Abstract
Highly hydrophilic pomelo peel is used as an activated carbon (AC) precursor so that KOH can be homogeneously absorbed within it. Subsequent cryodesiccation retains the original morphology of the pomelo peel and distribution of KOH, which provides the precondition of the one-step molecular level activation. The resulting AC has a high yield of 16.7% of the pomelo peel. The specific surface area of the AC prepared by the one-step molecular activation of cryodesiccated mixture of pomelo peel and KOH (CAC-1) is 1870 m2 g−1, which is higher than that of the AC by the one-step activation of oven-dried mixture (AC-1) and AC by the two-step calcination (AC-2). CAC-1 has the highest specific capacitance of 219 F g−1 at 1 A g−1 among all the three samples. Importantly, the CAC-1 electrode has a high packing density of 0.63 g cm−3. The aqueous supercapacitor based on CAC-1 has a volumetric cell capacitance of 30.7 F cm−3, which corresponds to 123 F cm−3 for a single electrode. When the ionic liquid of 1-ethyl-3-methyl-imidazolium tetrafluoroborate is used as electrolyte, CAC-1 shows maximum specific energy of 40.5 Wh kg−1 and energy density of 25.5 Wh l−1.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
With the exploitation of renewable energy, supercapacitor has become one of the most important electrochemical energy storage devices. Compared with secondary batteries, supercapacitors based on carbon materials have unique advantages of higher power and longer lifetime. 1–3 Among all carbon materials, AC is currently the most ideal commercial electrode material 4–6 for supercapacitors due to its low cost, large specific surface area (SSA), tunable pore structure, good electrical conductivity and excellent surface chemistry.
In a conventional approach, ACs are typically prepared by a two-step process consisting of carbonization and subsequent activation both at high temperature. 7,8 Biomass wastes as natural materials are often selected as the precursors of ACs. 9 For example, recently, the pomelo peel was carbonized and activated, both of which were carried out at high temperatures of 400°C–800°C. 10 Sometimes, in order to improve the electric conductivity and capacitive performance of ACs, even a post-synthetic step of vacuum treatment was supplemented, for instance, when bamboo was used as the precursor. 11 The processes at high temperatures increase the cost in the preparation of ACs. Additionally, the solid coke after carbonization is hydrophobic and cannot homogeneously contact with KOH. Therefore, high cost and heterogeneous mixing of carbon and activating agent are two main obstacles in the preparation of commercial ACs. Currently, one-step activation of precursors is desirable for the preparation of low-cost ACs. 12,13 However, the one-step activation derived AC generally has low yield and low packing density. 14 Therefore, it is still a challenge to prepare ACs with low cost, high yield and packing density.
Here, we reported an AC with high yield (16.7%) and packing density (0.63 g cm−3) by the one-step molecular level activation of hydrophilic pomelo peel for supercapacitors. The highly hydrophilic pomelo peel thoroughly sucked KOH solution and subsequent cryodesiccation retained the original morphology of the pomelo peel, which ensured the homogeneous distribution of KOH and provided the precondition of molecular level activation. The AC by the one-step activation after cryodesiccation had an SSA of 1870 m2 g−1 and a specific capacitance of 237 F g−1 at 0.5 A g−1 in 6 mol l−1 KOH, corresponding to a volumetric capacitance of 149 F cm−3. Besides, the one-step activation saved energy and cost in the production of AC from the commercial view. Its symmetrical two-electrode supercapacitor with aqueous electrolyte showed maximum specific and volumetric cell capacitances of 48.8 F g−1 and 30.7 F cm−3, respectively. CAC-1 also showed maximum specific energy of 40.5 Wh kg−1 and energy density of 25.5 Wh l−1 in cell with the 1-ethyl-3-methyl-imidazolium tetrafluoroborate (EMIMBF4) electrolyte.
