Abstract
Dye-sensitized solar cells (DSSCs) are promising photovoltaic technology with diverse indoor and outdoor applications. DSSCs can be integrated with curtains and glasses to power various devices, including wireless sensors, computer network peripherals, internet-of-things (IoT) devices, and wearable electronics. DSSCs have the potential to become the future source of energy. However, their efficiency, stability, and industrial production still need to be scaled up. The present review encompasses these elements and the various changes that have occurred inside the DSSC over the last few years, including device structure, TiO2 photoelectrode, novel electrolytes, new organic photosensitizers, efficient catalyst materials, and encapsulation strategies for sealing DSSC devices. We further discuss how the performance of each functional component of a DSSC has been enhanced as a result of the introduction of novel materials and manufacturing processes. In addition, we also briefly cover p-DSSCs and tandem DSSCs. Finally, the prospect of highly efficient and stable DSSCs is highlighted.
Export citation and abstract BibTeX RIS
In recent years, the surge in population, rapid economic development, and energy needs have dramatically increased. The majority of energy sources are currently non-renewable, such as coal, oil, fossil fuels, and natural gas, which generate over 80% of energy but have environmental consequences. Econometric simulations predict that fossil fuel stocks will be entirely depleted by 2042. 1 These concerns have sparked a widespread quest for alternative and environmentally friendly energy production methods. Among the numerous renewable energy sources, solar energy provides plentiful, clean, and ecologically sustainable power with tremendous potential for addressing significant power consumption demands. 2,3 Solar cells are devices that convert solar energy into electricity. The invention of a photovoltaic (PV) solar cell at Bell Labs in the United States in 1954 was a pivotal event in the science and technological world. Photovoltaic solar cells are classified into three generations. The first-generation solar cells were fabricated from single and polycrystalline silicon wafers, commonly known as conventional silicon cells. Over the last five decades, silicon-based PV systems have dominated the global PV industry. This is primarily due to their advantageous characteristics, including effective electricity production in direct sunlight and reasonable photovoltaic output reliability across all climates and technological advancements. The second-generation solar cells incorporated thin-film (amorphous/polycrystalline silicon) technology but have relatively poor photoconversion efficiency due to less material and lower absorption area. Nonetheless, there are many limitations to Si-based PV systems, such as their energy-intensive manufacturing methods, the poor conversion efficiency in low light intensities, and relatively higher maintenance. 3,4 As a result, their widespread application in building-integrated photovoltaics (BIPV), wearable gadgets, and indoor applications have been restricted. 4–6
In contrast to conventional silicon solar cells, dye-sensitized solar cells (DSSCs) are regarded as one of the promising third-generation photovoltaics due to their cost-effectiveness, ease of fabrication, and purification. A typical DSSC has four major components; a photoelectrode (PE), sensitizer dye, redox shuttle, and a counter electrode (CE). 7–9 Among various components, sensitizers are the key component of DSSC because they expand the optical response of devices and inject electrons into the titanium dioxide conduction band. Since the first report published on ruthenium-based DSSCs by Grätzel and colleagues, 10 numerous researchers have been working hard to develop more effective new photosensitizers. 11–16 Despite significant advancements in the DSSC field, their implementation has been restricted due to the device's weak absorption spectra in the near-IR regions, which might impair the device's power conversion efficiency (PCE). Several earlier studies proposed using two or three co-sensitized dyes to achieve multispectral absorption rather than a single dye. 17–20 Additionally, the selection of sensitizers is critical when incorporated into co-sensitized DSSCs. 21 A good co-sensitizer should have a suitable size in addition to compatible optical absorption, which would otherwise result in dye desorption, limiting the PCE of the cell. 22–25 The idea of co-sensitization extends beyond the production of dyes with high molar extinction coefficients and panchromatic absorption. With careful molecular engineering of the two dyes, a significant improvement in the cell's PCE can be achieved. In 1991, Grätzel and co-workers announced the first report on DSSC with 7.1% PCE. Since then, the DSSC design and PCE have evolved significantly (Fig. 1). 10,26–37
Figure 1. Progression of DSSCs power conversion efficiencies in the last two decades.
Download figure:
Standard image High-resolution imageDSSCs are extremely efficient in powering microelectronic devices like wireless sensors and wearable gadgets, especially when capturing ambient light. They are perfect candidates for indoor applications to power a range of microelectronics devices thanks to their comparatively low cost, abundant materials, and the ability to create slim, lightweight, flexible solar panels. Nevertheless, cell manufacturing techniques need to be upscaled to commercial production with excellent solar-to-electrical conversion efficiency and lasting stability to realize their widespread applications.
The present review discusses novel DSSC design concepts that have emerged in recent years that incorporate different redox electrolytes, judiciously tailored organic photosensitizers, catalyst materials, and the new possibilities for their incorporation into portable electronics, wireless sensor networks, and Internet of Things (IoT) applications. Furthermore, a brief overview of p-DSSCs and tandem DSSCs are also provided with their limitations. In general, this review offers the most current insights on the most recent research advances that emerged during the evolution of the advanced DSSC technology.
Novel Device Architectures
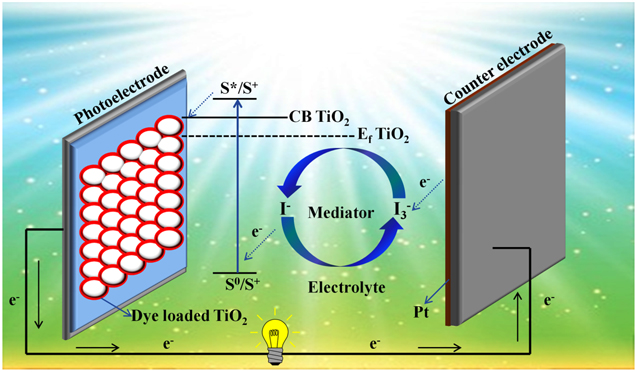
The conventional design of a DSSC employs two transparent conducting oxides (TCO) coated glass electrodes, usually covered with fluorine-doped tin oxide (FTO). 38 One of these glass substrates is coated with a 10–15 μm thick mesoporous nanocrystalline layer of a metal oxide semiconductor such as TiO2, ZnO, and SnO2, which functions as a photoanode. This nanocrystalline layer allows more dye molecules to be adsorbed, thereby improving the light-harvesting efficiency. The other glass substrate is usually covered with a catalyst such as platinum (Pt), which acts as a counter electrode (CE). The CE collects electrons from the external circuit and reduces the redox species that function as mediators in the sensitizer's regeneration after electron injection, which significantly impacts the PCE, long-term stability, and the cost of solar cells. The redox mediator influences the CE reactions used to pass photo-generated charges between the working and counter electrodes. The iodide triiodide redox-shuttle has been used as the redox mediator in the majority of the DSSCs. Inside a DSSC, the triiodides are generated near the working photoelectrode and reduced at the CE (Fig. 2).
Figure 2. A schematic and working principle of a classical DSSC.
Download figure:
Standard image High-resolution imageTo harvest optimum incident light and achieve optimum performance, dye anchored counter electrode (DACE)-based DSSC devices have recently attracted considerable attention from the scientific community. In this technique, both the working and counter electrodes are coated with metal oxide semiconductors and sensitized by two different dyes to facilitate enhanced light harvesting. The first report on DACE-based DSSC was communicated by Nam-Gyu Park et al. by incorporating N-719 and TA-St-CA sensitizers. They reported a 26% improvement in the photocurrent density relative to a traditional DSSC. 39 later, Raghavender et al. reported a PCE of 7.14% using different sensitizers (N-719 and PCH001), and carbon nanohorns (CNHs) coated DACE. 40 In a similar context, Giribabu et al. reported similar observations in their experiments based on PEDOT.PSS coated DACE and achieved 46% enhanced PCE relative to Pt-based DSSC. 41 Recently, Ganesh et al. synthesized dithionopyrrole-based TP–DTP and porphyrin-based Y351-S dyes for the application in DACE-based DSSC and reported a higher photoconversion efficiency of 8.72%. 42 These encouraging findings obtained with DACE indicate that DACE-based DSSC devices have significant potential for adoption in place of traditional DSSC systems.
