Abstract
The electrode reactions of Ag(I)/Ag and ferrocenium/ferrocene (Fc+/Fc) were investigated in an ionic liquid, 1-butyl-1-methylpyrrolidinium bis(fluorosulfonyl)amide (BMPFSA). The potential of Ag(I)/Ag depended on the logarithm of the concentration of Ag(I), as predicted by the Nernst equation, indicating the Ag(I)/Ag can be used as a reference electrode reaction in BMPFSA. The reversible electrode reaction of Fc+/Fc was observed in BMPFSA by cyclic voltammetry. The donor number of BMPFSA was estimated to be 13 from the difference in the formal potentials of Ag(I)/Ag and Fc+/Fc, indicating the coordination ability of FSA– was slightly stronger than that of bis(trifluoromethylsulfonyl)amide (TFSA–). The diffusion coefficients (D) of Fc and Fc+ were (5.7 ± 0.7) and (3.3 ± 0.2) × 10–7 cm2 s–1, respectively. The ratio of D of Fc+ against that of Fc was smaller than those in TFSA–-type ionic liquids, reflecting the higher charge density of FSA–. The standard rate constant (k0) of Fc+/Fc was estimated to be (5.4 ± 1.1) × 10–3 cm s–1. The apparent activation energy for k0 was close to the activation energy for D, suggesting the electrode reaction of Fc+/Fc can be regarded as the outer sphere electron transfer reaction with a very small reorganization energy.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
Amide-type ionic liquids have attracted attention in the field of electrochemistry (e.g., electrodepositions of transition metals 1–10 and rechargeable Li metal batteries 11–17 ) because they generally have non-flammability, low volatility, high electrochemical stability, and intrinsic ionic conductivity. Amide-type ionic liquids composed of bis(trifluoromethylsulfonyl)amide (TFSA–) are considered as the candidates of the non-aqueous electrolytes for redox flow batteries (RFBs), which are the large-scaled energy storage devices for renewable energies, because not only higher safety but also higher cell voltage than the conventional vanadium RFB using an acidic aqueous electrolyte are expected to be realized. 18–20 However, the high viscosity of TFSA–-type ionic liquids as compared with aqueous and organic electrolytes is disadvantageous to practical RFBs from the viewpoints on ionic conductivity and energy consumption for circulation. Therefore, ionic liquids having lower viscosity are demanded as the electrolytes for RFBs. Ionic liquids composed of bis(fluorosulfonyl)amide (FSA–) are known to have low viscosity with various organic cations. 21–24 In addition, FSA–-type ionic liquids have been investigated as the promising electrolytes for Li and Na secondary batteries, 11,13–17,25–27 because favorable solid-electrolyte interphase (SEI) is reported to form on anode materials as a result of cathodic decomposition of FSA–. Hwang et al. reported that stable SEI formed on Li metal in 30 mol% LiFSA-EMIFSA (EMI+ = 1-ethyl-3-methylimidazolium) to suppress the dendritic growth of Li, resulting in the better charge-discharge performance in Li|Li2FeP2O7 cell with 30 mol% LiFSA-EMIFSA than that with the organic electrolyte composed of ethylene carbonate, dimethyl carbonate (1:1 in vol%), and 1 M LiPF6. 16 Furuya et al. reported that homogeneous SEI formed in MPPFSA (MPP+ = 1-methyl-1-propylpyrrolidinium) and MOEMPFSA (MOEMP+ = 1-methyl-1-methoxyethylpyrrolidinium) containing a high concentration of LiFSA to suppress dendritic and whisker-like deposits. 17 FSA–-type ionic liquids have also been investigated as the electrolytes for Na secondary batteries for large-scaled energy storage devices. 25–28 The binary systems of NaFSA-EMIFSA and NaFSA-MPPFSA were reported to be liquid in the range of the molar fraction of NaFSA from 0.0 to 0.5 and have the electrochemical potential window as wide as 5.1 V. 25 The stability of EMIFSA against Na metal was reported to be higher than that of EMITFSA due to the formation of stable SEI, which consisted of the decomposition products of FSA–. 28 Forsyth et al. reported that the transference number of Na+ in NaFSA-MPPFSA was greater that 0.3, which was equivalent to those in commercially available organic electrolytes containing Li salts. 29 However, the ionic conductivity of NaFSA-MPPFSA was reported to decrease with increasing the NaFSA concentration. 30
Electrochemical behavior of metal ions in FSA–-type ionic liquids is important for applying the ionic liquids to the electrolytes for RFBs. The electrode reactions of some metal complexes, 18,19,31–35 lanthanides (Ln), 20 and nitroxylradicals 36–41 have ever been investigated in some ionic liquids. The redox reaction of ferrocenium/ferrocene (Fc+/Fc) has been studied in various non-aqueous electrolytes because its potential is recommended as a potential standard. 18,19,42–47 The SEI formation potentials in BMPFSA (BMP+ = 1-butyl-1-methylpyrrolidinium) and BMPTFSA in the presence of Li+ were determined to be –1.1 V vs Ag|Ag(I) by monitoring the redox reaction of Fc. 48 Yamamoto et al. reported the cyclic voltammograms of a Pt electrode in KFSA-, NaFSA-, and LiFSA-MPPFSA binary systems containing Fc and the diffusion coefficients (D) of Fc. 49 However, there are few reports about the electrode kinetics of Fc+/Fc in FSA–-type ionic liquids.