Experimental
Preparation of samples
The pomelo peel was chopped and dried before use. 1 g of pomelo peel was immersed into a 10 ml solution containing 0.45 g of KOH for 24 h. The wet solid was cryodesiccated in the chamber above the cold trap under 75 Pa. After cryodesiccation, the mixture was heated to 800 °C at 5 °C min−1 and held at the temperature for 1 h under Ar atmosphere. When it was cooled to room temperature, the powder was washed and dried to obtain the AC which was recorded as CAC-1.
For comparison, CAC-1a and CAC-1b samples were prepared by the same method but with 0.225 g and 0.90 g KOH respectively, while the AC-1 sample was prepared by the same method but by oven desiccation instead of cryodesiccation. Additionally, a conventional two-step method consisting of carbonization and activation with KOH both at 800 °C for 1 h was also carried out to obtain AC-2.
Characterization of samples
The morphology and elemental content of the samples were investigated by scanning electron microscopy (SEM, FEI Nova 400 Nano SEM). The elemental analysis of CAC-1 was executed by CHN and O modes in Elementar Vario el III. The elemental analysis of the ash was revealed by X-ray energy disperse spectrum (EDS). The N2 adsorption/desorption measurements were carried out at 77 K using automatic volumetric adsorption equipment (ASAP2020HD88). The surface element analysis was conducted by X-ray photoelectron spectroscopy (XPS) and the functional group by infrared (IR) spectrum. Graduated cylinders were used to measure the tapping density of the cryodesiccated and the oven-desiccated pomelo peel respectively after soaking in KOH solution by shaking.
Electrochemical measurements
A mixture of AC, acetylene black and binder, polyvinylidene difluoride (PVDF) or poly tetrafluoroethylene (PTFE), with a weight ratio of 75: 15: 10 was pasted on the nickel substrates as the working electrodes. The packing density of the electrode was calculated by Eq. 1,

where ρ is the electrode packing density, m is the mass of the mixture on the current collector weighted by balance (Mettler Toledo MS105DU), h is the thickness of the electrode and r is the radius of the electrode. For the three-electrode cells, a platinum foil and a Hg/HgO electrode were used as the counter and reference electrodes in 6 mol l−1 KOH as electrolyte. For the two-electrode tests, the two AC electrodes were assembled to coin-type symmetrical supercapacitors with 6 mol l−1 KOH and 1-ethyl-3-methyl-imidazolium tetrafluoroborate (EMIMBF4) electrolyte. Cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) were employed with a CHI 660E electrochemical workstation and the cyclic performances were measured by a LAND CT2001A system.
Results and Discussion
The IR spectrum of the pomelo peel in Fig. S1 (available online at stacks.iop.org/JES/168/060521/mmedia) shows the main functional groups of C–OH bond at 1054 cm−1, C=C bond at 1650 cm−1, C=O bond at 1748 cm−1 and hydroxyl at 3335 cm−1. After calcination in inert atmosphere, CAC-1 exhibited the C, O and H weight fractions of 87%, 4% and 1% respectively by the organic elemental analysis. After quick ash test of CAC-1 by rapid annealing in air, the ash with 7% weight percentage remained, consisting of 48% of O, 20% of Ca, 15% of Ni, 7% of Mg, 6% of S and 4% of Si in atomic percentage revealed by EDS. The other 1% component could be attributed to absorped water in CAC-1. Only Ni element in the form of Ni(OH)2 or NiO was possibly electrochemical active in aqueous electrolytes. The 30% Ni in weight percentage in the ash corresponded to 2% Ni in CAC-1. The specific capacitance of the commercial Ni(OH)2 was only 17 F g−1 (Fig. S2), meaning that the Ni in CAC-1 may produce a specific capacitance of 0.5 F g−1. Therefore, the contribution of the ash on capacitance can be negligible. The XPS C1s spectrum of CAC-1 in Fig. S3 shows three peaks which are attributed to C–C, C–O and C(O)O bonds at 284.6, 286.0 and 288.9 eV respectively, suggesting that residual oxygen functional groups append on carbon skeleton.