The traditional design of a DSSC incorporates a thin thermoplastic spacer (10–45 μm) between the photoanode and CE to prevent a short-circuit. 42,43 The photo-generated charges are shared between photoanode and CE during cell operation via a redox mediator-containing liquid electrolyte. The mediator diffuses into the mesoporous TiO2 electrode and also through the mesoporous spacer and the liquid electrolyte. Thus, the thermoplastic and spacer thickness has a direct effect on the PCE of DSSCs via mass transfer and the bulk electrolyte's diffusion resistance (RD). 43,44 At the moment, RD minimization can be accomplished only by altering the thermoplastic and porous insulator thickness or by using viscous solvent-based electrolytes for producing high-performance DSSCs. 44–46
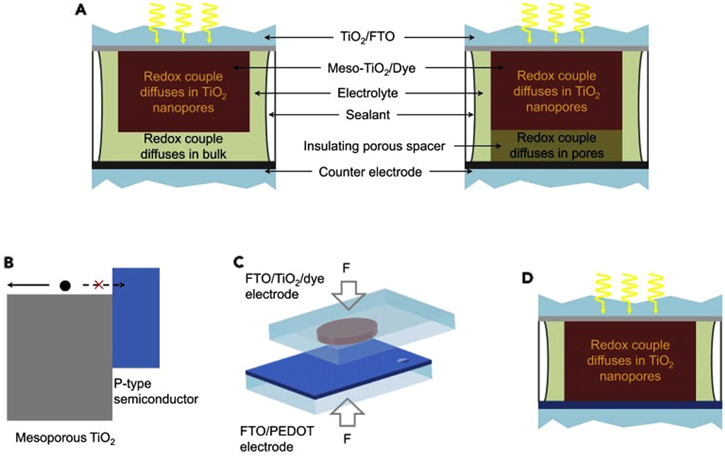
A recent work led by Cao et al. has considered this issue in their work. They developed a novel DSSC structure in which mesoporous TiO2 coated photoelectrode and poly(3,4-ethylene dioxythiophene) (PEDOT) covered CE were physically joined without using a spacer. 34 Further, an alternative Cu-based redox shuttle was used instead of the iodide/triiodide redox shuttle. Surprisingly, there was no electrical shunting when the electrolyte was injected, and the photoanode and CE were joined together. Besides, the whole device acted as a type II junction, wherein the sensitized nanostructured TiO2 layer served as an n-type semiconductor, and the other PEDOT-based layer served as a p-type semiconductor (Fig. 3). Consequently, the RD of the developed cell decreased considerably, thereby contributing to enhanced PCE of the cell. Moreover, the device exhibited a high PCE of 13.1% under standard AM 1.5 G, applied illumination. Furthermore, under ambient indoor lighting of 1000 lux, the same device demonstrated a superior photoconversion efficiency of 32% with a maximum output power density of 101 mW cm−2, which is impressive for powering IoT devices, sensors, and portable electronics. 34
Figure 3. (a) A conventional DSSC embodiment with separated electrodes is achieved by employing a thick frame of sealant or a porous insulating spacer to prevent any electrical shunts. (b) The alignment of the band edges at the type II junction between a mesoporous TiO2 layer and a p-type semiconductor layer. The p-type semiconductor acts as a charge collection layer that is electron-blocking and hole-selective. (c) By mechanically pressing the sensitized TiO2 electrode and the PEDOT semiconductor-based counter electrode into direct contact, a new DSSC embodiment is created. (d) In the DSSC with the contacted electrodes, the redox couple diffuses merely through the mesoscopic TiO2 film. (Reproduced from Ref. 34 with permission).
Download figure:
Standard image High-resolution imageLater, Lee et al. prepared highly efficient bifacial DSSCs using a Y123 dye anchored 10 μm thick TiO2 working electrode (6 μm main layer + 4 μm scattering layer), a Pt catalyst based CE (0.16 nm), and Co (II/III) based electrolytes in the ratio of 0.11/0.025 M. The device yielded an impressive PCE of 22.66%, 23.48%, and 24.52% at varying incident flux of 201.8, 607.8, and 999.6 lux, respectively. 47 In another study, Michaels et al. have prepared ambient light-operated DSSCs based on Cu (II/I) electrolyte to power IoT-based devices. The PCE of the DSSCs was surprisingly high (34%, 32.7%, and 31.4%) at different illuminations (1000, 500, and 200 lux). 6 The performance, high power density, and efficiency of these DSSCs make them a viable power-efficient option for many sensors, wearable, and Internet of Things (IoT) electronics. 48
In a recent report, Molla et al. have prepared a back-contact TCO-free novel DSSC assembly, wherein nanoporous TiO2 was coated over a flexible stainless-still mesh, which served as an efficient photoanode. The interfacial interaction between the SS-mesh and TiO2 results in the improved photovoltaic performance of the device. The device has produced a PCE of 5.26% by incorporating porphyrin sensitizer dye and Co-based electrolyte. 49
Regardless of these positive claims, DSSCs have been hampered by the following inherent issues(1): The device design is quite thick because of the two glass substrates, limiting its effective incorporation in IoT devices. 2 Increased cost due to two glass substrates, which maintain a significant share of all DSSC products. 3,45,50,51 The method of having a Cu redox and a hole transport layer between PE and CE is impractical since the Cu electrolyte's solvent must be evaporated for an extended period (72–96 h), which is problematic for quick batch manufacturing.6 (4) Drilled hole increases the non-functional region, overall cell resistance, and manufacturing cost by requiring extra thermoplastic and UV adhesive to seal them. 6,34 (5) The long-term chemical stability of the PEDOT catalyst is rarely investigated and therefore warrants deeper insight, as it was recently accused of causing electrolyte degradation under standard AM 1.5 G applied illumination. 52
Advanced Novel Redox Shuttles
The electrolyte is responsible for both charge transfer between the two electrodes and dye regeneration during DSSC operation. Electrolyte provides absolute ionic conductivity between the PE and CE. It has a significant impact on the solar cell's long-term stability and light-to-electric energy conversion efficiency. Along with device architecture advancements, electrolyte compositions using alternate redox shuttles have made tremendous progress in recent years and have substantially increased the PCE of DSSCs. 30,32,34,46,53,54
Despite their remarkable power conversion efficiency and superior stability, conventional I− /I3 −-based electrolytes have been rapidly replaced with new alternate redox couples to address their inherent constraints, such as their lower redox potential that certainly affects the stability and photovoltaic performance of the device. 29,55–57
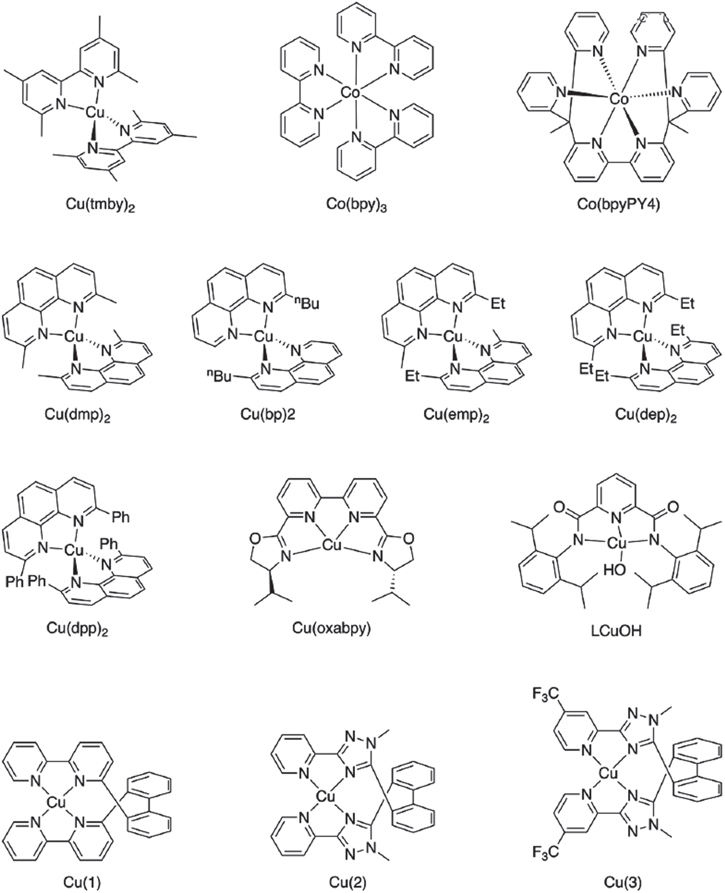
Recently, cobalt (Co) and copper (Cu)-based redox shuttles (Fig. 4) have gained significant interest owing to their rapid dye regeneration capability with a minimal driving force, minimized visible range absorption, ability to produce a higher open-circuit voltage (VOC), and well-matched properties with other catalyst materials (Fig. 5). In addition, they are capable of producing impressive photoconversion efficiencies under ambient light conditions. 36,37,58–64 Co-based redox shuttle offers surprisingly high open-circuit voltages due to their tunable redox potential. 59,65–71 In a recent report, Selvaraj and Shanmugam have synthesized novel cobalt redox and thiophene-based additive using a cost-effective approach and accomplished a PCE of 4.50% with an open-circuit voltage of 795 mV. 71 In a similar report, Kim et al. developed a flexible DSSC incorporating an organic dye (JH-1) sensitized hierarchically structured TiO2 photoanode and Co(III/II)(bpy-pz)3](PF6)3/2 redox system electrolyte. The device has exhibited an impressive photoconversion efficiency of 6.12% under full applied illumination with a current density of 9.17 mA cm−2 and a superior open-circuit voltage of 0.953 V. 72
Figure 4. Structures of Cu and Co-based redox electrolytes and hole-transporting materials used for the fabrication of highly efficient DSSCs. (reproduced from Ref. 57 with permission).
