Calculated critical chromium contents at 750 °C and 850 °C by Wagner's theory and the parameters for the calculation.
| T (°C) |

|
 (cm2 s−1) (cm2 s−1) |
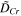 (cm2 s−1) (cm2 s−1) |

|
|---|---|---|---|---|

|

|

|

| 0.26 |
| 850 |

|

|

| 0.22 |
It should be mentioned that above oxygen permeability calculation considers the equilibrium condition of Ni/NiO which is the dominant situation at the early stage of the reaction. However, this situation could be replaced later when NiCr-spinel is formed, leading to the reduced oxygen permeability and therefore a lowered  In practical condition, the formation of these Cr-rich oxides could cause the depletion of Cr underneath the Cr-rich oxide layer, offsetting the beneficial effect of the reduced
In practical condition, the formation of these Cr-rich oxides could cause the depletion of Cr underneath the Cr-rich oxide layer, offsetting the beneficial effect of the reduced  By this consideration, above
By this consideration, above  calculation based on Ni/NiO equilibrium is the up limit of Cr concentration required for the transition from the internal to external oxidation of chromia.
calculation based on Ni/NiO equilibrium is the up limit of Cr concentration required for the transition from the internal to external oxidation of chromia.
Furthermore, the second critical level of chromium  was applied to determine whether there was sufficient chromium concentration to maintain chromia oxide layer growth:
19
was applied to determine whether there was sufficient chromium concentration to maintain chromia oxide layer growth:
19
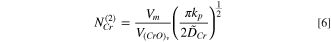
Here  is the parabolic rate constant for chromia layer thickening, which could be shown as:
is the parabolic rate constant for chromia layer thickening, which could be shown as:
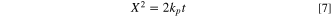
where  represents the scale thickness of chromia layer and
represents the scale thickness of chromia layer and  is time. The calculated values of
is time. The calculated values of  in Table IV indicated that only 617 alloy satisfies the condition for maintaining the growth of chromia scale at 750 °C, but the other two are marginal. However, all alloys at 850 °C satisfy the condition for maintaining the growth of chromia scale, which is consistent with the experimental results. As the commercial alloys contain several other alloy elements, these alloy elements could provide additional protection.
in Table IV indicated that only 617 alloy satisfies the condition for maintaining the growth of chromia scale at 750 °C, but the other two are marginal. However, all alloys at 850 °C satisfy the condition for maintaining the growth of chromia scale, which is consistent with the experimental results. As the commercial alloys contain several other alloy elements, these alloy elements could provide additional protection.
Table IV. The second critical chromium contents at 750 °C and 850 °C.
| T (°C) | 230 | 617 | 601 | |
|---|---|---|---|---|
| 750 |
 cm2 s−1 cm2 s−1
|

