Abstract
Bis(neopentyl glycolato)diboron (BNGDB) is used as a electrolyte additive to improve the stability of commercialized lithium ion batteries operating at a high charging-cutoff voltage. Adding of BNGDB lowers the oxidation potential of the electrolyte, which will lead to the formation of a uniform, stable, and low resistance cathode solid electrolyte interphase (CEI) on the cathode surface. This CEI film can suppress the loss of electrolyte by preventing further reaction between electrolyte and cathode material (LiNi0.6Co0.2Mn0.2O2), which improves cyclic stability and discharge capability of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells. With 0.5 wt% BNGDB addition, the pouch cell demonstrate a discharge capacity retention of 83.2% after 200 cycles in base electrolyte. This work brings new insight into the role of additives in electrolytes and can guide the design of more versatile electrolytes for commercialized lithium-ion batteries.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Due to the high energy density and substantial cycle performance, lithium ion batteries (LIBs) have been widely applied to many portable electronic devices, electric vehicles (EVs), and hybrid electric vehicles (HEVs). 1–3 However, to meet with the the fast updating speed of these commercials, battery systems with higher energy density and longer cycle life are demanded. Increasing the charging voltage of the layered cathode material is a key way to improve the energy density of lithium-ion batteries, 4–7 but this could cause a structural damage of the layered cathode material and an intensified oxidation and decomposition of the electrolyte, which will seriously reduce the cycle life of LIBs. 8–11
The carbonate salts and LiPF6 which exist in the commercialized electrolyte for LIBs are easily oxidized and decomposed under high voltage (4.3 V), 12,13 which result in the interface impedance increase between the electrode and electrolyte. Moreover, the redox reactions of LiPF6 during the charging/discharging cycles generate HF which tends to corrode the cathode material and causes the dissolution of metal ions, and thus leads to the loss of active materials. 9,14–16 In order to deal with these problems, additives, such as sulfur-containing molecules, 17–21 nitriles 22–25 and boronic acids 26–33 were introduced into the commercialized electrolytes to improve the stability of high-voltage LIBs, and an increase in battery cycle life has been reported. J. H. Kim et al. 27 found that lithium bis(oxalate)borate (LiBOB) electrolyte additive sequesters the radical by trapping the PF5 which tends to oxidize EC and DEC electrolyte solvents. Adding 1 wt % LiBOB to the electrolyte significantly improved the cycle life and Coulombic efficiency of the full-cells at 30 and 45 ℃. Y. Lai et al. 31 proposed tributyl borate (TBB) as an additive to improve the stability of LiCoO2 (LCO) cathode operating at a high charging-cutoff voltage. They believed that TBB can form a CEI film with B-containing compound and lower content of LiF on the LCO cathode surface, which is beneficial to improve the stability and decrease the interfacial impedance of LCO cathode. However, Most of boron compounds are very expensive, and the functionality of additives in most of these research work are merely tested on button batteries. It is still important to develop new additives which can be used in commercial high-voltage LIBs to improve their performance.
Herein, we report the functionality of BNGDB as a novel electrolyte additive in improving the stability of high voltage LIBs. We believe BNGDB as a biboron molecule can form a CEI film with more B-containing compounds, which lower the CEI film formation resistance on the surface of commercial LiNi0.6Co0.2Mn0.2O2 cathode. The LiNi0.6Co0.2Mn0.2O2/graphite pouch cell with BNGDB as an additive exhibit significantly improved cycling performance under a high voltage of 4.5 V, due to formation of a uniform, stable CEI and a decrease in the decomposition of the electrolyte.
Experiment
Electrolyte Preparation
1.1 M LiPF6 in ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/diethyl carbonate (DEC), (1/1/1 by weight) was used the base electrolyte (BE). LiPF6, EC, EMC and DEC were provided by Guangzhou Tinci Materials Technology Co., Ltd., China. The 0.25%, 0.5% and 1.0% BNGDB-containing electrolytes (made by Shanghai Aladdin Biochemical Technology Co., Ltd., 98%) were prepared in a highly pure argon-filled glove box, in which oxygen and water content were controlled below 1 ppm.