Download figure:
Standard image High-resolution imageFigure 5. The energetic in DSSCs concerning the redox potentials of the redox couples I−/I3 −, [Co(bpy)3]2+/3+, and [Cu(dmp)2]1+/2+ used in DSSCs. 64
Download figure:
Standard image High-resolution imageIn another research, Yan et al. showed a high-performance DSSC by supplementing the standard cobalt complex electrolyte with an organic electron donor, tris(4-methoxyphenyl)amine. The modified Co-based electrolyte significantly improved the photovoltaic parameters of the prepared DSSC. The fabricated DSSCs exhibited remarkably high PCE of 11.7% and 10.5% at 0.46 sun intensity and AM-1.5 simulated solar flux. Additionally, after 250 h of continuous operation under AM-1.5 full sunlight illumination, the prepared DSSC retained 90% of its initial efficiency. 73 These encouraging findings prompted further investigation of different cobalt redox-based electrolyte structures using carbon-based or polymer-based potential alternative catalysts, demonstrating excellent performance and remarkably high photoconversion efficiencies of 12%–13% when tested under maximum sunlight exposure. 32,46 To date, laboratory-scale DSSCs incorporating [Co(phen)3]2+/3+based redox couples and a co-sensitized (ADEKA-1 and LEG4 dyes) photoanode have exceeded over 14% PCE. This photoconversion efficiency is the highest reported so far for a DSSC employing Co-based electrolytes. 33
Nonetheless, considering the enormous potential for better conversion efficiencies, the main shortcomings identified with the Co redox operation include high charge carrier recombination and poor charge transport owing to heavy ligands, limiting short-circuit current densities under full sunlight. 74
As with Co-based redox couples, the ability to tune the redox potential of Cu redox complexes through ligand engineering enables better redox potential matching to the dye's HOMO level. Thereby allowing it to produce remarkably high (> 1) open-circuit voltages with reasonable short-circuit current densities. 6,38,63 As a result, the fabricated DSSCs containing these Cu-based redox electrolytes demonstrated extraordinarily high photoconversion efficiencies in both simulated full sunlight and low light environments. 6,38,57
Recently, there has been a rapid surge in copper-based electrolyte studies due to its small molecular size, deficient driving force, high diffusion coefficient, enhanced charge collection efficiency, and improved mass transport. 63,75–84 In this regard, Sourava et al. performed a comparative investigation between copper, cobalt, and iodine redox electrolytes on TiO2 DSSCs sensitized with the same dye (LEG4). The [Cu(dmp)2]1+/2+ electrolyte-based DSSC performed exceptionally well among the three electrolytes due to improved regeneration and higher charge collection efficiency. Additionally, the PCE of [Cu(dmp)2]1+/2+ based DSSC was superior relative to both [Co(bpy)3]2+/3+ and I−/I3 − based DSSCs. 81 Ellie et al. fabricated a CuI/II(tmby)2 (tmby = (4,4',6,6'-tetramethyl-2,2'-bipyridine)) redox electrolyte-based co-sensitized DSSCs by supplementing a less expensive π-A dye (5T) dye with a D-A-π-A dye (XY1) dye. The fabricated DSSCs were tested under different simulated incident fluxes of 1 sun and 0.1 sun conditions. The co-sensitized (5T + XY1) DSSCs exhibited superior solar-to-electrical power conversion efficiency of 9.5% and 10.2% with an open-circuit voltage of 1.04 V and 0.95 V at 1 sun and 0.1 sun conditions, respectively. Additionally, the same device achieved an impressive PCE of 29.2% when operated in ambient light conditions. 82 Saygili et al. designed and investigated two copper redox mediators [Cu(beto)2]1+ (beto = 4,4'-diethoxy-6,6'-dimethyl-2,2'-bipyridine) and [Cu(beto2Ox)2]1+ (beto2Ox = 4,4'-bis(2-methoxyethoxy)−6,6'-dimethyl-2,2'-bipyridine) with extended side chains in liquid state and solid-state DSSCs. They further claimed that the PCE of liquid state devices was over 10% with a high VOC > 1 V, whereas, for solid-state devices, it was significantly low. 83
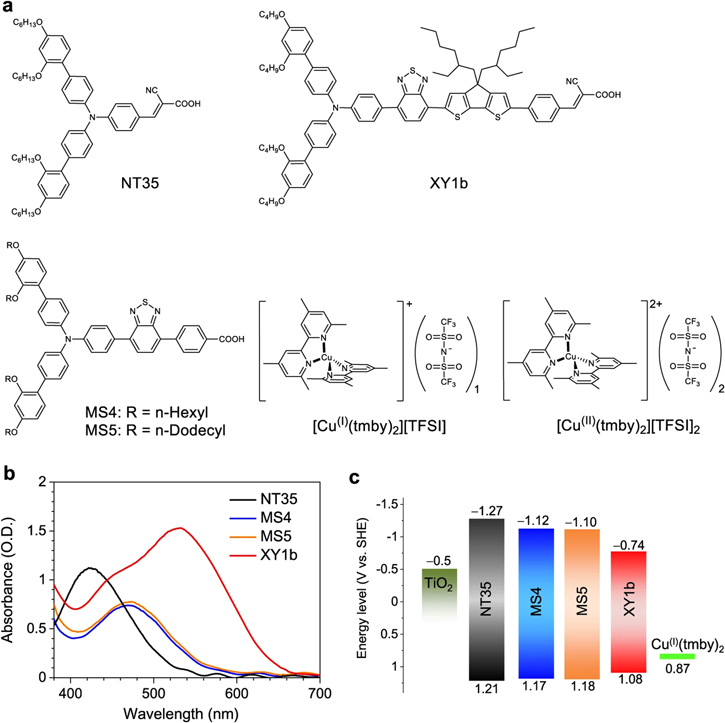
In a recent report, Zhang et al. designed and synthesized thoughtfully tailored organic dyes NT35, MS4, MS5, and XY1b along with copper complex (II/I) electrolyte for TiO2 DSSC (Fig. 6). Among all dyes, the synergistic combination of MS5 dye and copper (II/I) electrolyte yielded a remarkably high VOC of 1.24 V with a PCE of 8%. In addition, a co-sensitized DSSC sensitized with MS5, and another high-performing dye XY1b produced a superior PCE of 13.5% under AM 1.5 simulated solar radiation. Furthermore, a DSSC with an effective active area of 2.8 cm2 sensitized with MS5 and XY1b sensitizers accomplished an astonishingly high PCE of 34.5%, VOC of 0.98 V, and a maximum power output of 109.8 μW cm−2 under ambient light at 1000 lux. 37
Figure 6. (a) Molecular structures of various dyes and copper complex ([Cu(I)(tmby)2][TFSI] and [Cu(II)(tmby)2][TFSI]2, tmby = 4,4',6,6'-tetramethyl-2,2'-bipyridine; TFSI = bis(trifluoromethylsulfonyl)imide). (b) UV–Vis absorption spectra of various dyes used in this study. (c) Energy levels diagram of TiO2, dyes, and [Cu(I)tmby)2][TFSI]. 37
Download figure:
Standard image High-resolution imageAll the findings discussed above are promising and suggest that DSSCs incorporating copper (II/I)-based redox shuttles are an efficient and attractive choice for indoor light harvesting energy sources for low-power electronic devices. Moreover, the Cu redox shuttles have shown enough promise to be used as solid-state hole conductors, limiting the leakage issues concerned with liquid electrolytes. Future research may be projected towards Cu redox-based printable solid-state hole conductors, resulting in the development of not just fully printed lab-scale DSSCs but also large-scale fully printed DSSC modules, one of the aims of this prospective PV technology. Table I lists some of the high-performing DSSCs that have been fabricated using alternative electrolytes based on Co and Cu.