|

|

|

| 0.29 | 0.22 | 0.27 | |
| 850 |
 cm2 s−1 cm2 s−1
|

|

|

|

| 0.10 | 0.18 | 0.22 |
Effect of minor alloy elements on oxidation
Effect of Al
As discussed above, although the calculated results at 750 °C indicated that for all three alloys, only alloy 617 meets the conditions that can form a protective chromium oxide layer. However, the experimental results indicate the formation of a protective chromia layer with a small weight gain for all three alloys, showing a good oxidation resistance. This result could be related to the formation of Al oxides at the interface between the chromia and the matrix. According to Abbasi et al., 20 the addition of Al up to 2.4 wt% leads to reducing weight gains, and the formation of Al oxides along the boundary under chromia layer is responsible for the improved oxidation behavior. The diffusion of Cr and O ions is restricted by Al2O3, which reduces the thickness of Cr2O3 layer.
Wagner's theory is applied to calculate the critical level of Al for the formation of the protective Al2O3 layer on Ni-base alloys. The calculated results are shown in Table V, where all parameters for the calculation were obtained from Gust et al.
21
The solubility of oxygen was calculated based on the  at the equilibrium of Cr/Cr2O3 at the reaction temperatures. The calculated results show that an Al2O3 layer could form for both 601 (
at the equilibrium of Cr/Cr2O3 at the reaction temperatures. The calculated results show that an Al2O3 layer could form for both 601 ( ) and 617 (
) and 617 ( ) but could not form for 230 (
) but could not form for 230 ( ) at 750 °C, and all alloys could form an Al2O3 layer at 850 °C. This is basically consistent with the experimental results as shown by the formation of thin alumina layer, e.g. for alloy 617 (Fig. 8g). At 750 °C, the alloy 617 seemed to form densely packed Al2O3 precipitates (Fig. 7g), but relatively less densely packed Al2O3 particles were observed in the alloy 230 (Fig. 4h), confirming an increased tendency for alumina formation with the increasing temperature predicted by the calculation (Table V). For alloy 601 with relatively higher Al concentration, an obvious Al2O3 layer was formed beneath a chromia layer even at 750 °C (Fig. 10g). At 850 °C, large chromia precipitates penetrated deeply into the substrate, probably along the grain boundaries (Fig. 11g).
) at 750 °C, and all alloys could form an Al2O3 layer at 850 °C. This is basically consistent with the experimental results as shown by the formation of thin alumina layer, e.g. for alloy 617 (Fig. 8g). At 750 °C, the alloy 617 seemed to form densely packed Al2O3 precipitates (Fig. 7g), but relatively less densely packed Al2O3 particles were observed in the alloy 230 (Fig. 4h), confirming an increased tendency for alumina formation with the increasing temperature predicted by the calculation (Table V). For alloy 601 with relatively higher Al concentration, an obvious Al2O3 layer was formed beneath a chromia layer even at 750 °C (Fig. 10g). At 850 °C, large chromia precipitates penetrated deeply into the substrate, probably along the grain boundaries (Fig. 11g).
Table V. Calculated critical aluminum contents at 750 °C and 850 °C by Wagner's theory and the parameters for the calculation.
| T (°C) |

