Abstract
Reversible lithium (Li) deposition and stripping on conductive substrates like copper is vital for long-life high-energy rechargeable Li batteries. The reversibility is believed hindered by solid electrolyte interphase (SEI) formation and Li dendritic growth. Via in situ, operando cryo-microscopy and molecular dynamics simulation, we discovered amorphous Li before a disorder-order phase transition to bcc phase. The kinetics plays a significant role in Li nucleation and morphology evolution. This perspective on Li nucleation and growth from atomistic to nano- (<20 nm), meso- (20–100 nm), and micro-scales (>100 nm) provides a practical guidance on regulating dense Li deposits reversibly for long life performance.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
High energy density or specific energy rechargeable Li batteries are being targeted 1,2 and developed for the current electrification needs in transportation and energy storage. However, the lack of long service life could impede the progress and commercial potential of these batteries. Solid electrolyte interphase (SEI) formation and Li dendrite growth 3,4 have been cited as the major impediments of the rechargeable Li batteries for short cycle life. How to achieve reversible, highly efficient Li deposition and stripping has been a critical subject for decades. 4 More recently, attention to Li nucleation and growth becomes a focal point of interest. For instance, B. Thirumalraj, et al. 5 proposed a Li-SEI model for quantitatively understanding of the Li nucleation and growth mechanism associated with the SEI formation. As they postulate the current transients at various overpotentials initiate the nucleation and growth of Li metal on conductive substrates, the Li-SEI model considers a three-dimensional diffusion-controlled process with a simultaneous reduction of electrolyte decomposition due to SEI fracture as the basis for investigating the Li nucleation and growth mechanism. The rate constants of the Li deposition/stripping, electrolyte reduction, and SEI fracture were derived to model Li plating morphology and dendritic growth. Likewise, the Li nucleation and growth on gold-solid electrolyte interface was investigated by H. Wang et al., 6 and the preferential Li deposition was correlated with intrinsic defects of polycrystalline electrolytes, such as pores, grain boundaries, and impurities that cause non-uniform Li deposition. These defects exhibit faster Li deposition kinetics and high nucleation tendency. Nonetheless, most of the studies were conducted in the microscale to macroscale in observations, lacking the critical insights on the initial stage of the nucleation from the atomistic to mesoscale to enhance the understanding with a broader perspective of the fundamental issues that govern the microscopic behavior and macroscopic phenomena.
Current Status
Recent progress made by the authors 7,8 has shown promising results to achieve reversible and dense Li deposition and stripping to support long cycle life. 9 Despite such successes, finessing and perfecting the control of the Li morphology and reversibility during cycling remains as an art. Thus, there is a need calling for better understanding of the underpinning mechanism related to Li metal nucleation and growth.
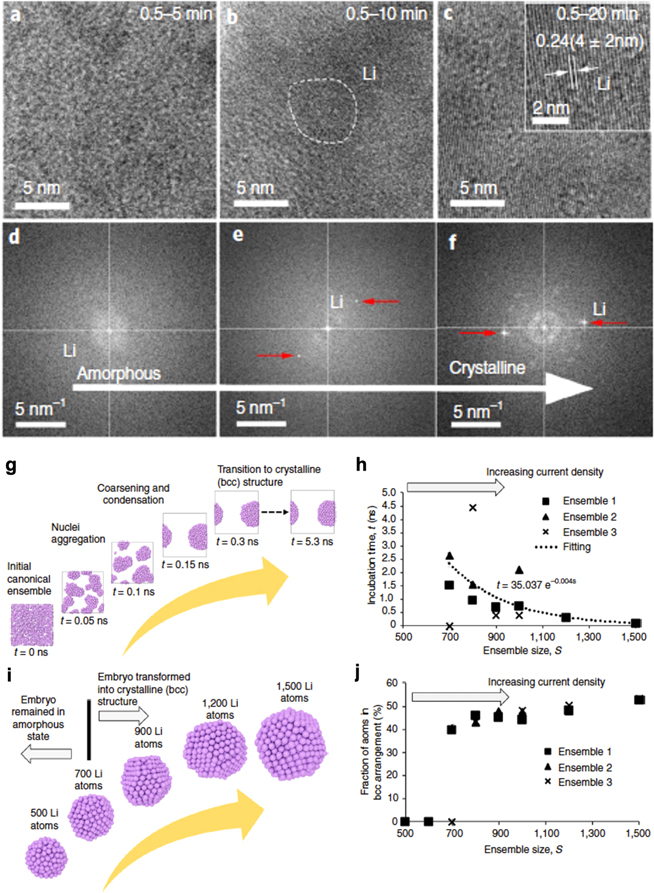
