Abstract
The performance of electrocatalytic CO2 reduction (CO2RR) depends not only on the catalytic material but also on the neighbouring chemical environment around the active sites. The surrounding local environment can perturb the electronic properties of active sites and alter the adsorption/desorption behaviour of reactant/intermediate/product, thus changing CO2RR characteristics. Herein, we studied electrochemical reduction of CO2 onto supported atomically precise [Au9(PPh3)8](NO3)3 clusters and observed an unusual increase in catalytic activity over time. Additionally, electrochemical activation of the electrodes by applying a more negative potential was found to improve activity of the electrode. Investigations using UV–vis and X-ray absorption spectroscopy revealed that these observations may be attributed to the interaction of the Nafion ionomer with the catalytic Au9 clusters. These interactions may cause partial blocking of the Au9 active sites, and the prolonged application of negative potentials leads to favourable interface reconstructions. In addition, a method was developed to minimise the interaction between the Au9 clusters and Nafion ionomer by first depositing a layer of carbon black followed by dropcasting the active catalyst. Our study highlights that polymeric binders modulate the electronic properties of the electrocatalysts, which can change the product distribution during CO2 electrolysis.

Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Electrochemical reduction of CO2 to fuels and chemical feedstocks using renewable energy resources can enable the recycling of CO2 in a carbon-neutral energy cycle. 1,2 Various metal electrodes have been studied for the electrochemical reduction of CO2, producing a range of products such as CO, formate, methane, ethane, ethene, methanol, etc. 3 The selectivity of products on a catalyst surface depends upon its ability to adhere to intermediates and relative overpotentials for the competing reactions. 4–6 Gold cathodes have been reported to produce CO selectively at a relatively lower overpotential, along with formate as a minor product. 7–9 To improve the CO2 reduction reaction (CO2RR) performance, materials with high surface area and tunable morphology and composition have been prepared and studied. 10
Recently, sub-nanometer metal clusters have attracted considerable interest in sensing and catalysis due to their size-dependent electronic, optical, and chemical properties. 11–15 Due to their well-defined size and composition, the size-selected clusters can be considered as "model catalysts" for understanding the catalytic process at the molecular level. 16 In CO2RR, Au25(SR)18 nanoclusters were found to selectively reduce CO2 into CO at an overpotential of 90 mV and demonstrated the peak production rate with nearly 100% selectivity towards CO at −1.0 V vs RHE. Depending upon the applied potential, the CO production rate was 7–700 times higher for Au25 clusters-based catalyst compared to the carbon-supported plasmonic gold NPs (diameters ∼2–5 nm). 14 Furthermore, the addition or substitution of one or more atoms is shown to substantially alter the catalytic activity and selectivity of the clusters for CO2RR. 17,18 It is also possible to modulate the electronic and chemical properties of clusters through the peripheral ligands, resulting in changes in the reactivity of clusters. For example, Narouz et al. demonstrated that the substitution of phosphine (PPh3) ligands with N-heterocyclic carbenes could improve CO2 to CO selectivity on [Au11(PPh3)8Cl2]Cl clusters. 19 Minor changes in catalyst composition could alter the catalytic performance of clusters; for example, the charge state of [Au25(SR)18]q (q = −1, 0, +1) species was found to influence the adsorption of reactants and intermediates, thereby modulating the selectivity of the reaction. Kim et al. demonstrated that the Au25 clusters-based catalyst could be employed in large-scale electrolysers to achieve current density up to 540 mA cm−2 (at −0.8 V vs RHE) and stable operation up to 3000 h. 20 Likewise, Au137(SR)56, [Au22H3(dppe)3(PPh3)8]3+, [Au11(dppp)5]3+, Au55(p-MBT)24(Ph3P)6](SbF6)3 and various Au and other metal clusters have shown promises to selectively reduce CO2 at lower noble metal loading. 20–23
Generally, for application in electrocatalysis, the clusters are immobilised onto a support, and their activity is accessed by dispersing the supported catalyst with a binder and casting it onto a working electrode substrate. 24 Although precise synthesis and characterisation of sub-nanometer Au clusters is possible, these clusters may undergo various structural and electronic rearrangements during electrode preparation steps, such as during immobilisation onto a support, 25 interactions with a polymer binder, 26 and sintering during solvent removal. 25 Ideally, electrode binders should be inert polymer matrices that act as both a physical binder for catalyst particles and a proton conduit that allows the conducive transport of reactants and products. However, the binders used in a catalytic layer can alter CO2RR characteristics by both modifying the catalyst surface and changing the local environment of the catalyst embedded in the porous network. 