|

|
 cm2 s−1 cm2 s−1
|
 Al, cm2 s−1
Al, cm2 s−1
|

|
|---|---|---|---|---|---|
| 750 | 12.9 |

|

|

|

|
| 850 | 12.9 |

|

|

|

|
Effect of Si
According to Huntz et al., 22 the role of silicon is to form silica by diffusing to the alloy surface in the early stage of oxidation, reducing the oxygen activity, unstablising iron and nickel oxides. When the oxidation continues, a duplex scale of Cr2O3 and SiO2 layers forms, improving the oxidation resistance. The experimental results confirm formation of SiO2 internal precipitates beneath a chromia layer (Fig. 4i). Further work by Evan et al. 23 revealed that, this silica layer also acts as a partial barrier to slow down the growth rate of the chromium oxide layer.
Furthermore, the addition of silicon can change the diffusion characteristics of the alloy subsurface area. Johnston
24
found that  increased by around 20% after adding silicon in Ni-Cr-Si alloys. Hence another explanation proposed by Li and Gleeson
25
was that the addition of silicon expands the atomic volume of Ni-based alloy resulting in the weakening of the metal bond strength and increasing the alloy lattice parameters. These factors will contribute to increasing
increased by around 20% after adding silicon in Ni-Cr-Si alloys. Hence another explanation proposed by Li and Gleeson
25
was that the addition of silicon expands the atomic volume of Ni-based alloy resulting in the weakening of the metal bond strength and increasing the alloy lattice parameters. These factors will contribute to increasing  The higher alloy interdiffusion coefficient of Cr could provide more Cr to compensate for the depletion of Cr due to the formation of chromia layer, thus promoting the regeneration of chromia layer and increase the oxidation resistance.
The higher alloy interdiffusion coefficient of Cr could provide more Cr to compensate for the depletion of Cr due to the formation of chromia layer, thus promoting the regeneration of chromia layer and increase the oxidation resistance.
The concentrations of Si (0.4 wt% for 203, 0.04 wt% for 617 and 0.2 wt% for 601) are rather low. A clear presence of silicon rich oxide was found for only alloy 203 with the highest Si concentration (Fig. 4i). The beneficial effect of this silica sublayer has been reported extensively by our group 26 and also many other researchers. 27 Based on the results in this work, it seems the minimum Si concentration for silica formation in this reaction condition should be ≥0.4 wt% for Ni-based alloys.
Effect of Mn
Douglass and Armijo 27 reported that even though MnO is more stable than chromium oxide, manganese has a higher tendency to form a continuous and dense manganese-rich oxide spinel with lower oxygen activity by the following equation:
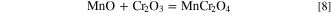
Furthermore, Naoumidis
28
proposed that diffusion coefficient of manganese in chromium oxide ( cm2 s−1 at 750 °C and
cm2 s−1 at 750 °C and  cm2 s−1 at 850 °C) is faster than that in alloys (
cm2 s−1 at 850 °C) is faster than that in alloys ( at 750 °C and
at 750 °C and  cm2 s−1 at 850 °C).
29
This process can be expressed as follows:
cm2 s−1 at 850 °C).
29
This process can be expressed as follows:
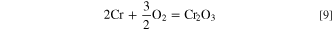
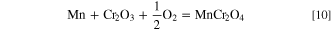
Due to the rapid diffusion of Mn through the chromia lattice, Mn-rich oxide formed in the outermost scale layer on the top of Cr-rich oxide layer for both alloys 230 and 601 (Figs. 4f, 5g, 10f, 11f) with relatively high Mn concentrations (0.5 and 0.61 wt%, respectively). The 617 alloy contains very little Mn content (0.04 wt%) which is not enough for Mn-rich oxide on the top of Cr-rich oxide layer. However, diffusion of manganese through the chromia layer was observed with increasing temperature to 850 °C (Fig. 8f). The low-valent Mn in the manganese-rich oxide layer will occupy the electron vacancies in the chromium oxide and play a role in reducing the concentration of defects, thereby improving the oxidation resistance.
Effects of other alloying elements
In general, all three Ni-based alloys have a good oxidation resistance, forming a protective chromia scale. For alloys 230 and 617, Ni-rich oxide containing some iron was observed on top of the Cr-rich oxide layer (Figs. 4, 5, 7 and 8). This situation could be attributed to the rapid diffusion of Ni and Fe ions through the chromia layer. An explanation was proposed by Yi et al.
30
that the outward migration of Ni is due to the release of stress by the addition of Al and Si during their internal oxidation process. Two different contrasts of oxide morphologies could be well observed by SEM in Figs. 5b and 8a. The bright contrast outermost layer was Ni-rich oxide and the dark contrast layer next to the matrix was Cr-rich oxide. From the XRD results in Fig. 2, Ni-rich oxide layer could be explained as consisting of a small amount of NiO particles on the outmost surface and (Fe, Ni, Cr)3O4 spinels underneath. It has been reported
31
that for this process, Cr2O3 forms first in the early stage and Ni and Fe ions diffuse along the grain boundary to form spinel and then NiO grows on the top of (Fe, Ni, Cr)3O4 spinels. For alloy 617, some Co diffuses outside, co-existing with the upper part of Cr-rich scale (Fig. 8h). This observation is explained by the fact that Co has much higher mobility than that of Ni ions in the oxide lattice which leads to the higher Co concentration near the scale-gas interface (