Figure 1 exhibits the unique aspects that we learned from the recent studies. 8 In Figs. 1a–1c, the cryogenic electron microscope (Cryo-EM) imaging results reveal the dependence of the Li deposit's morphology as a function of deposition time (5, 10, and 20 min) at 0.5 mA cm−2. The high-resolution images show the evolution of a distinct disordered structure in the short duration to an ordered lattice appearance in the prolonged deposition. The amorphous-to-crystalline nature of the second-order phase transition (SOPT) is reflected in the fast Fourier transform (FFT) patterns as shown in Figs. 1d–1f. Independently, using reactive molecular dynamics (MD) simulations with a process comprised (1) heating at 500 K for 0.1 ns to provide sufficient driving force for the nucleation, (2) quenching to 300 K with a cooling rate of 1 K ps−1, and (3) equilibrating at 300 K for 5 ns (details should be referred to Ref. 8), the temporal evolution of an ensemble with 700 Li atoms from a completely disordered state to an ordered aggregate of bcc lattice structure is shown in Fig. 1g as an illustration to explain the embryonic stage in the nucleation process, where the aggregate size is about 2–3 nm with a mass density on the order of 0.0534 g cm−3 (10% of the Li metal density). In Fig. 1h, the MD simulations further suggest that the incubation time for SOPT is ensemble-size dependent in the embryonic stage. Here, an ensemble is prescribed by a certain number of Li atoms used in the simulation case study (ensemble size), whereas in the initial state the atom's coordinates were assigned by a specific random number generation (RNG) script. Ensemble 1 of different sizes mean that these ensembles were produced by the same RNG script. In the simulation, the incubation time is of several nanoseconds (ns). Considering the current density of 0.5 mA cm−2, the mass transport rate is about 0.3 Li atoms per 1 ns across a 10 nm × 10 nm cross area (a reference space frame used in the simulation). Thus, for the experiment to reach 700 Li atoms in a dense deposition of 0.534 g cm−3, the actual duration should be on the order of 20 microseconds. Figure 1i shows that SOPT is ensemble-size dependent, where 700-atom aggregates of about 2–3 nm in size gave bifurcate results. In other words, ensembles that are larger than this size likely experience SOPT, whereas those smaller may remain in amorphous for much longer time. The cryo-EM imaging results suggest the same. Figure 1j indicates that the crystallinity of the nuclei is ensemble-size dependent, and the degree of crystallinity increases with the current density and/or deposition time. Figure 1 exhibits a few important implications that are worth noting here:
- i.The MD simulations indicate that the duration for an ensemble to stabilize as an embryo in order to grow into a crystal structure or remain amorphous in a glassy state is much longer than those of most classical models consider as "spontaneous." The implication of the ensemble-size dependence and the associated SOPT reflects the "unexpected" consequence of the impact from the mass transport as we emphasized in the comparison of the computational time in which a nanosecond range of phenomena in computation could take two-to-three orders of magnitude in real time (microseconds) to occur. If we multiply the length and areal scale in the observation from nanometers to micrometers, to reach a more refined state that can homogenize the observation for comparison would take even much longer time into the millisecond range or longer, as we often encounter in real life observation.
- ii.Furthermore, the quasi-equilibrium assumption usually subscribed in the conventional approaches may intentionally overlook and neglect this temporal aspect in their model description to simplify the development, simulation, and prediction of the model.
- iii.Combining these arguments, it becomes apparent that the multi-scale modeling practice to date suffers the scaling issues with incompatible presumptions and negligence of the mass transport aspect over the temporal and length scales in the kinetic regime.