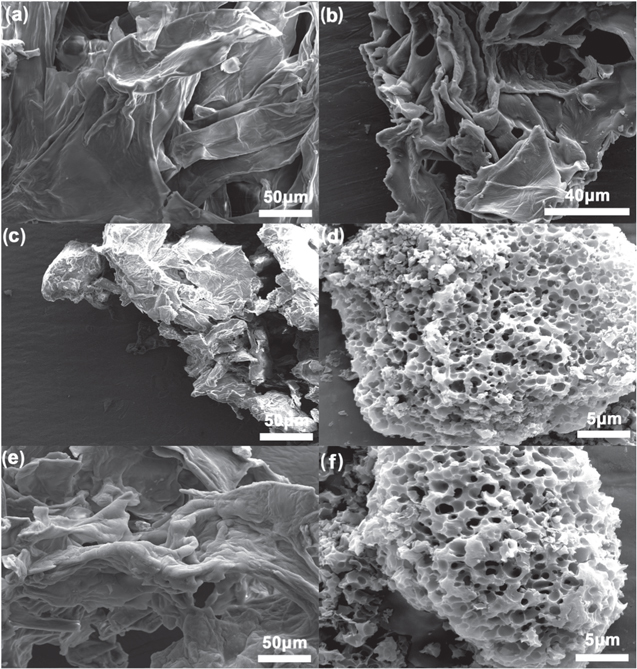
Figure 1a shows the SEM image of the pomelo peel as the carbon precursor with the dense wrinkle. The conventional two-step process obtained a similar morphology with shrunk size of ca. 126 μm without any macropores (Fig. 1b). After soaking the pomelo in KOH, the oven-desiccated pomelo peel sample was tightly stacked due to shrunk volume, compared with pomelo peel (Fig. 1c). The cryodesiccated pomelo peel after soaking in KOH basically maintained the appearance of the untreated pomelo peel and became even looser, which indicated the advantage of cryodesiccation (Fig. 1e). The retained morphology by cryodesiccation resulted in the homogeneous distribution of KOH in the pomelo peel, while the shrunk volume by the oven-desiccation led to the partial segregation of KOH during evaporation of water under ambient pressure. Many macropores with the average size of 1.0 μm and the average wall thickness of 0.8 μm are seen in the bulk carbon with size of 28 μm in Fig. 1d for AC-1. Figure 1f shows a similar porous structure for CAC-1 with a similar bulk size of 26 μm. The decreased bulk size of AC-1 and CAC-1 compared with AC-2 suggested that KOH could attack both the pomelo peel precursor and its carbonization products at different stages of the one-step activation process. However, the distribution range of macropores on the surface in CAC-1 was slightly narrower than that in AC-1, which may be attributed to the more homogeneous distribution of KOH in the pomelo peel by cryodesiccation.
Figure 1. SEM images of (a) pristine pomelo peel, (b) AC-2, (c) oven-desiccated pomelo peel after soaking in KOH, (d) AC-1, (e) cryodesiccated pomelo peel after soaking in KOH, and (f) CAC-1.
Download figure:
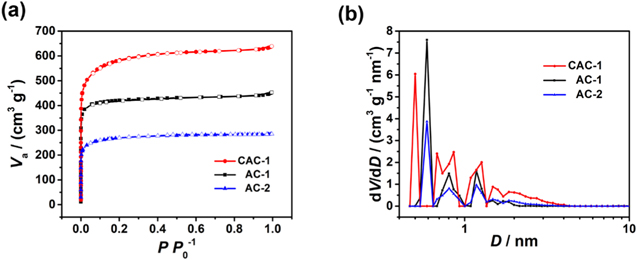
Standard image High-resolution imageThe adsorption and desorption isotherms of N2 and the pore size distributions of AC-1 and AC-2 and CAC-1 are shown in Fig. 2. All the samples in Fig. 2a exhibit type I isotherms, which correspond to the microporous structure. CAC-1 possessed the largest SSA of 1870 m2 g−1 and the largest total pore volume of 0.99 cm3 g−1. According to Eq. 2, 15 the apparent density of CAC-1 was calculated to be 0.69 g cm−3.