Table I. Some of the highly efficient DSSCs incorporating alternative Co- and Cu-based redox shuttles.
| Electrolyte | Dye | Light Intensity (mW cm−2) | PCE (%) | VOC (V) | Year | References |
|---|---|---|---|---|---|---|
| [Co(bpy)3](PF6)2, [Co(bpy)3](PF6)3, TPAA | D35 + Dynamo blue | 100 | 10.5 | 0.92 | 2016 | 73 |
| 46 | 11.7 | 0.89 | ||||
| [Co2+(phen)3](PF6 −)2, [Co3+(phen)3](PF6 −)3, TBAPF, TBPPF, TBP, TMSP | ADEKA-1 + LEG4 | 100 | 14.3 | 1.01 | 2015 | 33 |
| 50 | 14.7 | 0.99 | ||||
| [CoII(bpy)3](B(CN)4)2, [CoIII(bpy)3](B(CN)4)3), LiClO4, TBP | ZL003 | 100 | 13.6 | 0.95 | 2019 | 35 |
| [Co(bpy)3](TFSI)2, [Co(bpy)3](TFSI)2, TBP., LiTFSI | MK2 | 100 | 9.42 | 0.89 | 2017 | 43 |
| ([Cu(I)(tmby)2][TFSI], [Cu(II)(tmby)2][TFSI]2 | MS5 + XY1b | 100 | 13.5 | 1.05 | 2021 | 37 |
| 1000 lux | 34.5 | 0.98 | ||||
| 500 lux | 32.3 | 0.95 | ||||
| 200 lux | 32.4 | 0.92 | ||||
| [Cu(beto)2]1+, [Cu(beto2Ox)2]1+ | Y123 | 100 | 10.4 | 1.08 | 2020 | 83 |
| Cu(tmby)2TFSI, Cu(tmby)2TFSI2, TBP. | XY1:L1 | 100 | 11.5 | 0.98 | 2020 | 6 |
| 1000 lux | 34 | 0.91 | ||||
| 500 lux | 32.7 | 0.88 | ||||
| 200 lux | 31.4 | 0.84 | ||||
| ([Cu(I)(tmby)2][TFSI], [Cu(II)(tmby)2][TFSI]2 | D35 + XY1 | 100 | 11.3 | 1.03 | 2017 | 85 |
| 1000 lux | 28.9 | 0.79 | ||||
| 200 lux | 25.5 | 0.73 |
Novel TiO2 Photoelectrodes
While several semiconductor oxides (TiO2, ZnO, Nb2O5, SnO2, Zn2SnO4, SrTiO3, and WO3) in DSSCs have been investigated, the nanoparticle-driven charge transport layer of titanium dioxide (TiO2) is perhaps the most effective photoelectrode in the DSSC structure due to its numerous properties, including high chemical stability, ease of synthesis, porous structure, high surface area, printability, and durability. 85 Furthermore, recent years have seen the emergence of intriguing trends and tactics, with improved designs of conventional TiO2 photoelectrodes, for reaching world-class photovoltaic performance using improved molecular sensitizer dyes and redox electrolytes. 30,34,57,85 The first-generation DSSCs typically employ a 10–20 micrometer thick layer of nanoporous TiO2, sensitized with ruthenium-based sensitizers and coupled with I−/I3 − based redox shuttles to produce reasonably high short-circuit current densities (JSC). However, DSSCs with such thick nanoporous TiO2 layers, when operated with advanced redox shuttles (Co and Cu-based), could not produce acceptable current densities due to increased diffusion resistance (RD), thereby limiting the PCE of the DSSC. Nevertheless, numerous strategies are being developed to address the concern of poor light absorption by constructing thoughtfully tailored sensitizers with high absorption coefficients to harness the maximum portion of available sunlight. By sensitizing considerably thinner TiO2 photoelectrodes, these tailored dyes could yield similar JSCs. 86,87
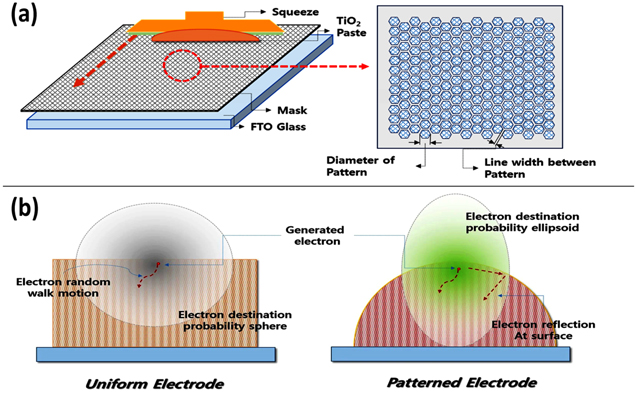
Moreover, various unique strategies, such as the fabrication of screen-printed patterned photoelectrodes and their porosity tuning, have been adopted for the performance improvement of the DSSCs. Yun et al. demonstrated a repetitive screen-printing process to create a patterned electrode (Fig. 7). This imprinted electrode had a hexagonal pattern and was densely arranged to minimize loss. 88 This patterned electrode boosted the PCE of the device by 12.7% due to increased electron diffusion and the electrode's improved photon distribution. Chen et al. produced hierarchical TiO2 nanotubes (HTNT) using a straightforward one-step hydrothermal technique and employed them as pathways for rapid electron transport in the photoanode of the DSSC. 89 Many small sections of TiO2 nanorods and one-dimensional (1D) long nanotubes trunks are included in the produced HTNTs. The efficiency of the champion device was about 18.5% higher relative to P-25-based DSSC. Furthermore, the TiO2 films with a range of TiO2 nanoparticle sizes were also used to overcome the diffusion constraints of Co and Cu-based bulky redox shuttles in the constructed DSSC devices. 87
Figure 7. (a) Schematic illustration of screen printing utilizing the mask for depositing patterned TiO2 paste on FTO glass. (b) Schemes demonstrating the influence of the pattern size on the diffusivity of the electron in uniform and patterned DSSCs photoanodes. (reproduced from Ref. 88 with permission).
Download figure:
Standard image High-resolution imageApart from these unique tactics, the concentration changes in the TiCl4 post-treatment approach for these mesoporous TiO2 layers were also proposed. 46,90 This approach has a proven effect on the JSC of the cell when examined with Co-based redox shuttles. This appears reasonable given the benefits observed with I−/I3 − redox electrolytes treated with uniform TiCl4 concentrations of 40–50 mM, including elevated dye adsorption and increased quantum yield. 90–92 Further, a concentration-dependent study of TiCl4 solution could enable accurate monitoring of diffusion properties of Co-based redox shuttles and, therefore, accordingly can provide effective pathways to achieve higher JSCs by minimizing the diffusion resistance. 87 Interestingly, these improved and thinner TiO2 photoelectrodes have demonstrated remarkably high PCEs of 12%–13% with Co-based redox shuttles and porphyrin sensitizers. 30,32 Additionally, when sensitized with advanced sensitizers and operated under ambient and standard solar illuminations, DSSCs based on Cu redox shuttles combined with these thinner and state-of-the-art photoelectrodes have recently demonstrated surprisingly higher PCEs. 82–84,93 Table II summarizes some of the best DSSCs developed using these upgraded TiO2 photoelectrodes in combination with different sensitizers, along with alternate redox electrolytes based on I−/I3 −, Cu, and Co.