Pouch Battery Assembly
The cathode slurry was prepared by mixing 97.3% LiNi0.6Co0.2Mn0.2O2 (Rongbai Technology Co., Ltd., Ningbo, China), 1.2% polyvinylidene fluoride (PVDF) as a binder, 0.5% carbon black conductive agent (SP), and 1.0% carbon nanotubes (CNT). After stirring at a standard number of revolutions for 6 h, it was uniformly applied to an aluminum foil (cathode) by a coater at an areal density of 340 g m−2. The anode slurry was artificial graphite (BTR Energy Materials Co., Ltd., Shenzhen, China), carboxymethyl cellulose (CMC), carbon black conductive agent (SP, produced in Japan, battery grade) and styrene butadiene rubber (SBR) with a mass ratio of 95.7%: 1.3%: 1.0%: 2%. After stirring at a standard number of revolutions for 6 h, it was uniformly applied to a copper foil (anode) by a coater at an areal density of 170 g m−2. The prepared materials were placed in a vacuum oven at 120 °C for 20 h before assembling the batteries. The cathode (558.0 mm long and 55.0 mm wide) and anode (708.0 mm long and 59.0 mm wide) together with a celgared 2400 separator (Xingyuan Material Technology Co., Ltd. Shenzhen, China, 750.0 mm long and 61.5 mm wide) were rolled up and then put into the specifc aluminum-plastic to obtain the cells without electrolyte with a nominal capacity of 2000 mAh (0.33 C). The batteries were again placed in a vacuum oven and dried at 85 °C for 48 h. After taking out, 6.5 g electrolyte was injected into the cells. Then the batteries were sealed under a pressure of 5000 N for later tests. Finally, the pouch cells would be 62 mm long, 54 mm wide and 5 mm thick.
Simulation
All theoretical calculations were conducted on the Gaussian 09 W package. The ground-state molecular structure of BNGDB were optimized by the DFT method and using B3LYP/6-311G basis set. The frontier molecular orbital energy of each solvent in the electrolyte was calculated at B3LYP/6-311G (d, p) level. Then we used the Hartree constant of 27.21 for unit conversions to get the final HOMO and LUMO values.
Electrochemical Performance Test
We used linear sweep voltammetry (LSV) to study the electrochemical window of electrolyte in a system with platinum as the working electrode and lithium metal as the reference electrode. Battery testing was performed by Gamry Reference 3000 electrochemical workstation (Metrohm, PGSTAT 302 N, Switzerland). The normal temperature and high voltage performance of 200 cycles in the range of 2.75 V–4.5V was tested on a multi-channel battery performance tester (Neware, CT-3008W-5V/6A, China) with a charging rate of 1.0 C and no pressure applied to pouch cells. A C-rate is a measure of the rate at which a battery is discharged relative to its maximum capacity. A 1.0 C rate means that the discharge current will discharge the entire battery in 1 h. The capacity retention is defined as the ratio of current capacity to initial capacity. Electrochemical impedance spectroscopy (EIS) before and after high voltage cycling was measured by the Solartron 1470E multi-channel electrochemical test system (AMETEK, Solartron-1455A instrument, UK). In the test, the impedance frequency ranged from 10 mHz to 100 kHz with an amplitude disturbance of 10 mV, and all test cells were charged at 4.5 V. 3 cells were evaluated at each set of conditions.
Characterization of Electrodes
To further explore the surface characteristics and elemental distribution of the electrodes, the recycled cells were disassembled in an argon-filled glove box. In order to remove the residual lithium salts and electrolyte on the electrode surface, the cut electrode piece was rinsed three times with pure dimethyl carbonate (DMC). The morphology of the electrodes surface were observed by a scanning electron microscope (SEM, US8010, Japan) and transmission electron microscope (TEM, JEM-2100HR, Japan). The surface chemical compositions of the electrodes were analyzed by X-ray photoelectron spectroscopy (XPS, Axis Ultra DLD, Kratos) with the Al Kα (1486.6 eV) line as the X-ray source. The spectral results were analyzed using XPS-peak software.