where Vt is the total pore volume, ρa and ρ0 are the apparent and true density. In contrast, AC-1 showed an SSA of 1383 m2 g−1 and a total pore volume of 0.70 cm3 g−1, while AC-2 the smallest SSA of 867 m2 g−1 and the smallest total pore volume of 0.44 cm3 g−1. As shown in Fig. 2b, the maxima of CAC-1 pore distribution lies at 0.50 nm, while both of AC-1 and AC-2 lie at 0.58 nm, suggesting that cryodesiccation of the mixture leads to more homogeneous distribution of KOH in pomelo peel. In the range of 0.7–2.0 nm, CAC-1, AC-1 and AC-2 exhibit similar pore size distributions but CAC-1 possesses more solvated ion-accessible pores in the same range.
Figure 2. (a) Nitrogen adsorption-desorption isotherms and (b) pore size distribution of AC-1, AC-2 and CAC-1.
Download figure:
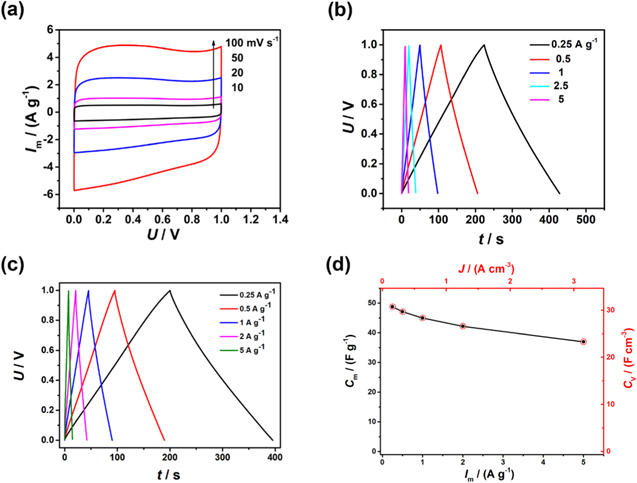
Standard image High-resolution imageThe electrochemical properties of the three comparative samples with the PVDF binder were measured by CV and GCD in three-electrode cells with the 6 mol l−1 KOH electrolyte at a potential range from −1 to 0 V vs Hg/HgO. At a scan rate of 10 mV s−1, all the three cyclic voltammograms (CVs) exhibit a quasi-rectangular shape with no redox peaks (Fig. 3a), indicating that all the samples have nearly ideal capacitive behavior. Apparently, CAC-1 has the largest CV area, indicative of the best specific capacitance. Figure 3b exhibits the GCD curves of CAC-1, AC-1 and AC-2 at a specific current (Im) of 1 A g−1. Consistently, CAC-1 had the highest specific capacitance of 219 F g−1 (calculated by the segment with shorter time referred by the arrow in Fig. S4), which was 33% higher than 165 F g−1 for AC-1 and 49% higher than 147 F g−1 for AC-2 at the same specific current. After the same cryodesiccation and activation, different mass ratio of KOH to pomelo peel gave rise to different properties. Fig. S5 shows the GCD curves of CAC-1, CAC-1a and CAC-1b. The specific capacitances of CAC-1a and CAC-1b were 182 F g−1 and 196 F g−1 respectively, which were evidently lower than 219 F g−1 of CAC-1. When the scan rate increases to 100 mV s−1, the CV of CAC-1 in the three-electrode cell show a distorted rectangle (Fig. 3c), suggesting that most of the charge storage might have occurred on the outer surfaces at higher scan rates because the cations has much shorter times to build a certain level of potential along the porous structure of the electrode. 16 Figure 3d shows that the GCD curves of CAC-1 at different specific currents. A maximum specific capacitance of 237 F g−1 was obtained at 0.5 A g−1. The corresponding areal capacitance was 166 mF cm−2, which was much higher than 15 mF cm−2 of hollow carbon spheres 17 and 10 mF cm−2 of porous carbons, 18 but lower than 1590 mF cm−2 of well-aligned carbon fibers. 6 In order to obtain the packing density, a CAC-1 electrode with the thickness of 69 μm was prepared with the PTFE binder, as shown in Fig. S6. The whole electrode packing density was calculated to be 0.63 g cm−3, which was close to its own apparent density (0.69 g cm−3), comparable to the apparent density (0.65 g cm−3) of the commercial YP50f and higher than the packing density (0.50 g cm−3) of the AC from pomelo peel by a two-step method. 10 Assuming that CAC-1, acetylene black and binders had the same true density, the volumetric capacitances of the CAC-1 material could be obtained according to Eq. 3.