2 The binder may contain functional groups which could perturb the electronic properties of the catalyst, and it may have stabilising or destabilising interactions with the reactant/intermediate/product, leading to a change in the intrinsic activity of the catalyst. 27 For example, it is previously reported that the Nafion ionomer, one of the most commonly used binders, is adsorbed onto Au and Pt surfaces via the sulphonate group and can change the electrochemical behaviour of the surfaces as observed by cyclic voltammetry. 28 Also, it has been found that the Nafion ionomer influences the reaction environment and slows the CO oxidation at the Pt(111) surface. 29 The reactants and products have to diffuse through the ionomer film to reach the active catalyst; this additional mass transport limitation affects the electrochemical process on the catalytic surface. 30,31 Recently, the method of incorporating the Nafion ionomer into the catalytic layer was found to influence the hydrogen evolution performance of reduced graphene oxide supported Au clusters, 32 with electrodes prepared using an ink containing supported Au clusters and Nafion ionomer showing poorer activity compared to the electrodes prepared by first casting supported clusters and then adding the Nafion ionomer, likely due to blockage of active sites pores or surface area. 32 For CO2RR, Andrews et al. 26 studied electrocatalytic CO2 reduction on Au25 clusters and 5 nm Au NP using Nafion and polyvinylidene fluoride (PVDF) binders. The authors found that electrodes containing Nafion binder exhibited higher selectivity for CO2 to CO conversion and a lower onset potential. X-ray photoelectron spectroscopy (XPS) measurements indicated that the sulfonate group could alter the electronic properties of Au and can result in favourable adsorption energy of the reaction intermediates. Lee et al. have demonstrated that the functional group of the polymeric binders can modify the catalyst surface properties and thus can significantly affect electrochemical CO2 reduction on the gold surface. 27
Despite the significant quantity of binder in catalyst layers (5%–30%) and the aforementioned experimental evidence, its role in CO2RR electrocatalysis has not been understood in depth. Additionally, very little has been studied about the interaction between the Nafion ionomer and the active catalyst, which is an important parameter for the design of an efficient CO2RR system. Here we investigate the influence of Nafion ionomer in the electrochemical activity of carbon-supported [Au9(PPh3)8](NO3)3 clusters (herein referred to as Au9) under potentiostatic conditions and use UV–vis spectroscopy and X-ray absorption spectroscopy to study the interactions between the Nafion ionomer and the Au9 clusters. A method to improve the activity of the catalyst was developed by electrochemically activating the catalyst at a more negative potential. Furthermore, the influence of the interactions was minimised by first spraying a layer of Vulcan carbon support, followed by drop-casting the Au9 clusters to create the electrocatalytically active electrode.
Experimental
Electrode with pre-deposited Au9 on Vulcan carbon (Au9/C electrode)
The clusters were deposited on carbon black (>99%, Cabot Vulcan XC-72R) following the previously reported methods. 33,34 Briefly, 2 g Vulcan carbon was dispersed in dichloromethane (DCM) (ca. 40 ml) via sonication and stirred under a nitrogen atmosphere for 2 h in a Schleck flask. [Au9(PPh3)8](NO3)3 clusters were dissolved in DCM (ca. 20 ml). The calculated amount of DCM solution was added to the stirring dispersion to achieve a 10% monolayer of Vulcan carbon surface (Fig. SI4a). The dispersion was stirred for 2 h; the clusters were observed to be deposited on the carbon support, as the colour of the mother liquor changed towards colourless. The solvent was then evaporated under reduced pressure using a Schlenk line, and the product was collected and stored in the fridge, avoiding exposure to light.
The cathode for electrochemical CO2 reduction studies was prepared by spraying an electrocatalyst ink onto a carbon felt (AvCarb C100, area 3.141 cm2). 24 The ink suspension was prepared by sonicating the ethanol and water mixture containing 1 mg ml−1 Au9/C catalyst and Nafion ionomer (5 wt%, 10 wt%, 20 wt%, and 30 wt%). The calculated amount of Nafion ionomer (Equation SI1) was added from a 10 mg ml−1 stock solution in ethanol. The catalytic ink was sprayed at 0.15 ml min−1 using an ultrasonic atomiser with a constant flow of compressed air onto a carbon felt placed on a hotplate at 120 °C (Fig. SI4b). The carbon felt was further heated at 120 °C for 30 min after spraying to remove traces of solvent. This procedure formed a uniform electrocatalytically active layer on carbon felt with different Nafion content. A uniform sprayed layer onto the carbon felt was obtained with a catalytic loading of 0.84 ± 0.06 mg cm−2 (measured gravimetrically).