Figure 1. Cryo-STEM observations of amorphous Li deposit and a rational explanation of a second-order phase transition by reactive molecular dynamics (MD) simulations. 8 (a)–(c) Cryo-EM imaging of Li deposits at 0.5 mA cm−2 for 5, 10, and 20 min (d)–(f) FFT patterns of the Li deposits showing amorphous-to-crystalline second-order phase transition (SOPT). (g) Temporal evolution of an ensemble of 700 Li atoms from a random distribution to a condensed phase in the nucleation process simulated by the MD method. (h) Incubation time vs ensemble size in the nucleation. (i) SOPT is ensemble-size dependent. (j) Crystallinity is ensemble-size dependent with increasing current density or deposition time.
Download figure:
Standard image High-resolution imageThe importance of the mass transport aspect in the nucleation and growth over the temporal and length scales is also evident in Fig. 1j. The reason underpinning the ensemble-size dependence that increases with current density and/or deposition time in crystallinity is because the increase in mass transport rate would shorten the incubation time for SOPT due to a higher rate of densification. The implications summarized above are further evident in Fig. 2.
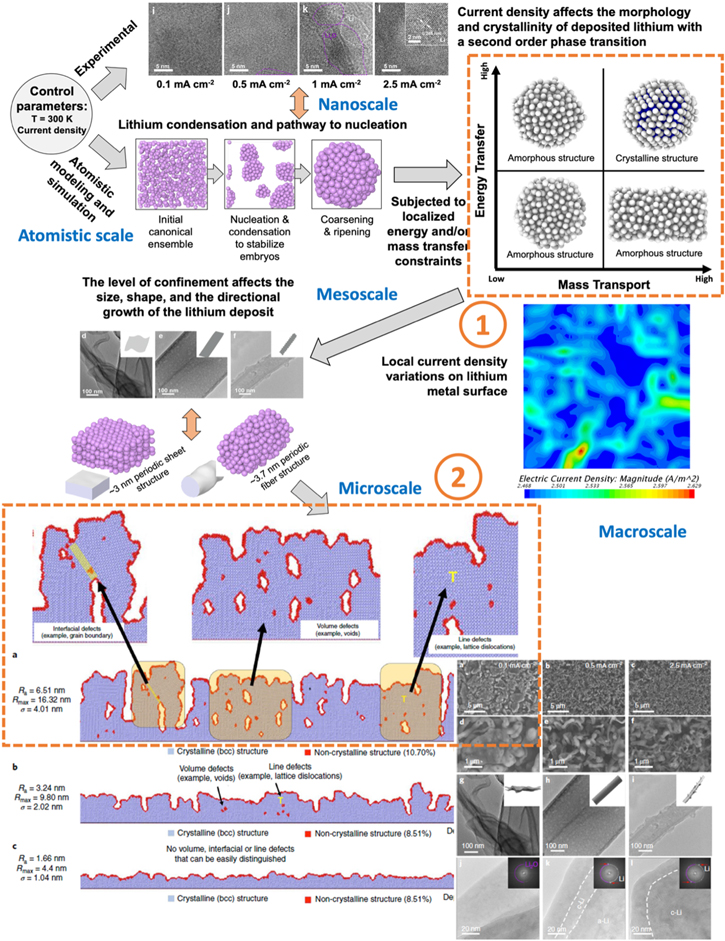
Figure 2. A multiscale perspective from the atomistic, nanoscale nucleation of Li to larger scales in the simulation to reflect real-life scenarios. 8 Even the same force field used in the MD simulation at nanoscale (see Area ①) could reflect dramatic variations of interactions at larger scales (see Area ②), as also shown by the cryo-EM observations, which is a surprise as the matter has not been discovered and explored fully in the past.