The redox potential of a metal ion and its metal has been known to depend on the donor number (DN) of the solvent. 20,50–52 The standard Gibbs energy of formation of a metal ion, Δf G°, is determined by the energies for sublimination, ionization, and solvation. The solvation of the metal ion depends on the solvent while the sublimination and ionization of the metal are independent of the solvent. The metal ion tends to be solvated by the solvent molecule more strongly with an increase in the DN of the solvent. Consequently, the redox potential determined by Δf G° of a metal ion becomes more negative with an increase in the DN of the solvent. A linear relationship between the half-wave potentials of Ln(III)/Ln(II) and the DNs of several organic solvents has been reported by Gutmann et al. 50,51 Yamagata et al. estimated the DN of a TFSA–-type ionic liquid according to the relationship. 20 The similar relationship has been reported for the formal potentials of Ag(I)/Ag in organic solvents by Gritzner. 52
In the present study, the electrode reaction of Ag(I)/Ag in BMPFSA was investigated in order to demonstrate the applicability of Ag(I)/Ag to a reference electrode in BMPFSA. Then, the electrode reaction of Fc+/Fc in BMPFSA was investigated to examine such parameters as D and the standard rate constants (k0) in the temperature range from 25 to 65 °C. The DN of FSA– was also estimated from the potential difference between Ag(I)/Ag and Fc+/Fc.
Experimental
BMPBr was prepared by the reaction of 1-methylpyrrolidine (Tokyo Chemical Industry, >98.0%) and butyl bromide (Tokyo Chemical Industry, >98.0%) in acetonitrile (FUJIFILM Wako Pure Chemical, >99.5%) for 12 h. The obtained BMPBr was purified by recrystallization in acetonitrile with ethyl acetate (FUJIFILM Wako Pure Chemical, >99.5%) for three times. The purified BMPBr was dried under vacuum (<10 Pa) at 100 °C for more than 24 h. BMPFSA was obtained by the metathesis reaction of BMPBr and LiFSA (Nippon Shokubai, >99.9%) in distilled water. Then, BMPFSA was separated by solvent extraction into dichloromethane (FUJIFILM Wako Pure Chemical, >99.0%) and washed with distilled water for three times. The washed BMPFSA was dried under vacuum (<10 Pa) at room temperature for more than 24 h and at 80 °C for more than 24 h. The water content in BMPFSA was found to be less than 10 ppm by Karl Fischer titration (Metrohm, 831 KF Coulometer). No impurities like bromide were detected in BMPFSA by a UV–visible spectrometer (JASCO, V-770ST) and linear sweep voltammetry using a Pt electrode. Fc (Kanto Chemical, >98.0%) was used as supplied. Fc+ and Ag(I) in BMPFSA were obtained by potentiostatic anodic oxidation of Fc and Ag (Nilaco), respectively, in BMPFSA. The hygroscopic regents were handled in an Ar-filled glovebox (Miwa MFG, DB0–1KP-K01 and DB0–1KP-K03) with a continuous gas purification apparatus (H2O < 800 ppb and O2 < 1 ppm).