Results and Discussion
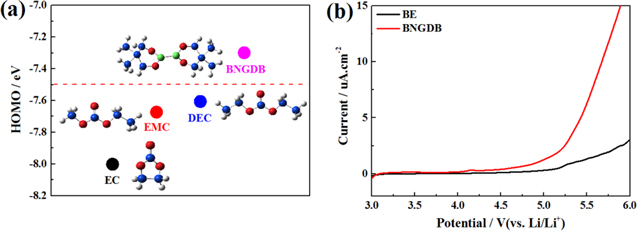
In order to give strong support for preferential oxidation of BNGDB among all three molecules in electrolyte, Gaussian-09w was used to calculate the highest occupied molecular orbital (HOMO) of these carbonate solvents and BNGDB. The calculation results were present in Fig. 1a. The HOMO energy of BNGDB is higher than that of the carbonate solvents (EC, EMC and DEC). It indicates that BNGDB is a better electron donor than other solvents in electrolyte, and thus BNGDB will be preferentially oxidized in the charging process. The Linear sweep voltammetry (LSV) measurement results of BE and BNGDB-containing electrolyte are shown in Fig. 1b. The oxidation of BNGDB-containing electrolyte occurred at 4.06 V, while the oxidation of BE occurred at 4.7 V. This lower oxidation voltage of BNGDB-containing electrolyte may benefit the formation of a uniform, stable, and low resistance CEI.
Figure 1. (a) Chemical structures and calculated HOMO/LUMO energies of EC, EMC, DEC, and BNGDB molecules. (b) LSV profiles for the BE and the BNGDB containing electrolyte.
Download figure:
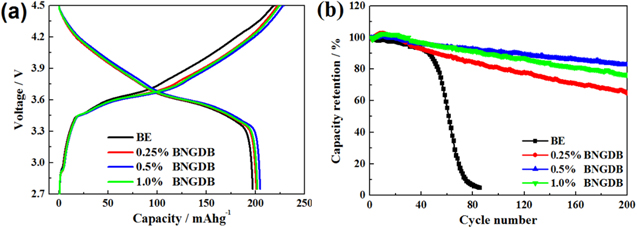
Standard image High-resolution imageFigure 2a shows the first cycle charge-discharge curve of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells with BE and electrolytes with different concentrations of BNGDB at room temperature with a charging rate of 0.2 C. The pouch cell with BE delivers an initial discharge capacity of 196.5 mAh g−1. However, the cells containing 0.25%, 0.5% and 1.0% BNGDB electrolyte demonstrate initial discharge capacities of 200.5, 204.5, and 201.7 mAh g−1, respectively. These results show that BNGDB additive can improve the initial discharge capacity of LiNi0.6Co0.2Mn0.2O2 electrode. The cyclic performances of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells with BE and electrolytes with various concentrations of BNGDB are displayed in Fig. 2b. When 0.25%, 0.5%, and 1.0% BNGDB were added, the capacity retentions are 65.2%、83.2% and 76.1%, respectively after 200 cycling, while the capacity retention is less than 40% after only 60 cycling with BE electrolyte. Note that the LiNi0.6Co0.2Mn0.2O2/graphite pouch cell with 0.5% BNGDB-containing electrolyte show the highest initial discharge capacity and the best cycle performances.
Figure 2. Performances of LiNi0.6Co0.2Mn0.2O2/graphite pouch cell at room temperature. (a) first cycle charge-discharge curve with a charging rate of 0.2 C , (b) 1.0 C cycle performance.