where Cv and Cm are volumetric and specific capacitances respectively. The maximum volumetric capacitance of the CAC-1 material in the three-electrode cell was 149 F cm−3 obtained at 0.35 A cm−3, which was higher than 98 F cm−3 of YP50f according to the results in the literature. 19
Figure 3. Three-electrode electrochemical performance in KOH electrolyte. (a) CVs of AC-1, AC-2 and CAC-1 at 10 mV s−1, (b) GCD curves of AC-1, AC-2 and CAC-1 at 1 A g−1, (c) CVs of CAC-1 at different scan rates, (d) GCD curves of CAC-1 at different specific currents.
Download figure:
Standard image High-resolution imageCompared with that of AC-2, the electrochemical performances of CAC-1 and AC-1 were significantly improved. The effectiveness of the one-step calcination was mainly because the pomelo peel had good absorption of KOH solution, which could homogeneously activate the carbon precursor. Among the three samples, CAC-1 showed the overwhelming superiority in specific capacitance. Microscopically, from the SEM images, only the cryodesiccated pomelo peel after soaking in KOH maintained the appearance of the untreated pomelo peel and became even looser because KOH was absorbed within it. Macroscopically, the tapping density of the cryodesiccated mixture was 0.14 g cm−3, which was smaller than 0.35 g cm−3 of the oven-desiccated mixture, meaning that partial segregation of KOH might occur during the evaporation of water accompanied by volume shrinking by oven desiccation. Therefore, cryodesiccation was beneficial to homogeneous distribution of KOH in pomelo peel. It was worth noting that the yield of CAC-1 was 16.7% of the pomelo peel, which was much higher than 2.7% for the AC by the one-step activation of agar and similar with 16.2% for the agar derived AC by the two-step method. 14 The high performance and yield illuminated prospect of CAC-1 as a commercial AC.
A symmetrical two-electrode coin-type supercapacitor was assembled with two pieces of the CAC-1 electrodes with 6 mol l−1 KOH as the electrolyte. Because of the compact structure of the two-electrode cell, the CV shows a rectangular outline even at 100 mV s−1 (Fig. 4a). According to the discharge curves in Fig. 4b, the specific capacitance of the CAC-1 cell with was 49.8 F g−1 at 0.5 A g−1, corresponding to 199 F g−1 at 1 A g−1 for a single electrode, which was close to 219 F g−1 in the three-electrode cell. At 0.25 A g−1, the specific capacitance of the CAC-1 cell was 51.3 F g−1, corresponding to 205 F g−1 for a single electrode. Figure S7 shows the cyclic performance of the CAC-1 cell. 112.6% of the initial capacitance could be retained for the CAC-1 cell at 2.5 A g−1 after 10000 cycles. When the specific current was altered to be 1 A g−1, followed by another continued 10000 cycles, 104.5% of the retention was obtained relative to the initial capacitance at the first cycle at 2.5 A g−1. It was worth noting that the CAC-1 cell showed progressively increased specific capacitances in the initial cycles, which was mainly attributed to the following two aspects. On one hand, the wettability at the electrolyte/electrode interface increased with cycles, leading to the thorough utilization of deep pores. 20 On the other hand, the residual oxygen content of 4%, as revealed by the elemental analysis, contributed more pseudocapacitance after cycles. 21 The good cyclic performance of the CAC-1 cell at two different specific currents indicated that the CAC-1 materials could be qualified for high rate capability and long-time stability.