Download figure:
Standard image High-resolution imageIn Fig. 2, the simulation of the embryonic stage of nucleation at the atomistic and nanoscale (<20 nm) is expanded to larger spatial and temporal scales to explore the evolution of the growth of the nuclei. As the embryo size-dependent crystallinity and incubation time for SOPT vary with current density and duration, the degree of complexity in the morphological evolution becomes sensitive to the local energy transfer and mass transport dependence, as shown in Area ① of Fig. 2. Such a sensitivity revealed in the meso-scale (20–100 nm) begins to impact the morphological evolution due to confinements in local energy transfer and mass transport conditions. The local confinements will affect the morphology evolution of the grains in size, shape, and crystallinity—as the origins of the inhomogeneity of the Li deposits in a multi-grain, multi-domain microstructure. The divergent evolution continues into microscale (>100 nm), where the microstructure of the Li deposits is being formulated, as shown by the cryo-EM and MD results, respectively and independently, in Area ② of Fig. 2. At this microscale to macroscale, the simulation results show the degree of mismatches in crystal orientation, mass density, growth rate that resulted in defect formations including line defects like dislocations (1-dimensional or 1-D), surface defects like grain boundaries (2-D) and volume defects like voids (3-D). The degree of such defect structure variations also depends on deposition rate and duration. Although cryo-EM and MD results might not be in complete sync numerically, they independently revealed the sensitivity to the kinetic divergency in the morphological and microstructural variations of the Li deposits—an aspect that was not elaborated in the original work but in this work as a subject for discussion.
Future Needs and Prospects
Figures 1 and 2 provide a unique, but complimentary, view that deviates from the one which is pursued by the conventional wisdom. This viewpoint emphasizes the kinetic regime in the Li nucleation process and progression. It focuses on how the kinetic aspects shape the nucleation and growth process during Li deposition at various scales with a framework of coherent view on kinetic divergency caused by localized energy transfer and mass transport kinetics. It presents a divergency in the kinetic regime, primarily due to local energy transfer and mass transport confinements and interactions over a broad range of spatial and temporal scales, where the phase formation and transformation could lead to diverse results in morphology and microstructure. This divergency in the kinetic aspects of the phase formation and transformation is not often discussed in the classical model where a unified framework is commonly sought as the goal for developing a basic understanding of the nucleation and growth whereas the time- and/or volume-averaging approach is applied. Combining this inhomogeneous, diverse kinetic view with the homogeneous approach in the classical model, we hope to provide a more inclusive and balanced perspective to a complicated process in the Li metal deposition (and stripping).
Several takeaways are summarized hereafter with this perspective to strike more balanced future research needs and directions. By emphasizing the kinetic divergency, we hope to:
- 1.Provide a more quantitative view to decipher what really matters in the control of the Li morphology during nucleation and growth in the electrochemical cycling. For instance, via the understanding of the embryonic stage to the mesoscale growth, by incorporating variations in the current density distribution cross the surface at the electrolyte-electrode interface (as shown in Fig. 2 between Area ① and ②) as perceived at the microscale, 10 one could quickly conceive the implication on the needs and the complicated challenge in perfecting electrode fabrication and formulation to achieve a desired performance of the electrode homogeneously during cycling.
- 2.Present the scenarios that imperfection is a natural process that percolates from nanoscale to macroscale. The local variations in energy transfer and mass transport rates could affect the morphology and microstructure evolution of the Li deposits, as shown in Area ② in Fig. 2. The distribution of the defect structure and defect density is a result of such a local random perturbation; thus, even the MD simulation used the same force field for each individual Li atom, the results might vary due to such perturbations. In other words, the localized perturbations lead to a diverse consequence over the temporal and spatial evolutions at scales via the perturbed interactions among Li atoms, as shown in Area ②. In contrast to the conventional time-averaging and volume-averaging approach, more in-depth understanding of this kinetic divergency and the resulting uncertainty would be useful in finessing the control of the morphology and microstructure evolution.
- 3.Raise the awareness of the intriguing observation that suggests, despite a well-designed experimental control at the macroscopic level, the intrinsic variability due to localized perturbations could remain as a tough challenge for any attempt to finesse the control of materials and electrodes over long-term stability. To control such an intrinsic variability and localized perturbation to improve life cycle performance of a system, particularly for rechargeable Li batteries, is something we need to master using extrinsic variables.