Preparation of electrode by drop-casting Au9 onto a carbon black layer
The cathodes for electrochemical CO2 reduction studies were prepared by spraying carbon black onto a carbon felt followed by drop-casting of [Au9(PPh3)8](NO3)3 clusters from DCM (Fig. SI4c). Briefly, the sprayed layer of carbon black onto a carbon felt was prepared, following the abovementioned method. The carbon felt was heated at 120 °C for 30 min after spraying to remove traces of solvent. The carbon felt was allowed to cool to room temperature, and Au9 clusters were then drop-casted from a 3 mg ml−1 solution of Au9 clusters in DCM. The electrode thus prepared was dried in a vacuum overnight to remove traces of DCM. These electrodes will be referred to as the Au9 DC electrode.
Electrochemical CO2 reduction studies
Electrochemical reduction of CO2 was carried out on a custom-made three-electrode flow cell. The cell consists of an Au clusters-based cathode, a Pt plate as the counter electrode, and a Ag|AgCl (saturated KCl) reference electrode. Constant potential electrolysis of CO2 was carried out at −1.3 V vs Ag|AgCl and −1.7 V vs Ag|AgCl reference electrode using a Gamry Reference 3000 potentiostat. 0.2 M KHCO3 (Sigma-Aldrich, 99.7%), used without any purifications and prepared with 18.2 MΩ cm deionised water, was used as catholyte. The electrolyte was bubbled with CO2 gas at 20 ml min−1 during the experiment using a mass flow controller Alicat, MC-20SCCM-D. To ensure that the electrolyte was saturated with CO2, purging was started almost 30 min before the experiment. The CO2 saturated KHCO3 catholyte was recirculated from a 100 ml reservoir at 20 ml min−1 through carbon felt, and the flow channels machined on the graphite block using a peristaltic pump (LongerPump® YZ1515x). The counter electrode and anolyte (1 M KHCO3) were separated from the working compartment with a fine porous frit. The reference electrode was held in a lugging capillary placed in the anode chamber. Nafion 117 membrane was used to separate the anode and the cathode chamber along with the flow of the proton. Electrochemical impedance spectroscopy was studied to determine the resistance between the cathode and reference electrode; this resistance was then compensated using positive feedback during potentiostatic electrochemical reduction. All the electrochemical measurements were carried out at room temperature.
The headspace of the electrolyte reservoir was vented directly to a gas chromatograph (SRI 8610C) equipped with a 6" haysep-D column, TCD, and methanizer-FID detectors to quantify H2 and CO during the electrolysis. Samples were injected into the column through the sampling loop continuously every 15 min. The liquid products of CO2 electrolysis were analysed by High-Performance Liquid Chromatography (Thermo Scientific™ UltiMate™ 3000) using the electrolyte after completion of the experiment.
X-ray absorption spectroscopy measurement and processing
X-ray absorption spectroscopy (XAS) measurements on clusters were carried out in fluorescence mode at the Australian Synchrotron in Clayton, Victoria, Australia, using a multipole wiggler XAS beamline (12-ID) operating with an electron beam energy of 3.0 GeV and a beam current of 200 mA (maintained using the top up mode). The Au-L3 data were collected using a Si(111) monochromator and focusing optics, and the measurements were performed at 10 K helium cryostat.
Raw data obtained at the beamline was converted using Sakura software 35 and processed using Athena software 36 by extracting the EXAFS oscillation χ(k) as a function of photoelectron wave number (k) following standard procedures. For all the clusters, the k-range was chosen from 2.5 to 15 Å–1. Fittings of EXAFS were performed using a fuzzy degeneracy approach, which allows grouping the path length in a bin of user-defined width. 37,38 The contribution of smaller hydrogen atoms in the EXAFS spectra is expected to be very weak due to the low scattering power of this species; thus, it is ignored in the FEFF calculations. 39 More details on the analysis of the XAS spectrum are given in the supplementary information.