All the electrochemical measurement was carried out with a three-electrode cell in the glovebox using a potentiostat (Hokuto Denko, HZ-7000). A Pt disk (Sanwa Kinzoku, 3 mmϕ) was used as a working electrode for cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy. The electrochemical impedance spectra were obtained at the open circuit potentials, in the frequency range from 50 k to10 Hz with an amplitude of 10 mV. Impedance data were analyzed with an EIS software (Hokuto Denko). A Ag wire (Nilaco) immersed in BMPFSA containing 0.10 M AgCF3SO3 (Tokyo Chemical Industry, >98.0%) was used as a reference electrode, which is denoted by Ag|Ag(I). The electrolyte of the reference electrode was isolated from the main electrolytes by a Vycor glass barrier. A spiral-shaped Pt wire (Sanwa Kinzoku) was used as a counter electrode. Potentiostatic anodic oxidation was carried out using a two-compartment cell with a diaphragm of a glass filter. The temperature was controlled by a Peltier device (AS ONE, EHC). The viscosity of the electrolyte was measured using a densitometer (Anton Paar, DMA4500M) and viscometer (SEKONIC, VM-10A-L). The molar volumes of ions were estimated by Gaussian16 at B3LYP/6–311 G++(d,p) level. 53
Results and Discussion
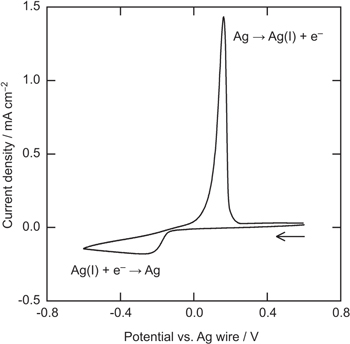
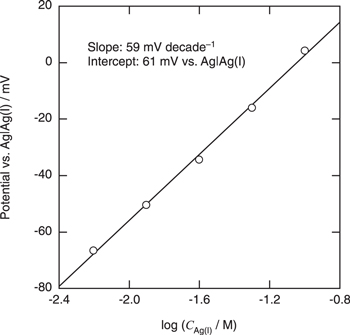
Figure 1 shows the cyclic voltammogram of a Pt electrode in BMPFSA containing 25 mM Ag(I) at 25 °C. The potential was measured against a Ag wire immersed in the electrolyte. A cathodic and anodic current observed around 0 V vs Ag wire were attributed to deposition and dissolution of Ag, respectively. 9 The similar cyclic voltammogram was observed for the electrode reaction of Ag(I)/Ag in BMPTFSA containing AgTFSA. 9 The overpotential for the Ag deposition was about 0.15 V, which is probably attributable to the nucleation overpotential on a Pt electrode, as observed in some TFSA–-based ionic liquids. 9 The open circuit potential of a Ag electrode was measured against Ag|Ag(I) in BMPFSA containing different concentrations of Ag(I) at 25 °C, as shown in Fig. 2. The Ag(I) concentration was calculated from the electric charge during the potentiostatic anodic oxidation assuming the current efficiency to be 100%. The open circuit potential depended linearly on the logarithm of the Ag(I) concentration. The slope of the regression line in Fig. 2 was 59 mV decade–1, which was close to the theoretical value for the one-electron transfer reaction. Accordingly, the potential of the Ag electrode was confirmed to be determined by the Ag(I) concentration according to the following redox equilibrium.
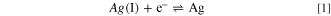
The formal potential of Ag(I)/Ag was 0.06 V vs Ag|Ag(I), which was consistent with the value measured against the Ag|Ag(I) reference electrode using BMPFSA containing 0.10 M AgCF3SO3. A linear dependence of the potential of a Ag electrode in BMPTFSA on the concentration of Ag(I) has also been reported, 9 indicating the potentials of Ag immersed in both BMPFSA and BMPTFSA containing Ag(I) are determined by the redox equilibrium, as represented by Eq. 1. Thus, the electrode reaction of Ag(I)/Ag was considered to be used as a reference electrode in BMPFSA.