Download figure:
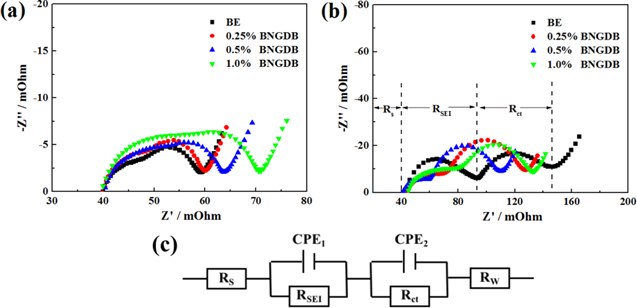
Standard image High-resolution imageIn order to explore the effect of BNGDB on the interface impedance of the pouch cell, electrochemical impedance spectroscopy (EIS) was used to analyze the whole battery. Figures 3a and 3b show the EIS of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells after 1 and 60 cycles in BE and 0.25%, 0.5%, 1.0% BNGDB-containing electrolytes. Figure 3c shows the EIS results which have been simulated by using the equivalent circuit model. In the equivalent circuit, RS is the solution resistance; the high-frequency semicircle, representing the surface-film resistance (RSEI); the intermediate frequency semicircle, representing the charge-transfer resistance (Rct) and the low-frequency straight line represents the Warburg resistance (Rw). 24,34 After 1 cycle, there is a slight difference in the interfacial impedance between the cells with 0% and 0.25% BNGDB-containing electrolyte. Meanwhile, the interfacial impedances of the batteries with 0.5% and 1.0% BNGDB-containing electrolyte are higher than the cells with 0% and 0.25% BNGDB-containing electrolyte. It indicates that while the concentration of BNGDB is more than 0.5%, BNGDB may affect the state of electrode interface during the initial cycle.The corresponding impedance values after 60 cycles are given in Table I. The resistances of the LiNi0.6Co0.2Mn0.2O2/graphite pouch cells with 0.25%, 0.5% and 1.0% BNGDB-containing electrolytes are lower than that of the cell with BE, and among these, for pouch cell with 0.5% BNGDB-containing electrolyte, the values of RS, RSEI and Rct are 41.17, 19.10 and 43.14 mΩ, showing the lowest resistance. These results indicate that BNGDB additive is beneficial to enhance the stability of CEI film on the cathode surface.
Figure 3. EIS of LiNi0.6Co0.2Mn0.2O2/graphite cells with BE and BNGDB in the electrolyte and charged to 4.5 V after (a) 1 cycle and (b) 60 cycles, (c) the corresponding equivalent circuit diagram.
Download figure:
Standard image High-resolution imageTable I. Equivalent circuit data of the LiNi0.6Co0.2Mn0.2O2/graphite pouch cells with 0%, 0.25%, 0.5% and 1.0% BNGDB-containing electrolytes after 60 cycles.
| Samples | RS mΩ−1 | RSEI mΩ−1 | Rct mΩ−1 |
|---|---|---|---|
| BE | 44.94 | 44.84 | 52.50 |
| 0.25% BNGDB | 43.33 | 29.36 | 48.53 |
| 0.5% BNGDB | 41.17 | 19.10 | 43.14 |
| 1.0% BNGDB | 43.97 | 37.93 | 46.17 |
During the charge and discharge processes of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells in 0.25%, 0.5%, 1.0% BNGDB-containing electrolyte, the loss of reversible lithium ion is very few. These conclusion was draw from the dQ/dV curves of LiNi0.6Co0.2Mn0.2O2/graphite pouch cells in Fig. 4. The peaks at 3.45 V and 3.63 V represent the lithium deintercalation/intercalation of the LiNi0.6Co0.2Mn0.2O2/graphite pouch cells in BE and BNGDB-containing electrolytes, respectively. After 60 cycles, the peak intensity of pouch cells in BE was significantly reduced, while that of the pouch cells in BNGDB-containing electrolyte nearly remained the same intensity.
Figure 4. The dQ/dV curves of LiNi0.6Co0.2Mn0.2O2/graphite cells with BE and BNGDB in the electrolyte after 1 cycle (a) and after 60 cycles (b).