Figure 4. (a) CVs of CAC-1 supercapacitor with thin electrodes at different scan rates, (b) GCD curves of CAC-1 supercapacitor with thin electrodes (0.7 mg cm−2) at different specific currents, (c) GCD curves of CAC-1 supercapacitor with thick electrodes (7 mg cm−2) at different specific currents, (d) specific and volumetric capacitances of (c) as function of the currents in KOH electrolyte.
Download figure:
Standard image High-resolution imageHowever, the typical mass loading of the CAC-1 electrodes with the PVDF binder was only 0.7 mg cm−2, which was far from the commercial requirement. When the PTFE binder was used, the average mass loading of the CAC-1 electrodes increased to 7 mg cm−2. Figure S8 shows the CVs of the supercapacitor with the thick electrodes, also demonstrating the ideal capacitive behavior. As shown in Fig. 4c, at 0.25 A g−1, the specific capacitance of the supercapacitor with the thick electrodes only decreased to 48.8 F g−1, corresponding to 195 F g−1 for a single electrode, which was 95% of the value for thin electrode with loading of 0.7 mg cm−2. As the packing density of the electrode was 0.63 g cm−3, the volumetric cell capacitance was 30.7 F cm−3 at 0.16 A cm−3, which corresponded to 123 F cm−3 for a single electrode (Fig. 4d). When the current density was 3.15 A cm−3, the volumetric cell capacitance was still 23.3 F cm−3.
In order to increase the energy of the CAC-1 based supercapacitor, EMIMBF4 was applied as the electrolyte to extend the potential window. 22 The CVs of CAC-1 in EMIMBF4 was investigated in a three-electrode cell with a silver reference electrode at 10 mV s−1 (Fig. S9). Figure S10 also shows the CVs in a two-electrode supercapacitor with the ionic liquid electrolyte within 3.5 V at different scan rates. Small positive current tails are seen at a potential window (cell voltage) of 3.5 V in both Figs. S9 and 10. According to Fig. S11, the specific cell capacitance of the supercapacitor with 3.5 V was calculated to be 32.5 F g−1 at 0.5 A g−1 with the coulombic and energy efficiencies of 94% and 73%. As the energy efficiency at 1 A g−1 with 3.5 V was also 73%, the cyclic performance of the CAC-1 cell with ionic liquid electrolyte was carried out with the condition. Figure S12 exhibits that 75.0% of the initial capacitance can be retained for the cell after 10000 cycles at 1 A g−1, suggesting the moderate durability of CAC-1 in EMIMBF4 with 3.5 V cell voltage. Nevertheless, the retention of CAC-1 was inferior to that of the AC prepared by an extra post-synthesis vacuum annealing, which suggested that the deterioration of cyclic performance was attributed to irreversible redox reaction between the O functional groups in CAC-1 and ionic liquid. 23 However, the coulombic and energy efficiencies at 0.25 A g−1 were only 87% and 67%, which were too low in real energy stored systems. Combining with the CVs of CAC-1 in a three-electrode cell in Fig. S9, the very stable potential window should be from −1.5 to 1.5 V vs Ag/Ag+. As shown in Fig. S13, the CVs of the CAC-1 supercapacitor with a 3 V cell voltage present rectangular profiles without tails even at the small scan rate. Figure 5a shows the GCD curves of the CAC-1 supercapacitor with a 3 V cell voltage. The specific cell capacitances of the supercapacitor were calculated to be 30.1 and 32.4 F g−1 at 0.5 and 0.25 A g−1, respectively. The coulombic and energy efficiencies increased to 93% and 75% at 0.25 A g−1. Although the energy efficiency was lower than that of the D-glucose derived carbon, 24 it was still much higher than agar derived carbon. 14 Such reduced energy efficiency was at least partly due to heat dissipation. 25
Figure 5. (a) GCD curves and (b) specific power and power density vs specific energy and energy density of CAC-1 supercapacitor with thick electrodes in EMIMBF4 with cell voltage of 3 V, compared with those in aqueous electrolyte.