Results and Discussion
Electrocatalytic reduction of CO2 on gold clusters (Au9/C)
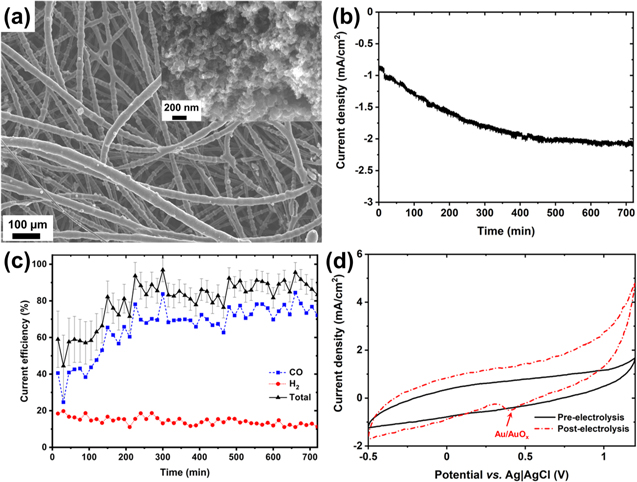
Atomically precise clusters ([Au9(PPh3)8](NO3)3) were synthesised following the reported procedure and deposited onto Vulcan carbon support. 33 More details on the synthesis, purification and characterisation of pure clusters are provided in the supplementary information. The electrodes were prepared by spraying Au9/C catalyst with 5 wt% Nafion (Fig. SI4b), with the carbon fibres found to coat uniformly with a layer of Au9/C aggregates (Fig. 1a). This uniform deposition over the carbon fibres within the felt allows for easy electrolyte penetration and gas release during CO2 electrolysis. The SEM image (Fig. 1a, inset) clearly shows that nanometer-sized carbon aggregates containing Au9 clusters which are embedded in the Nafion matrix forming a uniform network conducive to electron transport.
Figure 1. (a) Scanning electron microscopic (SEM) image of electrocatalytic layer prepared by spraying Au9/C with 5 wt% Nafion ionomer; Potentiostatic (−1.3 V vs Ag|AgCl) CO2 reduction on the Au9/C catalyst with 5 wt% Nafion in the catalytic layer: (b) current density, (c) gaseous product distribution for CO2 reduction, and (d) CV before and after 12 h of electrolysis experiment; in 0.2 M KHCO3 saturated with CO2.
Download figure:
Standard image High-resolution imageThe activity of Au9/C catalyst deposited carbon felt was assessed using potentiostatic reduction at −1.3 V vs Ag|AgCl (sat. KCl) in a three-electrode system. CO and H2 were major products for the electrolysis (Fig. 1c), along with a small fraction of formate, consistent with other gold-based cathodes. 7,40–43
The overall current density and CO Faradaic efficiency were observed to improve over time, reaching almost 75% after 12 h of electrolysis (Figs. 1b and 1c). The increase in both current density and selectivity towards CO suggests the availability of more active sites over time. The increase in activity over the electrolysis run is contrary to the previous reports, 44–46 where the catalyst undergoes deactivation during electrolysis due to various reasons such as metal impurities in the present aqueous electrolyte, 47–49 surface poisoning due to chemical species, 50–52 change in surface morphology of catalyst over time. 46,53 It should be noted that in the beginning, the total current efficiency was not closer to 100% (Fig. 1c); this can be the result of the lower current density (< 1 mA cm−2). The production rate for the products will be low for such current densities to be detected by Gas Chromatography efficiently.
Due to ultrasmall size Au9 clusters (ca. 0.8 nm), it is expected that most of the Au atoms are on the surface (ideally, 100% Au atoms are on the surface from the known crystal structure); hence a bigger Au oxide reduction behaviour was expected. Interestingly, the as-made electrode does not show typical Au oxide reduction behaviour 54 in cyclic voltammogram (Fig. 1d), likely due to considerably different oxidation behaviour of molecule-like Au clusters compared to bulk gold or inaccessibility of electrolyte to Au clusters, which may be located in pores of Vulcan XC-72R carbon black. 24,55 However, after 12 h of electrolysis at −1.3 V vs Ag|AgCl, the typical Au/AuOx peak can be seen, suggesting the sintering of at least a fraction of the Au clusters into bulk-like Au nanoparticles (which are known to exhibit the Au/AuOx redox feature), in line with the previous reports. 24,56
Unusually, although Au particles are growing in size during CO2 electrolysis, the catalytic activity is found to increase, suggesting that despite the lower dispersion of gold atoms in the electrode, the activity of the gold sites is increasing. Clearly, the performance of an electrocatalytic layer depends not only on the number of catalytic particles (or active sites) but also on their accessibility to the reactants. Previously it has been found that sulfonate groups in Nafion ionomers get adsorbed onto the Pt surface, resulting in blockage of active sites hence an increased overpotential or poor activity. 29,57–59 Here, the increase in catalytic performance could result from a favourable interface restructuring during prolonged electrolysis.
As the interfacial rearrangements triggered by electrolysis are expected to be accelerated at a higher electrolysis current, the activity of the catalyst was accessed before and after the electrochemical treatment of the electrode at a significantly more negative potential. The electrochemical CO2 reduction was performed at −1.3 V vs Ag|AgCl, then the applied cathode potential was decreased to −1.7 V vs Ag|AgCl for 2 h, and finally, the potential was stepped back again to −1.3 V vs Ag|AgCl (Fig. 2a).