 cm2 s−1).
32–34
cm2 s−1).
32–34
It is noted that alloy 230 contains up to 14% W. Some studies have reported that the addition of W reduces the oxidation resistance of Ni-base alloys. 35,36 Because W is a high valance element, when these metal ions were mixed with Cr2O3, the metal vacancies increased and then the diffusion rate of other metals through the chromia layer increased. 37 As a high valence element with a low diffusion rate, W could also retard the formation of Cr2O3 because of low oxygen activity. However, the large size of W could slow down the diffusion of other elements including the Al2O3 formation. 38 Therefore, the effect of W on oxidation is not clear in this research. The experimental results (Fig. 4g) show (W, Cr)-rich oxides in the matrix because of the inward diffusion of oxygen and no outward diffusion of tungsten. Furthermore, because the 230 alloy contains low content of Al, it is not clear if W slowed the formation of Al2O3. However, it was observed that more Ni, Fe and Mn diffused into the outermost layer of the chromia layer.
Unlike 230 and 617, alloy 601 performs differently. Only a small amount of nickel and iron diffusion was observed, and no NiO formed. This is because the addition of a higher amount of chromium and aluminum increases its oxidation resistance. 39 Furthermore, the outward diffusion of Ti and Mn was evident in the experiment results (Figs. 10h, 10f, 11h and 11f). The increase in temperature is conducive to the formation of titanium oxide at the position of the external interface but less inside the chromia layer. Lenglet et al. 40 have explained that Ti oxides formed at the external interface proved that Ti diffuses through the chromia scale. Meanwhile, Naoumidis et al. 28 explained that the high concentration of Ti dissolved in chromium oxide indicates that lattice diffusion could be responsible for the growth of Ti-rich oxides in the outer layer. Some authors 41,42 also proposed that titanium promotes the formation of additional cation vacancies in the chromium oxide scale when Ti4+ ions replace Cr3+ ions. Since the matrix bulk cation diffusion and grain boundary control the growth of the chromium oxide layer, the increase of cation vacancies in the chromia scale will result in faster chromium diffusion and higher kinetic rates. 43
Figure 13 schematically shows these element effects by classifying them into two categories, in general. The first category is able to form sublayered oxides (e.g. Al and Si). The second is able to integrate with chromia and to form even an additional spinel layer on the top of chromia, when the concentration is high. All these additional oxide formations increase the corrosion resistance of the chromia-forming alloys as discussed above.
Figure 13. Schematic diagram summarising the varied alloying effects on surface oxide formation.
Download figure:
Standard image High-resolution imageEffects of temperature on oxidation behavior
A comparison of oxide thickness at different temperatures in Table VI reveals that as the temperature increased, the scale thickness also increased. So, the increase in oxidation rate was observed with increasing temperature. Only the alloy 601 was observed with the formation of a thin Al2O3 layer in the IOZ at 750 °C, but a significant amount of aluminum oxide was observed in the internal oxidation zone at 850 °C. The huge volume expansion due to internal oxide formation generates high stress in the lattice of the alloy matrix. The stress relief process led to the outward transport of the alloy from the IOZ. 44 Therefore, the alloy particles were observed at the interface between the IOZ and the external oxide layer at 850 °C for all Ni-base alloys (Figs. 3b, 5b, 6, 8a, 9, and 11a). The morphology of most of the oxides was similar at both temperatures. Even though the diffusion of some elements such as Mn, Ti, Al was not observed by SEM but can be revealed by TEM analysis at a higher magnification. It is worth noting that no outward diffusion of Mn was observed in alloy 617 at 750 °C even under TEM observation. However, as the oxidation rate increases, Mn diffuses outward through the chromia layer. Therefore, diffusion of Mn into the chromia layer was observed at 850 °C.
Table VI. Oxide layer thickness (μm) after 500 h reaction at 750 °C and 850 °C.
| Alloy | Outer layer | Inner layer | IOZ | |||