Download figure:
Standard image High-resolution imageBased on the GCD curves in Figs. 4b and 5a, the relationships between power and energy of the aqueous and ionic liquid cells are plotted in Fig. 5b respectively. Accordingly, the highest specific energies of the cells with ionic liquid (3 V) and aqueous (1 V) electrolytes are 40.5 and 6.8 Wh kg−1 at specific powers of 375 W kg−1 and 125 W kg−1, respectively. Similarly, the highest specific powers of the cells with ionic liquid and aqueous electrolytes are 7500 and 2500 W kg−1 at specific energies of 23.8 and 5.1 Wh kg−1, respectively. The maximum energy densities of the cell with the ionic liquid and aqueous electrolytes were calculated to be 25.5 and 4.3 Wh l−1 respectively, while the maximum power densities to be 4725 and 1575 W l−1, respectively. The specific energy (34.3 Wh kg−1) at 1 A g−1 of the CAC-1 cell with EMIMBF4 was higher than 21.6 Wh kg−1 at the same specific current for the AC derived from pomelo peel by the two-step calcination 26 because larger potential window of 3 V could be achieved for CAC-1. Based on a weight ratio of 30% for AC material in a packaged supercapacitor device, a practical specific energy of 12.2 Wh kg−1 for a packaged device was expected, which was beyond 5 Wh kg−1 for a commercial application.
Conclusions
Homogeneous distribution of KOH in the pomelo peel has been realized by immersing hydrophilic pomelo peel into the KOH solution and subsequent cryodesiccation. Based on this, the one-step molecular level activation has been developed. The resulting CAC-1 has advantages of high yield (16.7%) and high packing density (0.63 g cm−3) in addition to low cost due to the one-step process at high temperature. Compared with AC-2 prepared by the conventional two-step method and AC-1 by the one-step activation of oven-dried mixture, CAC-1 has the highest SSA of 1870 m2 g−1 and the highest specific capacitance of 219 F g−1 at 1 A g−1 in the KOH electrolyte of all the three samples. Because of the high packing density of the CAC-1 electrode, the volumetric capacitance is 149 F cm−3, which is much higher than 98 F cm−3 of YP50f in three-electrode cells. The maximum specific cell capacitance of the two-electrode CAC-1 cell is 48.8 F g−1, corresponding to 195 F g−1 for a single electrode. The highest volumetric cell capacitance is 30.7 F cm−3, which corresponds to 123 F cm−3 for a single electrode. When the ionic liquid of 1-ethyl-3-methyl-imidazolium tetrafluoroborate is used as electrolyte, CAC-1 shows a maximum specific energy of 40.5 Wh kg−1 and energy density of 25.5 Wh l−1. High yield, high packing density and low cost indicates that the pomelo peel derived CAC-1 has a commercial promise for supercapacitors.
Acknowledgments
Y. Li, Z. Sun and L. Zhang contributed equally. We sincerely thank the reviewers for the suggestive comments to greatly improve the quality of the manuscript. This work was supported by National Natural Science Foundation of China (21905216), Natural Science Foundation of Hubei Province, China (2019CFB131), State Key Laboratory of Materials Processing and Die & Mould Technology, Huazhong University of Science and Technology (P2019–014). T. Jiang thanks National Natural Science Foundation of China (51602234) and Y. Li and Z. Sun thank Science and Technology Innovation Fund of Undergraduate (18ZRA010).