Figure 2. Potentiostatic (at −1.3 V vs Ag|AgCl) CO2 reduction on Au9/C catalyst before and after electrochemical activation at −1.7 V vs Ag|AgCl with different amounts of Nafion in the catalytic layer: (a) the process of electrochemical activation, (b) average CO Faradaic efficiency, and (c) average current density, in 0.2 M KHCO3 saturated with CO2.
Download figure:
Standard image High-resolution imageThe electrochemical activation of the Au9/C electrode is clearly seen to improve both the current density and CO Faradaic efficiency (Figs. 2b and 2c). One explanation for these results is that this activation procedure alters the morphology of the Au9-Nafion interface, thus enabling better reactant access to or less blocking of the active sites. This increase in active site availability is then preserved with the potential returned to −1.3 V vs Ag|AgCl, and thus the restructured catalyst exhibits higher activity. It is worth noting that while the CO Faradaic efficiency before and after electrochemical activation is almost the same irrespective of the amount of Nafion in the catalyst layer, the increase in current density after electrochemical activation was less for the higher amount of Nafion in the electrode (Figs. 2b and 2c). This would be expected for the activation process, which relies on "un-blocking" sites poisoned by Nafion, as a higher amount of Nafion in the layer would make almost certainly make the activation process more difficult.
Investigating the interactions between Au9 clusters and Nafion ionomer
In order to investigate possible interactions between the clusters and Nafion ionomer, methanol solutions containing the clusters and ionomer were prepared. Upon mixing, the characteristic colour of the Au9 clusters in methanol was reduced, and a precipitate was obtained within seconds. UV–vis spectra of the supernatant were recorded to quantitively estimate the fraction of precipitate with a different mass ratio of Nafion ionomer and Au9 clusters (Fig. 3a).
Figure 3. UV–vis absorption spectra of a solution prepared by mixing [Au9(PPh3)8](NO3)3 and Nafion ionomer in the different weight ratios: (a) UV–vis spectra (b) peak absorption of the Nafion-clusters supernatant at 314 nm, in methanol, (c) the XANEs spectra of Au9 clusters and Au9-Nafion precipitate at Nafion to Au9 ratio of 0.75:1 (Inset: the first derivative of the XANES spectra of Au9 clusters and Au9-Nafion precipitate) and (d) magnitude of the Fourier transform of EXAFS of Au9 clusters and Au9-Nafion precipitate at Nafion to Au9 ratio of 0.75:1 (uncorrected for the phase shift).
Download figure:
Standard image High-resolution imageThe Au9 clusters show UV–vis absorption peaks at 443, 375, 352, and 314 nm, consistent with the previous reports. 60,61 After mixing the Au9 clusters with the Nafion ionomer, while the supernatant showed UV–vis absorption peaks at the same wavelengths as that of the pure Au9 cluster solution, the peak intensities decreased. The absorbance at 314 nm (which is proportional to the concentration of Au9 clusters in the solution) reveals that the Nafion: Au9 ratio had a strong influence on the concentration of Au9 clusters that remain in the solution (Fig. 3b). This suggests that the interactions between Au9 clusters and Nafion ionomer lead to the formation of a precipitate, whilst a fraction of clusters remains in solution and that this interaction is strongest at the Nafion:Au9 weight ratio of 0.75:1. This change in the UV–vis spectra of Au9-Nafion mixture is significantly different from previously reported Au25-Nafion system, 26 where the authors observed that one peak shifts to higher wavelength and some of the peaks disappear upon mixing cluster and Nafion ionomer, and this attributed these changes to alteration or agglomeration of intact Au25 clusters.