Table II. Some of the best DSSCs developed using upgraded TiO2 photoelectrodes combined with different sensitizers, I−/I3 −, Cu, and Co-based alternate redox electrolytes.
| Photoelectrode | TiO2 film thickness (μm) | TiCl4 conc. (mM), pre and post treatment | Electrolyte | Sensitizer Dye | PCE (%) | Stability of the device @ full sun | References |
|---|---|---|---|---|---|---|---|
| TiO2 | 14 | Not reported | I−/I3 − | NKX-2569 | 6.8 | Not reported | 94 |
| 14 | Not reported | I−/I3 − | NKX-2600 | 5.9 | |||
| TiO2 | 4.5 | 40 mM, Post | I−/I3 − | CDNA | 4.72 | Not reported | 95 |
| TiO2 | 12 | 50 mM Pre + 50 mM Post | I−/I3 − | N719 | 8.33 | Not reported | 96 |
| TiO2 | 6 | 53 mM, Pre | [Cu(beto)2]1+, [Cu(beto2Ox)2]1+ | Y123 | 10.4 | Not reported | 83 |
| TiO2 | 4 | 50 mM Pre + 40mM Post | ([Cu(I)(tmby)2][TFSI], [Cu(II)(tmby)2][TFSI]2 | MS5 + XY1b | 13.5 | DSSC exhibited 7% of loss in PCE @ 45 °C after 1000 h | 37 |
| 4 | XY1b | 11.8 | |||||
| TiO2 | 4 | Not reported | Cu(tmby)2TFSI, Cu(tmby)2TFSI2, TBP. | XY1:L1 | 11.5 | Not reported | 6 |
| TiO2 | 3.5 | Not reported | Co(bpy)3(TFSI)2, Co(bpy)3(TFSI)3, TBP | SM315 | 13.0 | No significant loss in PCE was detected @ 25 °C after 500 h | 32 |
| 3.5 | Not reported | SM371 | 12.0 | ||||
| TiO2 | 4.5 | Not reported | [Co2+(phen)3](PF6 −)2, [Co3+(phen)3](PF6 −)3, TBAPF, TBPPF, TBP, TMSP | ADEKA-1 + LEG4 | 14.3 | Not reported | 33 |
| TiO2 | 6 | 40 mM Pre + 40 mM Post | [CoII(bpy)3](B(CN)4)2, [CoIII(bpy)3](B(CN)4)3), LiClO4, TBP | ZL003 | 13.6 | DSSC shows 15% loss in PCE after 50 days of operation | 35 |
| TiO2 | Not reported | Not reported | Co(bpy)3 2+/3+ | SGT-149 + SGT-021 | 14.2 | DSSC exhibited 17% of loss in PCE @ 50 °C after 1000 h | 36 |
Advanced Printable Dyes
The development of advanced and stable dye formulations for effective light harvesting has always been a key emphasis area in DSSC research, and it continues to be so today. 97–99 However, little focus has been paid to conventional and prolonged dye-sensitization methods, which certainly hinder their speedy production and limit their widespread applicability. 100–102
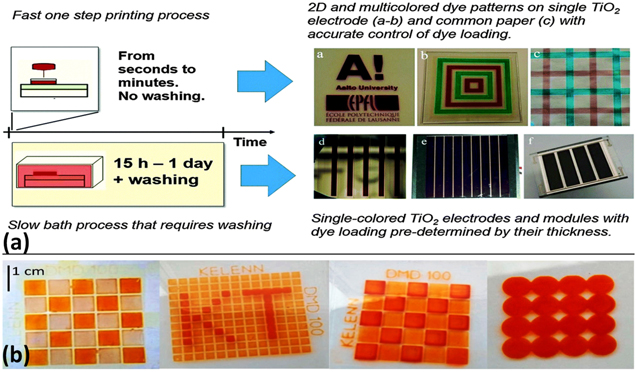
Hashmi and colleagues showed fast sensitization of TiO2 photoelectrodes using a scalable and proven inkjet printing technique wherein the dye loading over the photoelectrodes could be precisely controlled (Fig. 8a). 103 In terms of the method by which the dye molecules are adsorbed onto the surface of TiO2 nanoparticles, inkjet printing is fundamentally comparable to the classic soaking procedure. The sole difference is that the former uses a far more concentrated dye solution and exhibits excellent control over the droplet deposition. More importantly, in addition to rapid dye adsorption and a shorter sensitization period, this method offers several advantages, such as precise control over the transparency of the TiO2 photoelectrode by adjusting the dye amount and achieving panchromatic absorption by the co-sensitization with different dyes, which helps in producing digitally printed DSSC modules for indoor and outdoor applications. 104–107 Additionally, inert ambient requirements for sensitizing TiO2 photoelectrodes may be avoided if the inkjet sensitization strategy is employed. This is because the dye ink is kept enclosed in the cartridge and is deposited from a very thin nozzle in the form of tiny droplets, thereby reducing the potential threat of contamination and moisture. As a consequence, this functional dye solution will undoubtedly aid in the long-lasting photovoltaic performance stability of manufactured DSSCs under a variety of adverse environmental circumstances.
Figure 8. (a) A schematic illustration for rapid sensitization of DSSC photoelectrodes using inkjet printing and the traditional sensitization method. (b) various patterns printed on FTO photoelectrode using DMD technique (reproduced from Refs. 103 and 108 with permission).
Download figure:
Standard image High-resolution imageNevertheless, the low chemical compatibility of dye solvents with the cartridge print head nozzles is a potential concern linked to the inkjet printing of dyes. Using strong solvent-based dyes may cause undesired chemical reactions, clogging the cartridge print head nozzles and reducing accuracy, which is highly undesirable for producing high-performance DSSCs.
Recently, Raissi and co-workers have shown advantageously fabricated DSSC photoelectrodes (Fig. 8b) by the digital material deposition (DMD) technique. 108 Using this unique and rapid technique, authors have shown the prospects for deposition and sensitization of TiO2 thin films on a single platform. Interestingly, DSSCs incorporating this technique have demonstrated superior solar-to-electrical performance and less preparation time than DSSCs fabricated using the classical approaches. Printing using digital technology is distinct from inkjet printing in that it uses a three-dimensional platform to manage an extrusion phenomenon that can print a more extensive range of materials and viscosities ranging from 5 cPs to 40,000 cPs. Contrarily, inkjet printing uses several tiny nozzles per wafer to print only low viscous inks (from 5 cPs to 20 cPs). 108
Notably, DMD technology allows solar cells to be produced faster and more efficiently than equivalent printed films using inkjet techniques. This technology simplifies the production process of DSSC by decreasing material consumption and offers a unique option for the production of screen printing, spray coating, and inkjet printing techniques for DSSC devices. Although numerous successful demonstrations of DMD and inkjet techniques with several materials and viscosities have been published, further research is necessary to discover additional suitable solvents that can allow printing technology of the dye phase for the future generation of printable DSSCs.
Advanced Catalyst Materials for CE
Despite significant advancements in redox electrolytes, sensitizer dyes, and photoelectrodes, a fascinating change in defining conventional catalyst materials suitable to be employed with advanced redox shuttles has arisen in recent years. 84,85 Since the birth of DSSC, researchers have shown tremendous interest in platinum (Pt) based catalysts for producing efficient DSSCs. 109–114 Due to their high catalytic activity and mechanical and chemical stability, Pt counter electrode-based DSSCs have exhibited excellent PCEs surpassing over 11% when tested with traditional I−/I3 − redox shuttle electrolytes. Additionally, DSSCs incorporating Pt as CE have demonstrated strong stability, exhibiting no degradation in efficiency when exposed to long-term stability testing under a variety of simulated circumstances. 61,115 However, when tested with Cu and Co-based redox shuttles, Pt catalyst exhibits poor catalytic activity. 116–120 Further, the use of platinum as a catalyst in CE contributes significantly to the overall cost of DSSCs.