Download figure:
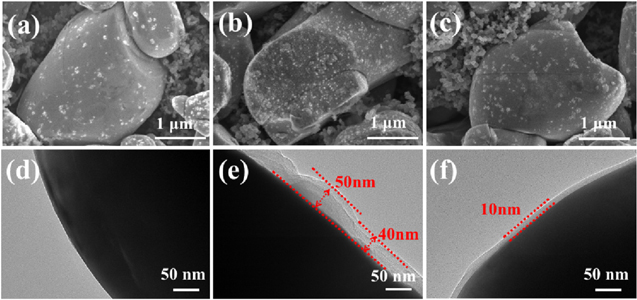
Standard image High-resolution imageIn order to compare the morphological and compositional differences of LiNi0.6Co0.2Mn0.2O2 electrode surfaces before and after cycling, SEM and TEM characterizations are adopted. Before cycling, the surface of LiNi0.6Co0.2Mn0.2O2 electrode is smooth (Fig. 5a). TEM image shows that there is no CEI film on the surface of LiNi0.6Co0.2Mn0.2O2 electrode (Fig. 5d). After 60 cycles, the surface of LiNi0.6Co0.2Mn0.2O2 electrode in BE become rough (Fig. 5b), while that in 0.5% BNGDB-containing electrolyte remain smooth (Fig. 5c). TEM image shows that the surface of LiNi0.6Co0.2Mn0.2O2 electrode in BE is covered by a layer of film with thickness of 40–50 nm (Fig. 5e). The growth of this thick layer originated from the decomposed BE reacting with cathode material during the charge and discharge processes, which is also the reason for poor stability of the pouch cells. On the other hand, the surface of LiNi0.6Co0.2Mn0.2O2 electrode in 0.5% BNGDB-containing electrolyte is still smooth and cover by a thin and uniform film, which is only 10 nm thick (Fig. 5f) after 60 cycles. This shows the fact that by adding BNGDB additive, the decomposition of electrolyte is suppressed and LiNi0.6Co0.2Mn0.2O2 electrode has been protected.
Figure 5. SEM and TEM images of fresh LiNi0.6Co0.2Mn0.2O2 (A, D) and LiNi0.6Co0.2Mn0.2O2 after 60 cycles in base (B, E) and 0.5% BNGDB-containing (C, F) electrolytes.
Download figure:
Standard image High-resolution imageThe interfacial compositions of LiNi0.6Co0.2Mn0.2O2 electrode before and after 60 cycles in the electrolyte without and with 0.5% BNGDB are characterized with XPS. As shown in Fig. 6, B element is observed on the surface of cycling LiNi0.6Co0.2Mn0.2O2 electrode in 0.5% BNGDB-containing electrolyte, indicating that the BNGDB additive actively participates the film-forming reactions. For the spectra of O 1 s, peaks located at 529.5 eV, 531.6 eV and 533.4 eV are correspond to M-O (LiNi0.6Co0.2Mn0.2O2), C=O (Li2CO3), C–O (ROCO2Li), respectively. 35,36 After 60 cycles, the intensity of M–O on LiNi0.6Co0.2Mn0.2O2 electrode in the electrolyte without and with 0.5% BNGDB are both obviously lower than fresh prepared LiNi0.6Co0.2Mn0.2O2 electrode. It indicate that interface film formed by the reaction between decomposed electrolyte and the LiNi0.6Co0.2Mn0.2O2 electrode. In the spectra of F 1 s, there are three main peaks located at 684.7 eV (LiF), 686.9 eV (LixPOyFz) and 687.7 eV (PVDF). 37 The intensity of LiF of LiNi0.6Co0.2Mn0.2O2 electrode cycled in 0.5% BNGDB-containing electrolyte is obviously lower than that in BE. Moreover, in the spectra of P 2p, the intensity of LixPFy (136.1 eV) observed on the LiNi0.6Co0.2Mn0.2O2 electrode cycled in 0.5% BNGDB-containing electrolyte is lower than that in base electrolyte. 38 The F 1s and P 2p XPS spectra results together show that BNGDB-containing electrolyte inhibit the formation of LiF and LixPFy during the cycling.
Figure 6. XPS images of fresh LiNi0.6Co0.2Mn0.2O2 and LiNi0.6Co0.2Mn0.2O2 after 60 cycles in BE and BNGDB-containing electrolytes.
Download figure:
Standard image High-resolution imageConclusion
We use BNGDB as an electrolyte additive to improve the stability of commercial LiNi0.6Co0.2Mn0.2O2/graphite pouch cells with 4.5 V high-voltage. Incorporation of BNGDB can form a CEI film with B-containing compound and lower content of LiF and LixPFy on the LiNi0.6Co0.2Mn0.2O2 cathode surface, which is beneficial to improve the stability and decrease the interfacial impedance of LiNi0.6Co0.2Mn0.2O2 cathode. It can effectively protect the cathode and avoid subsequent decomposition of electrolyte cycling at high voltage. Therefore, BNGDB is a promising candidate for practical application in rechargeable high voltage LIBs.
Acknowledgments
This study was supported by Guangzhou Automobile Group, China.