The precipitate obtained from the Au9 clusters and Nafion ionomer was studied using X-ray absorption spectroscopy. The X-ray absorption near edge structure (XANES) spectra of the Au9 cluster and the Au9-Nafion precipitate are significantly different compared to a metallic Au foil (Fig. 3c). The absorption edge of the Au9 clusters is shifted to a higher energy, and clusters show a higher white line intensity compared to metallic gold, which is in line with previous reports on ultra-small clusters. 62–66 A more detailed discussion comparing XANES of the Au9 clusters with bulk metal has been given in the supplementary information. Interestingly, XANES of the precipitate shows an absorption edge (determined by the peak in the dI/dE spectrum, Fig. 3c, inset) at a lower energy compared to that of the Au9 clusters, and the "white line" for the Au9-Nafion precipitate is less intense compared to Au9 clusters. This is most likely because the interactions between positively charged Au9 clusters (i.e. [Au9(PPh3)8]3+) and negatively charged Nafion ionomer would lower the overall charge per Au atom, resulting in a shift in the absorption edge and lowering the "white line" intensity. 67 The change in electronic properties of clusters was previously observed as the origin of an XPS 4f7/2 shoulder when Au25 clusters mixed with Nafion ionomer. 26 The difference becomes clearer in EXAFS (Fig. 3d), which suggests that the local environment around the Au atoms has changed in the precipitate formed after mixing. The EXAFS of the precipitate was fitted with one Au–O, one Au–P, and two Au–Au bonding environments, and the result suggests a bonding between cluster core and O from the Nafion ionomer (Table I and Fig. SI8). The structure of the Au species in the precipitate has notably distorted compared to pure Au9 clusters; a detailed description of fitting the clusters is given in the supplementary information. Previously, based on the change in XPS and UV–vis spectra, Andrews et al. proposed that mixing ultra-small clusters with Nafion may have resulted in coordination between clusters and the sulphonate group. 26
Table I. Structural parameters EXAFS fitting for Au9-Nafion precipitate.
| Scattering path | Coordination number | R (Å) | σ2 (Å2) |
|---|---|---|---|
| Au–O | 0.40 ± 0.26 | 2.098 ± 0.066 | 0.003 |
| Au–P | 0.73 ± 0.19 | 2.259 ± 0.014 | 0.001 |
| (Au–Au)1 | 5.19 ± 1.14 | 2.709 ± 0.009 | 0.007 |
| (Au–Au)2 | 1.73 ± 0.54 | 2.868 ± 0.027 | 0.006 |
In order to investigate the change in the Au9/C electrodes (prepared using 5 wt% Nafion ionomer) during electrolysis, the Au L3 edge X-ray absorption spectra of the electrodes in as-made form, after potentiostatic (−1.3 V vs Ag|AgCl) CO2 reduction and after electrochemical activation were studied. The gold species in all the electrodes tested had absorption edges (determined by the peak of the derivative spectra) at higher energies compared to the bulk gold but lower than the edge energy observed for the pure Au9 clusters (Fig. 3c). The shift in absorption edge for the electrodes relative to bulk gold suggests the presence of a fraction of Au9 cluster-like species. Moreover, the systematic decrease in the absorption edge shift (compared to bulk gold) of the electrode after electrolysis and after electrochemical activation suggests a decrease in the population of Au cluster-like species in samples. 67–69 The "white line" of all the electrodes is more intense than that of the bulk gold but less intense than that of Au9 clusters (Figs. 4b and SI9a), suggesting that gold species within the electrodes have a higher density of unoccupied d states compared to the bulk gold. 69,70 The XANES features of fcc gold (regions c and d) are visible in the spectra of all three electrodes, but they are attenuated compared to the bulk gold, which is due to the lower average coordination number of Au atoms in the electrode of interest compared to the bulk gold (i.e. CN = 12). 67 The intensity of XANES features at c and d increases after electrolysis and increases further after electrochemical activation, suggesting an increase in bulk gold-like species in the sample population. As previously demonstrated, 34 linear combination analysis using Au9 clusters and bulk gold as the main component can be used to study the agglomeration of clusters (Fig. SI10). Here, we observed that even the as-made electrodes have a fraction of bulk-like gold (∼20%), and the fraction of bulk-like gold (based on the linear combination fitting of the XANES spectra) was observed upon electrolysis, with the electrochemical activation further increasing the bulk-like gold features to 40% (Fig. SI10d). The agglomeration of clusters into bulk-like nanoparticles after CO2 electrolysis is in line with the appearance of the Au/AuOx peak in the cyclic voltammogram after the constant potential electrolysis experiment (Fig. 1b).
Figure 4. Comparison of pre-deposited Au9/C electrodes: as-made, after electrolysis at −1.3 V vs Ag|AgCl for 2 h, and after electrochemical activation (a) normalised XANES spectra, and (b) the magnitude of Fourier transform (FT) of Au LIII EXAFS (uncorrected for the phase shift). FT EXAFS intensity of Au foil was reduced 2.5 times to fit in the figure.