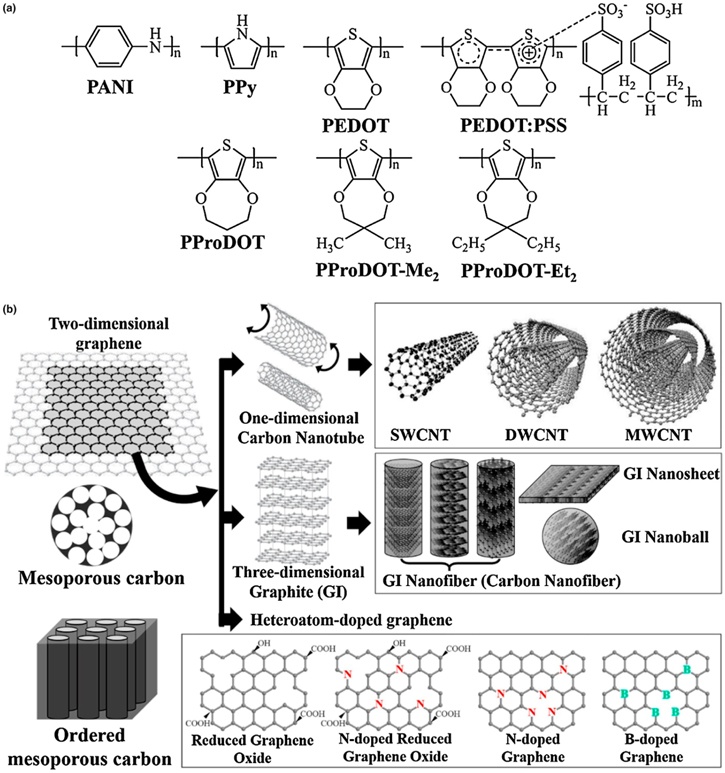
To address these limitations, conducting polymers and carbonaceous materials have emerged as a promising choice due to their high catalytic activity, excellent electrical conductivity, low cost, and superior stability for the regeneration of redox electrolytes. The conducting polymers benefit from greater transparency, which enables them to be utilized as bifacial DSSCs that can be lighted from both sides, implying that conducting polymers are highly adaptable to various DSSC applications. Among a variety of conducting polymers (Fig. 9a), including polyaniline (PANI), polypyrrole (Ppy), poly(3,4-ethylene-dioxythiophene) (PEDOT), poly(3,4-ethylene dioxythiophene): poly(styrene sulfonate) (PEDOT: PSS), etc., the PEDOT catalyst has exhibited proven electrocatalytic stability and improved PCEs when tested with Cu and Co-based advanced redox electrolytes under simulated indoor and outdoor lighting conditions. 6,34,85 However, the electrochemical stability of the PEDOT catalyst is still unresolved, as very few reports have been published on the long-term stability of the DSSC incorporating PEDOT catalyst and Cu or Co-based redox electrolytes. 6,121 This undoubtedly raises concerns about the steady and prolonged photovoltaic functioning of these advanced DSSCs in indoor applications such as IoT and portable microelectronics or when subjected to natural outdoor conditions for bulk power production.
Figure 9. Schematic structures of (a) various conducting polymers and (b) carbonaceous materials for the counter electrode of DSSCs. (reproduced from Ref. 122 with permission).
Download figure:
Standard image High-resolution imageRecently, Zhang et al. demonstrated the long-term stability (500 h) of a DSSC employing electrodeposited PEDOT catalyst-based CE in conjunction with Cu-based redox electrolyte under ambient light conditions. The device was co-sensitized with organic photosensitizers (MS5 + XY1b) and tested under various simulated indoor and outdoor conditions. 37 In another study, steady charge transfer resistance (RCT) was reported in DSSCs fabricated using an organic dye (Y123) and Co-based electrolytes over a prolonged period (2000 h) for PEDOT catalyst-based CEs. 52 Nonetheless, the degradation of redox electrolytes was predicted due to a progressive rise in diffusion resistance, which negatively impacted the short-circuit current density (JSC). Thus, further progress in elucidating the electrochemical nature of the PEDOT catalyst to increase its chemical stability is highly anticipated in the near future.
On the other hand, several carbonaceous materials (Fig. 9b) such as graphene and carbon nanotubes (CNTs) have emerged as a potential alternative to Pt-based catalysts due to their low cost, high surface area, superior electrical conductivity, and precision printability (Fig. 10). Recent findings using graphene, single (SWCNTs), and multi-walled carbon nanotubes (MWCNTs)-based CEs have displayed similar catalytic activity, electrochemical stability, and solar-to-electrical conversion efficiency, as well as the promise for significantly higher conductivity relative to Pt-based CEs. 123–127 Furthermore, with Co-based advanced electrolytes, these carbonaceous materials have demonstrated astonishingly high PCEs of more than 14% when integrated as CE in DSSC. 128
Figure 10. Characteristic properties of high-performing carbonaceous counter electrode in DSSCs.
Download figure:
Standard image High-resolution imageTo advance the use of carbonaceous catalyst-based CEs, Kavan et al. studied the electrochemical performance of Gr-based CEs in DSSCs using Cu-based electrolytes and claimed similar catalytic activity to those based on electrodeposited PEDOT catalysts and exceptionally superior to Pt catalysts. 127 Furthermore, the same researchers revealed a highly functional composite catalyst that was produced by combining Pt, platinum oxide (PtOx), graphene oxide, and graphene nanoplatelets and studied as CE in DSSCs with Cu-based redox shuttles. 129 The synthesized composite outperformed the Pt, PEDOT, and pure graphene-based catalyst materials by exhibiting excellent catalytic activity, resulting in extremely high PCE ∼9.5%–11.3% when evaluated under different applied illuminations. 129 Among various carbonaceous materials, graphene nanoplatelets have been extensively studied as catalysts with Co and Cu-based redox electrolytes. Due to their excellent catalytic performance and low charge transfer resistance, these catalysts outperformed conventional Pt catalysts as observed in pertinent DSSCs. 127,130
However, the electrodeposited PEDOT and graphene nanoplatelet-based catalysts addressed in this study have constraints associated with their precise deposition techniques, preventing them from fabricating large-area modules for bulk energy generation. 6,61 This casts doubts over their large-scale production, reproducibility, and reliable photovoltaic performance.
In this regard, Hashmi and co-workers recently presented a more realistic solution to the problem of scalable DSSC manufacturing using improved sets of ingredients in printable single-wall carbon nanotubes (SWCNT)-based CEs. 44 When tested with Cu electrolytes, the printed SWCNTs surpassed Pt/TCO in terms of catalytic activity, displaying extremely low charge transfer resistance (RCT = 2–2.9 Ω cm2). Interestingly, the developed DSSC demonstrated excellent PCEs of 7.5% and 8.3% under simulated full and half-sun incident light. In a very recent report, Michal et al. demonstrated the fabrication of screen-printed CEs using carbon dispersions in diverse mass ratios. Among all dispersions, a DSSC based on 100% carbon black and an I−/I3 − redox shuttle outperformed the others with a PCE of 3.05%. 131 Notably, despite the underlying PCE, these findings are impressive and present the prospects for their ease of fabrication and precision printing of future generations of DSSCs.
All these encouraging results facilitate the adoption of more cost-effective methods in the near future. As mentioned above, various carbon allotropes and their composites, in conjunction with conventional I−/I3 − electrolytes, also enable the production of more economical and cost-effective CEs that can be printed using proven scaling technologies like screen printing or inkjet printing. Although electrodeposited PEDOT catalyst films have been tested and proven using Co and Cu-based redox electrolytes, no research group has established their scalability. In contrast, the synthesis of printable carbonaceous pastes has been proven in many studies and is also commercially accessible from various material suppliers. Nevertheless, these low-cost and printable carbon composites ought to be explored with Cu-based advanced electrolytes to produce scalable DSSC modules as a future step in DSSC research.
Moreover, the development of more combinations of carbonaceous materials and PEDOT, which are already reported for rigid and flexible DSSCs using I−/I3 − electrolytes, may also be anticipated in the future. 132,133 Nonetheless, considering the prevailing trend toward increasing PCEs through new and advanced materials, future research for these futuristic DSSCs may also be predicted on developing reliable and robust DSSCs with long-term stability associated with each material combination could be published with certified efficiency and stability tests. Interestingly, several fascinating long-term stability studies under standard environmental stress conditions could be anticipated, for example, observing the degradation of advanced Cu redox electrolytes from PE and CE substrates in DSSCs or their corrosive nature if a metal supports either PE or CE. In this way, an optimum electrolyte-catalyst material combination may contribute significantly to the high performance and stable next-generation DSSCs. Table III highlights the best and most current DSSC efficiencies achieved by combining conventional I−/I3 − and new Co- and Cu-based redox electrolytes with different catalyst materials.