- 4.Construct a clear stepwise mechanistic view of how to decipher the critical factors that control the nucleation and growth of Li deposits. For instance, although the impacts from the electrolyte (e.g., the solvation effect) and the charge transfer kinetics at the electrode-electrolyte interface were often considered as key factors that affect the morphology and dendrite growth of the Li deposits, we have not found these aspects would greatly change the perspective on nucleation and growth as described hereabove. This observation is also supported by the early work of T. Abe, et al., 11 in which it has been shown that the "de-solvation" of Li ions from the solvated complex is the slowest, rate-determining step in the charge transfer kinetics at the graphite-electrolyte interface. The kinetic barrier (or the activation energy associated with de-solvation) is however rather constant, disregarding the electrolyte solvation species. In the classic view of charge transfer kinetics, the impact from the electrolyte is considered relevant to the solvation structure and electrochemical potential difference at the interface. Yet, the work by T. Abe and his co-workers in many examples continued to show that the solvent species do not alter the activation energy of the de-solvation in a noticeable manner. Thus, even we fully understand that such aspects will affect the localized perturbations in general as discussed above, and they remain as an important subject to be discussed, it is useful to cognize that Li nucleation is a step after the charge transfer. During the nucleation and growth, the kinetic impact is more relevant to the Li transport, including those in the porous electrode structure and through SEI. So, Li transport kinetics is far more important than the charge transfer kinetics in shaping the morphology and microstructure. We also want to mention that our perspective on kinetic divergency already implied the cause of dendrite formation and growth over temporal and length scales. Compounded with SEI formation and speciation distribution on a localized scale with kinetic divergency, we fully appreciate the complexity of such phenomena evolve over time and length scales. Such discussion is beyond the scope of this perspective and needs to be resolved with a more refreshing look by in situ , operando experimental characterizations and model simulations.
- 5.Develop a proper temporal perspective between the energy transfer and mass transport kinetics in a multiscale framework. As we just argued that the rate of change in the state of a matter is often controlled by mass transport kinetics (over broad spatial and temporal scales), the rate of corresponding energy transfer is much faster (as in the charge transfer kinetics) than that of the mass transport. Thus, homogenizing the classical view of energy transfer kinetics with the mass transport kinetics would help us develop a more balanced perspective to regulate the morphological evolution of the Li deposition and stripping over repetitive cycling.
- 6.Correlate the temporal evolution of the morphology and microstructure in a quantitative manner with regards to the spatial distribution of the homogeneity of the Li deposits (e.g., defect speciation and density, degree of crystallinity, etc.), so we may possess relevant information to elucidate and identify the controlling factor(s) to master the control of the morphology and microstructure evolution. Thus, the early illustration of correlating the computational results with the experimental ones in the temporal correspondence could help us design experiments to verify and validate the model predictions. More advanced data regression methods could help us optimize the experimental design and simulation fidelity in a shorter duration.
Conclusions
This perspective provides a unique view on Li metal nucleation and growth process from atomistic scale to macroscale. It explains the divergence in localized perturbations, and via percolation and convergence, in the kinetic regime in shaping the resulting morphological and microstructural evolution during the deposition. We reiterate the importance of the mass transport kinetics over the classical view of charge transfer kinetics on how they influence the Li nucleation and growth. Understanding the origin of this localized perturbation could help us determine the factors to control the deposition process and to obtain more homogeneous deposits. Mastering such a morphology and microstructure control is vital to the performance and life of a Li metal electrode in rechargeable Li batteries. Applying the basic understanding to other metal deposition processes could fundamentally change the principle of synthesis method and molecular level control of these energy materials to produce desired reliability and functionality.
Acknowledgments
This work is supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the U.S. Department of Energy through the Advanced Battery Materials Research Program (Battery500 Consortium). Idaho National Laboratory (INL) is operated by Battelle Energy Alliance under Contract No. DE-AC07–05ID14517 for the U.S. Department of Energy. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a nonexclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for U.S. Government purposes. The authors would like to thank Jun Liu, Jie Xiao, Jason Ji-Guang Zhang, Wu Xu, Xia Cao, and colleagues in Pacific Northwest National Laboratory (PNNL) and the General Motor technical team led by Dr Mei Cai in assisting the experimental work and participating in technical discussions.