Download figure:
Standard image High-resolution imageOne explanation for the as-made electrode containing a significant fraction of bulk-like gold is that during the electrode fabrication process, the electrodes are heated to 120 °C in order to dry the ink sprayed onto the electrode substrate. Even though it is known that the Au9 clusters in crystalline form remain stable up to 200 °C (Fig. SI3), 71 our results suggest that a fraction of Au9 clusters deposited onto carbon starts agglomerating at 120 °C, which is in line with a report from Longo et al. 25 The EXAFS of the three electrodes is also significantly different from the as-made clusters, with these electrodes exhibiting a peak around 2.7 Å, corresponding to the bulk-like gold features 24 (Fig. 4b). The EXAFS spectra of electrodes were fitted with two Au–Au scattering environment groups, similar to the EXAFS spectra of Au9 clusters and one Au–P/O bond (Fig. SI11). The EXAFS fitting parameters shown in Table II suggest that the clusters upon electrolysis undergo structural changes without significant change in Au–P/O coordination, and Au–P/O coordination decreases significantly upon electrochemical activation (Table II). Although the as-made electrode is significantly agglomerated, it is expected that the clusters will lose some phosphine ligands, but surprisingly it maintains the Au–P/O coordination number closer to the crystalline Au9 clusters (i.e., CN = 0.889, see Table SIIII). This suggests that a fraction of Au atoms bind with the sulfonate group from the Nafion ionomer, as observed in the physical mixing of the clusters and ionomer. While this data suggests that the activity of the electrode scales inversely to the fraction of Au cluster-like species within the electrode (the as-made electrode has the highest fraction of intact Au cluster-like species but is the least active), it is important to consider the availability of the gold sites for the reaction. It can be seen that the CN for the Au-P/O decreases with electrolysis and is lowest after activation. In principle, agglomeration of clusters to larger particles would avail lesser surface atoms for not only ligand coordination but also for the reactant. This should result in a lower activity upon electrochemical activation. Contrary to this, we observed higher activity which could be explained by the hypothesis that the bulky sulfonate group bonded to the metallic core can block access of reactants to the active sites, but when the electrochemical potential is applied, the Au9-Nafion matrix realigns itself, and Au–P/O coordination is lost, allowing the reactants to reach the Au core, and resulting in the higher conversion. Adsorption of the Nafion ionomer onto the surface and blockage of active sites has been extensively documented in the literature. 26,28–32
Table II. Structural parameters of the pre-deposited Au9/C electrodes from EXAFS spectra fittings; here, CN is the coordination number, and R is radial distance.
| Au9/C as-made electrode | Au9/C after electrolysis | Au9/C after Echem activation | ||||
|---|---|---|---|---|---|---|
| CN | R (Å) | CN | R (Å) | CN | R (Å) | |
| Au–P/O | 1.04 ± 0.13 | 2.298 ± 0.008 | 0.97 ± 0.12 | 2.301 ± 0.008 | 0.660 ± 0.15 | 2.297 ± 0.015 |
| (Au–Au)1 | 1.99 ± 0.48 | 2.699 ± 0.017 | 1.00 ± 0.43 | 2.688 ± 0.028 | 1.663 ± 0.57 | 2.686 ± 0.022 |
| (Au–Au)2 | 4.94 ± 0.68 | 2.863 ± 0.005 | 5.86 ± 0.69 | 2.862 ± 0.003 | 7.751 ± 0.93 | ± 0.003 |
Drop-casting Au9 clusters onto a carbon black - Nafion catalytic layer
From the above discussion, it is clear that the Nafion ionomer interacts with Au9 clusters, which results in the partial blockage of active sites in the Au9/C catalyst. These interactions are likely to occur in the catalytic ink dispersion, where both the Nafion ionomer and the Au9/C catalyst are present (as discussed in the experimental part and Fig. SI4a). 32 These interactions can be minimised by first spraying a film of carbon black with Nafion ionomer and then drop-casting the Au9 clusters to the dry layer, thereby avoiding the solution phase interactions between the Au9 clusters and Nafion ionomer. In addition, using a low boiling point solvent for drop-casting the active clusters onto a pre-made layer of carbon black will have the advantage of uniform and efficient delivery of clusters throughout the catalytic layer. Thus, the cathodes were prepared by spraying a film of carbon black onto the carbon felt, followed by drop-casting Au9 clusters from the dichloromethane (DCM) solution to achieve an Au loading of 90 μg cm−2.
No significant difference in morphology was observed between the carbon black - Nafion layers and the layers on which Au9 clusters were dropcast (Figs. 5a and 5b). These layers were also similar in morphology to the spray-coated layers produced from the Au9/C (Fig. 1a). The electronic and structural characteristics of the as-made Au9 drop-cast electrodes, as observed from XAS, are similar to that of the Au9 clusters (Fig. SI12), with the XANES spectra of Au9 clusters and the Au9 drop-cast electrode being nearly superimposable (Fig. SI12b). Additionally, the EXAFS fitting parameters of the as-made electrode are close to that of the crystalline Au9 clusters (Fig. SI13 and Table SIV), which is not unexpected due to the very low gold loading (90 μg cm−2) on the high surface area support layer. 25 This further suggests that the interactions between Au9 clusters and Nafion ionomer in dropcasted electrodes were not significant enough to change the geometrical and electronic properties of the clusters.