Table III. A partial list of literature studies on the DSSCs with different catalyst materials.
| Catalyst (CE) | Redox couple | Dye | RCT (Ω cm2) | η (%) | η of Pt (%) | Stability | References |
|---|---|---|---|---|---|---|---|
| Graphene | I−/I3 − | N719 | 6.02 | 8.19 | 8.89 | Not reported | 134 |
| SWCNT | I−/I3 − | N719 | 6.72 | 7.61 | 8.49 | 91% (after 600 h) | 135 |
| DWCNT | I−/I3 − | N719 | 3.13 | 8.03 | 94% (after 600 h) | ||
| MWCNT | I−/I3 − | N719 | 10.0 | 7.06 | 90% (after 600 h) | ||
| CNTs | I−/I3 − | N719 | 1.98 | 9.34 | 9.09 | Not reported | 136 |
| Carbon | I−/I3 − | N719 | 2.96 | 9.10 | Not reported | Not reported | 137 |
| Graphene | Co2+/Co3+ | Y123 | 3.3 | 9.4 | 8.2 | Not reported | 138 |
| Carbon | Co2+/Co3+ | 51–57dht.1 | 2.92 | 9.53 | Not reported | Not reported | 139 |
| Boran/Graphene composite | Co2+/Co3+ | JK-303 | 1.41 | 9.21 | 8.45 | Not reported | 140 |
| N-doped graphene nanoplatelets | Co2+/Co3+ | JK-225 | 1.73 | 9.05 | 8.43 | Not reported | 141 |
| SWCNT | Cu(I/II) | Y123 | ∼2.1–2.9 | 7.5 | 6.2 | Not reported | 44 |
| PEDOT | I−/I3 − | N719 | 10.54 | 8.50 | 8.75 | Not reported | 142 |
| PEDOT | Co2+/Co3+ | Y123 | 2.5 | 8.62 | 8.24 | Not reported | 143 |
| PEDOT | Cu(I/II) | XY1 + L1 | Not reported | 11.5 | Not reported | 16 h 1000 lux at daytime + 8 h in dark for 12 days | 6 |
| PEDOT | Cu(I/II) | XY1b+Y123 | Not reported | 13.1 | Not reported | ∼100 h | 34 |
| PEDOT | Cu(I/II) | MS5 + XY1b | Not reported | 13.5 | Not reported | ∼500 h | 37 |
Advanced Encapsulation Strategies
In addition to ongoing research to improve the photovoltaic conversion efficiencies of DSSCs, numerous traditional strategies and those utilizing novel encapsulating materials are being used to develop stable and robust PV devices. 55,56,115 The sealing of DSSCs requires careful consideration of architectural parameters to ensure excellent performance and economic advantage. Robust encapsulation materials and methods are needed to protect the functional architecture of the DSSCs from external influences to achieve long-term stability and performance. 45,144
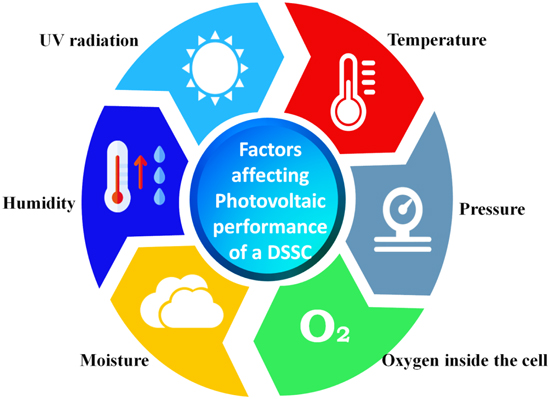
In order to meet these concerns, a sealant must endure the fluctuating climatic conditions over the lifecycle of the DSSC. It should offer substantial structural protection to withstand external and internal stresses that might cause harm to the functional components of the DSSC. The usual degradation factors (Fig. 11) in DSSCs include moisture and oxygen penetration into the device functional region, UV sensitivity, electrolyte spillage, and electrolyte vaporization when exposed to harsh environmental conditions and simulated indoor conditions. 144–146
Figure 11. Illustration of various environmental factors that affects the photovoltaic performance of DSSC devices.
Download figure:
Standard image High-resolution imageFor DSSCs utilizing the iodide/triiodide redox system and ruthenium sensitizers, the best stability data to date has been reported by Desilvestro and co-workers. Their team investigated electrolytes with various solvents (HSS, 3-methoxypropionitrile, and -butyrolactone) to explore the high-temperature stability of such systems, which resulted in relative PCE values of 83%, 60%, and 20% after 1000 h at 85°C in the dark. 147
Peng Wang et al. presented promising findings on the stability of organic sensitizers. 148 They employed co-sensitized organic dyes C268 and SC-4 in conjunction with an electrolyte including DMII and EMII ionic liquids and sulfolane. After 1000 h of 1 sun irradiation at 60 °C, their solar cells lost just 3% of their original PCE (10.1%). For the same system, a 1000-hour stability test in the dark at 85 °C resulted in a 9% loss. Yameng et al. examined the photostability of blue dye R6-sensitized and Co (II/III) electrolyte-based DSSCs and reported a PCE of 12.6% under standard conditions. 149 The DSSC also displayed exceptional photostability, retaining 90% of its original value after 1000 h of continuous exposure to full sunshine at 60 °C. The long-term stability of Z907-sensitized devices with Co(bpy)3 was studied by Jiang et al. 150 Using MPN as an electrolyte solvent, PCE maintained 91% of its initial value after 2000 h of continuous sunlight exposure with open circuit cells.
Furthermore, in contrast to traditional thermoplastics, novel alternatives like impermeable encapsulation to prevent oxygen and water infiltration and metal finger deterioration are necessary for allowing DSSCs to be commercialized for large modules in the near future. 151 In this regard, novel methods are being used to isolate liquid electrolytes in cell channels, wherein PE and CE are bonded together, as demonstrated in the innovative work carried out by Cao et al. with UV curing adhesive for the insulation of liquid electrolytes. 34 The prepared device is then sealed with the same UV-curing adhesive following the electrolyte insertion through drilled holes. When exposed to full sunlight intensity at 45 ºC, the encapsulated device demonstrated brief initial stability of 10 h. 34
In another report, Michaels and co-workers demonstrated a comparable device design with minor changes in the final sealing process. They sealed the device using the conventional DSSC encapsulation technique, wherein the drilled holes were sealed using the traditional thermoplastic and a glass cover strip, followed by electrolyte insertion between the cell channel. The prepared device performed exceptionally well and displayed steady and extended photovoltaic performance for powering an IoT device under ambient lighting conditions. 6 More recently, Zhang et al. demonstrated further progress with enhanced photovoltaic performance; they presented a device design identical to that described by Cao et al. wherein both the electrodes were mechanically pressed together without a spacer and subsequently sealed with UV-curing adhesive. 37 Remarkably, the device demonstrated outstanding PV performance and excellent stability after continuous illumination of 500 h under indoor light conditions. 37 In another report, sun and colleagues synthesized a stable Cu compound containing a pentadendate ligand that did not exhibit easy ligand exchange. 152 The device was sealed using a 25 μm thick surlyn film that retained 90% of its starting value after 400 h at one sun condition, compared to 80% for Cu(tmby)2 electrolyte-based device. All these findings discussed above indicate that the stability of DSSCs is not a big concern, provided all the functional components of DSSCs are designed to perform under stressful conditions.
Although these techniques and their outcomes seem promising, the aforesaid innovative sealing procedure may not be chosen as the standard protocol for DSSCs sealing just yet, as additional experimentation under more demanding conditions is required to validate its absolute limits of resilience. On the other hand, the manual sealing technique appears to be impractical for producing scalable large DSSC modules made of mechanically pressed PE and CE. Nonetheless, the UV—curing adhesive may be immediately screen-printed across sequentially linked functional surfaces, resulting in a durable and resilient solid-state monolithically designed novel device architecture along with printable advanced Cu redox electrolytes (discussed in Section 2) for the rapid production of these advanced DSSCs.
P-DSSCs
Each component of p-DSSCs, including photoanode, photocathode, sensitizer, and redox electrolyte, plays a crucial role in the operation of p-DSSCs. Researchers have made several notable attempts to increase device performance by altering these components. 153–168 To improve the performance of DSSCs, it has been suggested that the TiO2-based photoanode can be paired with a second photoelectrode in a tandem device. When two devices (n-type and p-type) are used in tandem, the VOC is equal to the total of their individual values. As a result, greater light can be collected more effectively. However, p-type DSSCs are significantly less efficient than n-type DSSCs, thereby limiting the efficiency of p-n DSSCs. Because of this, the search for more efficient p-type DSSCs has intensified during the last two decades. In such devices, the bulk of charge carriers in the semiconductor are holes (h+), and the current flows in the opposite direction compared to TiO2-based DSSCs. Following light stimulation of the dye, electron transfer from the semiconductor's valence band occurs to decrease the dye (Fig. 12).
Figure 12. A schematic illustration of the electronic process occurring in a p-DSSC. The black line represents light-induced photoexcitation, whereas the green lines represent the electrons (and holes) in an ideal device. Red lines indicate unwanted recombination reactions. (reproduced from Ref. 153 with permission).