Figure 1. Cyclic voltammogram of a Pt electrode in BMPFSA containing 25 mM Ag(I) at 25 °C. Scan rate: 10 mV s–1.
Download figure:
Standard image High-resolution imageFigure 2. Open circuit potentials of a Ag electrode in BMPFSA containing different concentrations of Ag(I) at 25 °C.
Download figure:
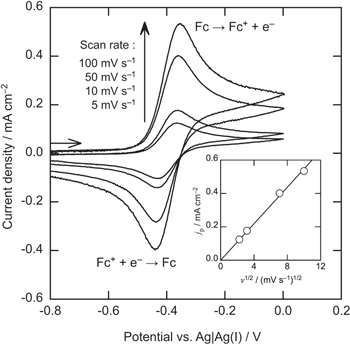
Standard image High-resolution imageFigure 3 shows the cyclic voltammograms of a Pt electrode in BMPFSA containing 10 mM Fc at various scan rates at 25 °C with IR compensation. The anodic and cathodic current peaks observed around –0.4 V vs Ag|Ag(I) were assigned to the oxidation of Fc and the reduction of Fc+ formed during the anodic scan, respectively. The difference between the anodic peak and half-peak potential, Ep—Ep/2, was 59 mV, which was close to the theoretical value for the reversible process (56.5 mV). The anodic and cathodic peak potentials were almost constant regardless of the scan rates. The anodic peak current density was proportional to the square root of the scan rate. Thus, the redox reaction of Fc+/Fc in BMPFSA was considered to be reversible. The peak current density for the reversible system is given by Eq. 2,
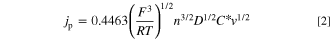
where jp is the current density, C* is the bulk concentration of the reactant, and v is the scan rate. The diffusion coefficient of Fc in BMPFSA was estimated to be 4.1 × 10–7 cm2 s–1.
Figure 3. Cyclic voltammograms of a Pt electrode in BMPFSA containing 10 mM Fc at 25 °C with IR compensation. The dependence of the anodic peak current density on the square root of the scan rate is shown in the inset.
Download figure:
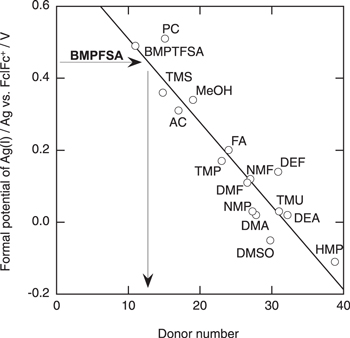
Standard image High-resolution imageFigure 4 shows the potential of a Pt electrode in BMPFSA containing Fc and Fc+ at different concentration ratios at 25 °C. No significant change in the cyclic voltammograms measured repeatedly was confirmed in the absence of Li+, 48 while the formation of a surface film and its effects on the electrode reaction of Fc+/Fc on a Pt electrode have been studied in some TFSA–-based ionic liquids. 47 The potential was linearly dependent on the logarithm of the concentration ratio of Fc+ to Fc. The slope of the regression line was 62 mV decade–1, which was close to the theoretical value for the one-electron transfer reaction. The formal potential of Fc+/Fc in BMPFSA was estimated to be –0.39 V vs Ag|Ag(I). Thus, the formal potential of Ag(I)/Ag was calculated to be 0.45 V vs Fc|Fc+. Figure 5 shows the dependence of the formal potential of Ag(I)/Ag vs Fc|Fc+ on the DN of the solvent. 52,54 The donor number of BMPFSA was estimated to be 13 from the obtained formal potential of Ag(I)/Ag vs Fc|Fc+. The donor number of BMPFSA was larger than that of BMPTFSA (7 ± 2 and 11), 20,54 indicating the coordination ability of FSA– to a cationic species is stronger than that of TFSA–. The similar tendency has been reported in some solvate ionic liquids with a solvatochromic probe. 55,56
Figure 4. Open circuit potentials of a Pt electrode in BMPFSA containing different concentration ratios of Fc+ and Fc during intermittent potentiostatic oxidation of Fc at 25 °C.