Figure 5. SEM image of (a) the film prepared by spraying carbon black with 5 wt% Nafion ionomer onto the carbon felt, (b) catalytic layer prepared by spraying carbon black with 5 wt% Nafion ionomer on carbon felt followed by drop-casting of Au9 from DCM (Au loading 90 μg cm−2); Potentiostatic (at −1.3 V vs Ag|AgCl) CO2 reduction on Au9 dropcasted onto carbon black-Nafion film with different amounts of Nafion ionomer in film (c) CO Faradaic efficiency; and (d) current density, in 0.2 mol l−1 KHCO3 saturated with CO2.
Download figure:
Standard image High-resolution imageThe dropcasted Au9-based electrodes demonstrated nearly 80% selectivity towards CO2 to CO conversion at −1.3 V vs Ag|AgCl (for electrodes with 5% Nafion) before electrochemical activation. Interestingly, the activity of the dropcasted electrodes before electrochemical activation is notably higher compared to the Au9/C-based electrodes. Due to the absence of notable interaction between the sulfonate group of Nafion and the metal core of Au clusters, the blockage of active sites remains low, which can explain the higher activity of the dropcasted electrodes. In addition, unlike the electrodes produced from the Au9/C catalyst, the dropcasted Au9 electrodes do not show similar activation behaviour, with the activity before and after the electrochemical activation procedure being almost the same (Figs. 5c and 5d). The improvement in activity of the Au9/C catalyst is most likely caused by the potential-driven restructuring of the Au9-Nafion interface where the cluster core was blocked by interaction with the sulfonate group from Nafion. The dropcasted Au9 clusters-based electrodes do not have such interactions; therefore, no significant change in activity was observed upon electrochemical activation at higher potential. However, based on cyclic voltammetry (Fig. SI14) and the XAS of the electrodes before and after electrolysis (Fig. SI12), it is clear that a fraction of the clusters undergo aggregation after electrochemical CO2 reduction. 54
Interestingly, the activity and selectivity of electrodes formed by dropcasting the Au9 clusters onto pre-made carbon black–Nafion layers are found to be strongly dependent on the Nafion content of the support layer (Figs. 5c and 5d), suggesting that the dropcast Au clusters still interact with the Nafion, but in a way which is not amenable to activation. When the Au9 clusters are dropcasted from DCM to the sprayed carbon black-Nafion layer, the clusters in solution are in contact with the Nafion ionomer momentarily, which could be the reason for pronounced interaction at higher Nafion content.
Conclusions
The carbon-supported Au9 clusters were studied for a potentiostatic electrochemical reduction of CO2 on the Au9/C catalyst, CO and H2 were observed as major products. The activity of the Au9/C catalyst was found to increase over time, suggesting the activation of the catalyst over time. Investigation of the physical mixture of Au9 clusters with Nafion ionomer using UV–vis spectra in methanol suggests the Au9 clusters interact with Nafion ionomer, resulting in precipitation. X-ray absorption spectroscopy of the precipitate suggests the Nafion ionomer binds with the cluster metallic core. These interactions between the Nafion ionomer and Au9 clusters result in shielding of the active site in the Au9/C catalyst, therefore, incomplete utilisation of the catalyst. The Nafion-Au9 cluster interface restructures itself upon the application of potential for a long time, thus exposing more active sites and improving activity, and the process of activation was accelerated upon the application of more negative potential. For this reason, electrochemical treatment of catalytic film at higher potential (−1.7 V vs Ag|AgCl) creates favourable electrode microstructures resulting in improvement in the CO2RR performance. Preparing the cluster-based electrode by first spraying a layer of carbon black with lower amounts of Nafion followed by dropcasting of Au9 clusters was found to minimise the interactions. However, due to the availability of a large amount of free ionomer for interaction with Au clusters at higher Nafion content in the catalytic layer, lower activity of the catalyst is observed. These electrodes do not show notable changes upon electrochemical activation.
Acknowledgments
This research was undertaken on the X-ray absorption spectroscopy beamline at the Australian Synchrotron, part of ANSTO. The authors thank Dr. Rosalie Hocking, Ms Brittany Kerr, and Mr. Jaydon Meilak for their help in carrying out the experiments. SKS acknowledges the funding support of the UC Connect PhD scholarship.
Supplementary data (1.9 MB PDF)