Download figure:
Standard image High-resolution imageThe first p-DSSC was reported in 1999, by Lindquist and colleagues, who employed erythrosin B as the photosensitizer and a layer of NiO as a p-type semiconductor. The PCE for this device was 0.0076%. Enhanced NiO quality and development of a dye, particularly for NiO, allowed this to be improved to 0.41% efficiency by 2010. However, the efficiency of tandem cells was significantly constrained because of the poor p-DSSC efficiency compared to n-type devices. The fast charge recombination at the dye/NiO and NiO/electrolyte interfaces is a crucial factor limiting the efficiency of p-DSSCs. Creating photosensitizers that facilitate charge separation and novel iodide-free redox mediators can significantly enhance device efficiency. In this regard, Bach et al. developed highly efficient p-DSSCs by incorporating [Co(en)3]2+/3+ based electrolytes. 169 Under simulated sunlight, the developed p-DSSC exhibited a PCE of 1.3% with an open-circuit voltage of 709 mV. Powar et al. synthesized nickel(II) oxide micro balls with a highly crystalline nanostructure (NiO-Bs) for p-DSSC. An excellent photocurrent density of 7.0 mA cm−2 was achieved with an IPCE of 74% and an overall PCE of 0.46%. 170 later, the same group developed a molecularly engineered donor-acceptor porphyrin-based dye ZnP1 for p-DSSC and obtained a PCE of 0.92% under standard one sun applied illumination. 171 This was the highest PCE reported for porphyrin-based p-DSSCs. Recent work by Zhou et al. used triphenylamine as the donor and a di-carboxylic acid group as the anchoring group for p-DSSCs to develop and synthesize new D-A dyes cTPAPh-NO2 and cTPABT-PyCN2, with PCEs of 0.103%. 172 Despite these encouraging findings, the PCEs of p-DSSCs are still insignificant. Therefore, more research is needed to determine how p-type devices might benefit from developing dyes that are both affordable and have excellent charge separation capabilities.
Tandem DSSCs
When compared to a single photoelectrode, tandem DSSCs have the potential to improve solar cell efficiency. Photons with greater energies are collected by the top electrode, whereas the bottom electrode captures lower ones. However, the photocathodes' poor performance restricts the tandem DSSC's performance. The early investigations concentrated on demonstrating the idea that the VOC of the tandem DSSC is the total of the individual n-type and p-type DSSCs, but low photocurrents and low fill factors plagued the devices. As the redox mediator, these initial tandem DSSCs commonly used I−/I3 −, but its substitution with metal complexes and commercial photo enhancement has resulted in enhanced performance. 173 To complement cutting-edge photosensitizers for TiO2 devices, significant progress has been achieved in developing dyes that absorb in the red to NIR area of the solar spectrum. Gibson et al., for instance, developed a tandem cell with up to 5.2 mA cm−2 using the cationic charge-transfer dye CAD3 on NiO and a standard charge-transfer dye D35 on TiO2. 174 Bonomo et al. reported a 2% PCE for p-DSSCs using P1-sensitized NZNC as the photocathode and nanostructured titania (TiO2) sensitized with squaraine VG10-C8 as the photoanode. 175 Bach et al. used PMI-6T-TPA as the dye and Fe(acac)3 as the electrolyte and achieved a better efficiency of 2.42%. 176 With a photocathode made up of NiPcTs on NiO supported on carbon fabric, the most efficient tandem cell to date (≈ 9.76%) was reported by Deepa and colleagues. 177 In order to ensure compatibility with the CdS quantum dots, a polysulfide electrolyte was employed as the electrolyte for the photoanode, which was constructed from a copper@carbon dot conducting core/shell.
Although substantial progress has been made in boosting the photocurrent density by developing new photosensitizers and the photovoltage by developing new redox mediators, the valence band position of the p-type semiconductor continues to limit the efficiency of tandem devices. In order to increase tandem devices' built-in potential, a semiconductor with a lower valence band than NiO or a material with a higher-lying conduction band must be substituted for TiO2. An efficiency of over 20% might be feasible if an alternate p-type transparent semiconductor with a valence band 0.5 V deeper than NiO could be discovered. However, there is yet no straightforward replacement for NiO as stated above.
Summary and prospects
Over the last two decades, major advances have been achieved in DSSCs, especially in terms of performance, durability, and cost benefits, by incorporating new and advanced materials into device fabrications. Accordingly, this review briefly highlighted the current advancements in the DSSC's device architecture, advancements made in photoelectrodes, printable dyes, novel redox shuttles, catalytic materials, and advanced device sealing strategies. The advanced research trends described in this study on the fabrication of next-generation DSSCs indicate that significant strides have been taken towards novel and improved materials, which eventually boost the PV performance of these solar devices in the long run. These novel materials enable the fabrication of innovative DSSC architectures, including mechanically pressed liquid junctions, back-contact TCO-free assembly, and solvent-free solid-state DSSCs. Various strategies have been adopted to improve the functionality of the mesoporous TiO2 photoelectrodes, such as the deposition of a transparent layer and a light-scattering layer of mesoporous TiO2 of an appropriate thickness (∼4 μm) followed by its pre and post-treatment using TiCl4. In contrast to the traditional TiO2 photoelectrode preparation, novel approaches such as modification of TiO2 by certain polymers and carbonaceous materials have been extensively explored and utilized as a template for producing high surface, ideal pore size, and light-scattering functional TiO2 films.
Similarly, the development of judiciously tailored printable organic photosensitizers is equally important for the rapid mass production and commercialization of efficient and stable next-generation DSSCs. The co-sensitization strategy utilizing several photosensitizers with compensating absorption spectra could be a handy strategy to expand the absorption threshold of the sensitizers in the visible and near-infrared portions of the solar spectrum. Further, by incorporating Cu-based advanced electrolytes as solid-state hole conductors in DSSCs, the solar-to-electrical conversion efficiency and stability of the pertinent DSSCs were significantly improved relative to classical DSSCs fabricated in conjunction with I−/I3 − based liquid electrolytes. The present goal in Cu-based quasi-solid-state DSSCs is primarily focused on their precise printability, diffusion rate enhancement, and long-term stability, which would be a key issue for the future study of these advanced quasi-solid-state electrolytes. So far, Pt-free electrocatalytic films based on conducting polymers (such as electrodeposited PEDOT, PANI, PPy, etc) and several carbonaceous materials (such as graphene, rGO, CNTs, carbon black, etc) have been successfully developed and implemented as highly efficient counter electrode for Pt-free DSSCs, and the majority of them have proven their stability and superior electrocatalytic activity towards the regeneration of Co and Cu-based advanced redox shuttles. Thus, the DSSCs incorporating these novel catalysts outperformed the classical DSSCs with Pt-based CE in terms of PV performance.
Additionally, appropriate encapsulation of all the aforementioned functional components of DSSCs is critical for their protection against degradation and exposure to various climatic conditions. UV-curing glue has emerged as a viable approach for preserving the DSSCs' functional design to address this issue. However, more testing under more demanding environments is necessary to establish the system's ultimate robustness limitations. As a result, rapid research and development by scientists and engineers are anticipated in the near future, which may expedite the broad adoption of next-generation DSSC technology at an affordable price. With intensified efforts dedicated to DSSCs, the commercialization of DSSCs is predicted within a few years and will open up new prospects for microelectronics devices, such as IoT sensors, wearable gadgets, and portable electronics.
In contrast to n-type DSSCs, P-DSSCs are uncommon since their PCEs are negligible. Most p-type DSSC performance advancements have stemmed from introducing new dyes and electrolytes. To achieve efficiencies comparable to thin-film solar cells, the VOC must be increased by approximately 0.5 V to equal that of n-type DSSCs. This demands a transparent, conductive, stable, and non-toxic substitute for NiO. Very few materials possess all of these characteristics. A trade-off between current and voltage in p-type DSSCs has to be understood for progress, but thus far, it has been unclear what the restrictions are beyond NiO itself. In order to exploit the full potential of p-n tandem DSSCs, a coordinated effort of materials development and state-of-the-art spectroscopy are required.
Acknowledgments
The authors would like to express their gratitude to all participants in this study, and the members of the Physics departments at Medi-Caps University, Indore, Govt. Holkar Science College, Indore, and S.N.G.P.G. College, Khandwa, for their assistance and support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.