Download figure:
Standard image High-resolution imageFigure 5. Relationship between the formal potentials of Ag(I)/Ag vs Fc|Fc+ and the donor numbers of the various media. The data for the organic electrolyte systems and BMPTFSA are taken from Ref. 52, 54, and respectively. Abbreviation for solvents; PC = propylene carbonate, TMS = tetramethylene sulfone, AC = acetone, MeOH = methanol, FA = formamide, TMP = trimethyl phosphate, DEF = N, N-diethylformamide, NMF = N-methylformamide, DMF = N, N-dimethylformamide, TMU = N, N, N, N-tetramethylurea, NMP = 1-methyl-2-pyrrolidinone, DMA = N, N-dimethylacetamide, DEA = N, N-diethylacetamide, DMSO = dimethyl sulfoxide, HMP = hexamethylphosphoric triamide.
Download figure:
Standard image High-resolution imageFigure 6 shows the chronoamperograms and Cottrell plots of a Pt electrode in BMPFSA containing 5 mM Fc and 5 mM Fc+ at 25 °C. The limiting current density was obtained at the potential more positive than –0.3 V and more negative than –0.5 V vs Ag|Ag(I). The diffusion coefficient was calculated from the Cottrell equation,
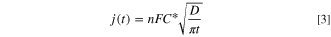
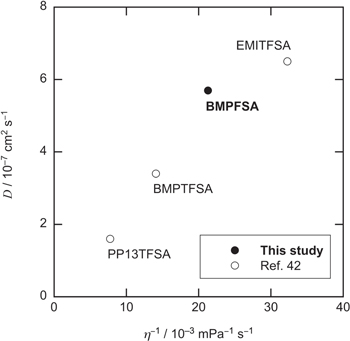
where j(t) is the current density at the time t. The diffusion coefficients of Fc and Fc+ in BMPFSA were estimated to be (5.7 ± 0.7) and (3.3 ± 0.2) × 10–7 cm2 s–1, respectively. The diffusion coefficients of Fc and Fc+ in some ionic liquids at 25 °C are listed in Table I. Figure 7 shows the dependence of the diffusion coefficient of Fc on the fluidity (the reciprocal of viscosity, η). The diffusion coefficient of Fc in BMPFSA was on the regression line, indicating that the diffusion of Fc is proportional to the fluidity, as predicted by the classical Stokes-Einstein relation. On the other hand, although the size of Fc+ is regarded as the same as that of Fc, the diffusion coefficient of Fc+ was smaller than that of Fc, probably reflecting the coulombic interaction between FSA– and Fc+. 18,19,32–35,42 The ratio of the diffusion coefficient of Fc+ to that of Fc, D(Fc+)/D(Fc), reflects the coulombic interaction of Fc+ and the anion of the ionic liquid. D(Fc+)/D(Fc) is considered to decrease with an increase in the charge density of the anion. The volumes of FSA– and TFSA– were estimated to be 0.14 and 0.20 nm3, respectively, by a molecular orbital calculation. 53 D(Fc+)/D(Fc) in BMPFSA was smaller than those in TFSA–-type ionic liquids because the charge density of FSA– is higher than that of TFSA–. 42 The Stokes-Einstein products, DηT–1, of Fc and Fc+ in BMPFSA were estimated to be 9.0 and 5.2 × 10–10 g cm s–2 K–1, respectively, which are slightly larger than those determined by chronoamperometry in some TFSA–-based ionic liquids, as listed in Table I. 18,47 However, the smaller values of the Stokes-Einstein products for Fc and Fc+ were reported in TFSA–-based ionic liquids using a Pt rotating disk electrode, 47 suggesting the dependence of the observed diffusion coefficients on the electrochemical technique used for the measurements.
Figure 6. Chronoamperograms of a Pt electrode in BMPFSA containing 5 mM Fc and 5 mM Fc+ at 25 °C. The insets show the Cottrell plots for the anodic and cathodic potential steps.