|---|---|---|---|---|---|---|
| 750 °C | 850 °C | 750 °C | 850 °C | 750 °C | 850 °C | |
| 230 | 1.2 ± 0.1 | 1.4 ± 0.3 | 1.1 ± 0.2 | 2.1 ± 0.4 | 0.2 ± 0.1 | 1 ± 0.2 |
| 617 | 0.8 ± 0.2 | 1 ± 0.7 | 0.7 ± 0.2 | 2.7 ± 0.2 | 0.7± 0.2 | 4.8 ± 2.1 |
| 601 | 0.9 ± 0.1 | 1 ± 0.1 | 1.8 ± 0.4 | 4.1 ± 0.8 | 3.1 ± 0.1 | 11.2 ± 4.2 |
Carburization
Except primary carbides from raw alloys, almost no newly formed carbides were found after 500 h exposure in Ar-20CO2 at 750 °C for both 617 and 601, but some carbide formation was observed along the grain boundary of alloy 230. At 850 °C, the newly formed carbides were found in all three Ni-base alloys after the reaction. According to ThermoCalc calculation, the volume fraction of primary carbides is expected to be decreased from 750 °C to 850 °C. Therefore, the increased carburization observed at 850 °C should be contributed by the CO2 gas.
In addition, other alloying elements also affect the diffusion of carbon. The beneficial effect of Al on decreasing carburization was reported by Searcy et al. 45 The high binding energy of Al2O3 results in a low rate of atomic mobility within it. So Al2O3 could be accounted as a good barrier to hinder carbon diffusion into the matrix. In this work, the formation of Al2O3 was observed in all three alloys, resulting in a reduction in carburising kinetics and the density of carbide precipitates. Meanwhile, the additional formation of silicon and manganese oxides decreases the diffusion of carbon. 46,47 In addition, the protective effect of alumina, silica, chromia and manganese oxide is attributed to their very low solubility of carbon, 48 and therefore low carbon permeability in these oxides. As a result, they provide a barrier effect to resist carbon penetration.
Conclusions
Three Ni-based commercial alloys were reacted in Ar-20CO2 at 750 °C and 850 °C, and their corrosion behaviors at both temperatures were examined. All three alloys showed a good oxidation resistance by forming a protective oxide scale, with low weight gains. The internal Al2O3 precipitates formed beneath a thin chromia layer for three alloys. For alloys 230 and 617, NiO and Cr-rich spinel were formed at the outermost layer, but for 601 less iron and nickel outward diffusion was observed at both temperatures. Furthermore, some other elements were also observed to diffuse into the chromia layers of these Ni-base alloys such as Mn, Ti, and Co.
Wagner's theory was applied to examine the critical chromium concentration for forming a protective chromia. This prediction indicated marginal concentrations for chromia formation at both temperatures for the test alloys. The observation of protective chromia formation can be attributed to the effect of some other alloying elements, e.g. Al, Mn, Ti, Si etc Because of the temperature effect on diffusion, the predicted result from Wagner's theory showed that the critical chromium concentration needed to form and maintain a protective chromia layer decreased with increasing the oxidation temperature, which is consistent with the experimental observation.
The Al and Si oxides formed an additional protective barrier between the matrix and the chromia layer, preventing the diffusion of iron and nickel outwards and therefore increasing the oxidation resistance. Manganese reduced oxidation and carburization by combining with chromium oxide to form the outermost spinel oxide layer, increasing corrosion resistance. Titanium diffused through the chromia layer, accelerating chromium diffusion and obtaining higher kinetic rates. The effect of tungsten is unclear from this work but could affect oxidation kinetics by increasing the metal vacancies.
No significant carbide formation was found for Ni-based alloys because of very slow carbon diffusion in Ni-based alloys. At 750 °C, carburization due to reaction was found for alloy 230 but not for alloys 617 and 601 after 500 h reaction. The variation of carburization for different alloys can be attributed to the formation of other oxides of aluminum, manganese and silicon which can impede the inward diffusion of carbon resulting in the reduction of carburizing kinetics. However, increasing temperature to 850 °C, carbide formation was observed for all three alloys.
Acknowledgments
Financial support by Australia Research Council under the Discovery Program is highly acknowledged.