Download figure:
Standard image High-resolution imageTable I. The diffusion coefficients (D) of Fc and Fc+, the ratio of D, the Stokes-Einstein product, Dη/T–1, the standard rate constant (k0) of Fc+/Fc, and the viscosity (η) of BMPFSA and TFSA–-type ionic liquids at 25 °C.
| System | D(Fc) /10–7 cm2 s–1 | D(Fc+) /10–7 cm2 s–1 | D(Fc+)/D(Fc) | D(Fc)ηT–1/ g cm s–2 K–1 | D(Fc+)ηT–1/ g cm s–2 K–1 | k0(Fc+/Fc) / 10–3 cm s–1 | η / mPa s | References |
|---|---|---|---|---|---|---|---|---|
| BMPFSA | 5.7 ± 0.7 | 3.3 ± 0.2 | 0.58 | 9.0 | 5.2 | 5.4 ± 1.1 | 47 | This study |
| BMPTFSA | 3.4 ± 0.6 | 2.1 ± 0.2 | 0.62 | 8.1 | 5.0 | 4.3 ± 0.2 | 71 | 42 |
| EMITFSA | 6.5 ± 0.2 | 4.2 ± 0.2 | 0.65 | 6.8 | 4.4 | 15 ± 8 | 31 | 42 |
| PP13TFSA | 1.6 ± 0.2 | 1.0 ± 0.1 | 0.63 | 6.9 | 4.3 | 2.3 ± 1.1 | 129 | 42 |
Figure 7. Relationship between the diffusion coefficients of Fc and the reciprocal of the viscosity (fluidity) of the media. The data for EMITFSA, BMPTFSA, and PP13TFSA were taken from Ref. 42.
Download figure:
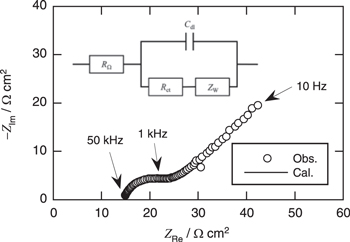
Standard image High-resolution imageFigure 8 shows the Nyquist plots of a Pt electrode in BMPFSA containing 5 mM Fc and 5 mM Fc+ at 25 °C. The obtained data were analyzed using the Randles model, as shown in Fig. 8. The charge transfer resistance, Rct, was estimated from the diameter of the semicircle, and the standard rate constant was calculated from (4)

where  and
and  are the bulk concentrations of Fc+ and Fc, respectively, and α is the transfer coefficient.
are the bulk concentrations of Fc+ and Fc, respectively, and α is the transfer coefficient.  can be regarded as
can be regarded as  or
or  for
for  =
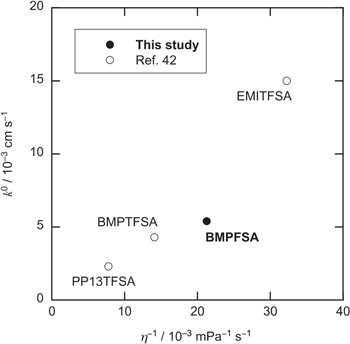
=  The standard rate constants of Fc+/Fc and the viscosities of some ionic liquids are also listed in Table I. The standard rate constant for outer sphere electron transfer reactions is represented by the following equation.
The standard rate constants of Fc+/Fc and the viscosities of some ionic liquids are also listed in Table I. The standard rate constant for outer sphere electron transfer reactions is represented by the following equation.
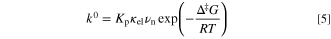
where Κp is the precursor equilibrium constant, κel is the electronic transmission coefficient, νn is the nuclear frequency factor, and Δ‡
G is the Gibbs energy for activation. Since νn is proportional to the fluidity (1/η),
57
k0 increases linearly with an increase in the fluidity of the electrolytes.
42,58
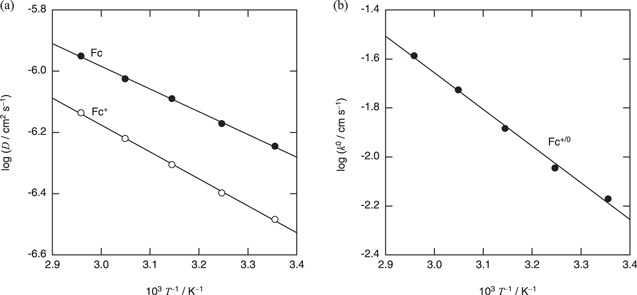
Figure 9 shows the relationship between k0 of Fc+/Fc and the fluidity. The value of k0 of Fc+/Fc in BMPFSA was larger than those in BMPTFSA and PP13TFSA (PP13+ = 1-methyl-1-propylpiperidinium) and smaller than that in EMITFSA, as expected. As described above, the diffusion coefficients of Fc and Fc+ are essentially proportional to the fluidity. Thus, the temperature dependence of νn is expected to be the same as that of the diffusion coefficient. Figure 10a shows the Arrhenius plots for the diffusion coefficients of Fc and Fc+ in BMPFSA. The activation energies for the diffusion coefficients of Fc and Fc+,  and
and  were calculated to be 14 ± 2 and 17 ± 1 kJ mol–1, respectively, which were close to that for the viscosity (Ea(η), 19 kJ mol–1). Thus, the standard rate constant can be represented as follows
were calculated to be 14 ± 2 and 17 ± 1 kJ mol–1, respectively, which were close to that for the viscosity (Ea(η), 19 kJ mol–1). Thus, the standard rate constant can be represented as follows
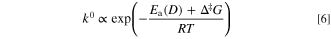
where Ea(D) is  or
or  Therefore, the apparent activation energy for the standard rate constant, Eapp(k0), corresponds to the sum of Ea(D) and Δ‡
G. Figure 10b shows the Arrhenius plots for the standard rate constant of Fc+/Fc in BMPTFSA. Eapp(k0) of Fc+/Fc in BMPFSA was 18 ± 2 kJ mol–1, which was close to
Therefore, the apparent activation energy for the standard rate constant, Eapp(k0), corresponds to the sum of Ea(D) and Δ‡
G. Figure 10b shows the Arrhenius plots for the standard rate constant of Fc+/Fc in BMPTFSA. Eapp(k0) of Fc+/Fc in BMPFSA was 18 ± 2 kJ mol–1, which was close to 
 and Ea(η), indicating that the contribution of Δ‡
G to the rate constant was very small. Therefore, the electrode reaction of Fc+/Fc in BMPFSA is regarded as the outer-sphere redox reaction with a very small reorganization energy, as reported by Hupp et al.
59
and Ea(η), indicating that the contribution of Δ‡
G to the rate constant was very small. Therefore, the electrode reaction of Fc+/Fc in BMPFSA is regarded as the outer-sphere redox reaction with a very small reorganization energy, as reported by Hupp et al.
59
Figure 8. Nyquist plots of a Pt electrode in BMPFSA containing 5 mM Fc and 5 mM Fc+ at the open circuit potential. Temperature: 25 °C. The inset shows the Randles-type equivalent circuit used for analysis.
Download figure:
Standard image High-resolution imageFigure 9. Relationship between the standard rate constants of Fc+/Fc and the reciprocal of the viscosity (fluidity) of the media. The data for EMITFSA, BMPTFSA, and PP13TFSA were taken from Ref. 42.
Download figure:
Standard image High-resolution imageFigure 10. Arrhenius plots for the (a) diffusion coefficients of Fc and Fc+ and (b) standard rate constants of Fc+/Fc in BMPFSA.
Download figure:
Standard image High-resolution imageConclusions
The redox reactions of Ag(I)/Ag and Fc+/Fc were investigated in BMPFSA. The potential of Ag(I)/Ag was found to be proportional to the logarithm of the concentration of Ag(I), indicating the electrode reaction of Ag(I)/Ag can be used for a reference electrode in this ionic liquid. The donor number of BMPFSA was estimated to be 13, which is slightly larger than that of TFSA–. The diffusion of Fc and Fc+ in BMPFSA was found to be mainly governed by the viscosity of the ionic liquid. However, the diffusion of the positively charged species is affected by the coulombic interaction with the anion, as reported in other ionic liquids. The ratio of the diffusion coefficient of Fc+ to that of Fc in BMPFSA was smaller than that in TFSA–, reflecting the higher charge density of FSA–. The electron transfer reaction for Fc+/Fc can be regarded as the outer sphere electron transfer reaction with a very small reorganization energy, suggesting the coulombic interaction between Fc+ with FSA– is enough small. Thus, the potential of Fc+/Fc is considered to be usable as a potential standard in FSA–-type ionic liquids.
Acknowledgments
S. K. thanks Kato Foundation for Promotion of Science for financial support.