Abstract
Rapid on-site measurements of arsenic (As) are essential for the timely remediation of As-contaminated groundwater for both municipal and emergency response applications. Current field tests suffer from either complicated end-user instructions or a lack of accuracy and specificity. The system presented here combines a whole-cell bacterial biosensor with an electrochemical measurement that provides enhanced accuracy and signal intensity compared to traditional bacterial-detection approaches. When integrated within a customized hardware system, this whole-cell sensor demonstrated excellent specificity and sensitivity. This fast, sensitive, and easy-to-use approach is a viable alternative for on-site arsenic testing.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
Despite the high toxicity of arsenic (As) at trace levels, measuring this metal accurately in the field remains a challenge. 1–3 The presence of arsenic in drinking water occurs from its natural presence in surface and groundwaters, 4 or as a result of human activities such as industrial applications, 5 the use of certain pesticides, mining, 6 or wood treatments. 7 The consumption of arsenic contaminated water has been linked to multiple serious health consequences including bladder, lung and skin cancer, 8–10 skin hyperkeratosis, 11 and more.
Currently, laboratory analysis is the primary method of choice for the measurement of As. There are many available methods for the accurate detection of arsenic in a laboratory setting, with the preferred methods involving sample pretreatment, either via acidic extraction or acidic oxidation digestion. 3 After pretreatment, the sample can be measured using different techniques, such as atomic absorption spectroscopy (AAS), 12 or inductively-coupled plasma mass spectrometry (ICP-MS). 13 However, these instrumental procedures cannot be performed on-site, necessitating the storage and shipment of samples, and are expensive, time consuming, and require sufficiently trained laboratory individuals. Field kits have been used extensively for the qualitative determination of arsenic in water, 1,14–16 as the measurement can be conducted on site and decisions can be made promptly. Of those kits, the most widely used in low-income communities are the easy-to-use and inexpensive colorimetry tests; however, the accuracy of these kits has been questioned. 1 That is especially true when used to classify water samples based on relevant drinking water safety standards. The World Health Organization (WHO) has recommended an upper limit of 10 parts per billion (ppb) in drinking water. 17 This low limit makes field analysis challenging, as the resulting tests need to be extremely sensitive. In solution, arsenic occurs in two oxidation states: a trivalent form, arsenite [As(III)], and a pentavalent form, arsenate [As(V)]. 18 Since arsenic speciation determines both its bioavailability and its potency as a toxin, there is much interest in the literature for speciation studies of arsenic. However, these studies are difficult and expensive to perform, and thus they are not "first response measures." 2 As(III) has been found to be 60 times more toxic than As(V), 19 and therefore As(III) has been chosen as the target species for our biosensor system.
Whole-cell arsenic biosensors are typically genetically modified bacteria that couple induction of the arsenic-responsive ars promoter to the expression of a reporter gene for a quantifiable output. These biosensors are primarily based on the ars promoters of Escherichia coli (K-12 genome and R773 plasmid), 20,21 Staphylococcus plasmid pI258, 22 and Bacillus subtilis genome, 23 with reporters such as β-galactosidases (e.g., LacZ), fluorescent proteins (e.g., GFP), or luciferases (e.g., Lux). 24 For a comprehensive review of arsenic biosensors, see French et al. 25 and Diesel et al. 26 Unfortunately, regulations regarding the use of these genetically modified microorganisms outside the laboratory hinder the commercial development of such biosensors in environmental analysis. 27
Herein, we demonstrate a novel integrated system for the field analysis of arsenic by coupling a genetically modified bacterial strain that produces the electrochemically detectable 4-aminophenol in the presence of As(III). The electrochemical signal is measured using single-use cartridges with screen-printed electrodes that are incubated and measured in a field-ready battery operated device. This system shows arsenic specificity and sensitivity under the 10 ppb WHO recommended limit for drinking water and was designed to be a contained system that would facilitate regulatory approval. This biosensor has received regulatory approval for field use in Canada (NSN No. 19393) and the USA (MCAN No. J-18–0041), making it one of the first such genetically engineered biosensors to be approved for widespread field deployment.
Experimental
Materials
Arsenite standards were freshly prepared using 1,000 ppm As(III) standard (Sigma-Aldrich; Cat No. 72718–100 ml). Chemicals 4-aminophenyl-beta-D-galactopyranoside (PAPG) and carbenicillin (CAR) were purchased from Gold Biotechnology and Fisher-Scientific, respectively (Cat No. A-280–500 and BP26485). Reagents for media preparation and the interference study were purchased from Sigma-Aldrich or Bioshop Canada Inc. All chemicals were used as supplied without further purification. Arsenic response and interference studies were performed in distilled water. Cartridge bodies and self-sealing silicone caps were made using Smooth-Cast™ 326 and Mold Star™ 15 Slow, respectively. Screen-printed electrodes (SPEs) were purchased from JE Solutions Consultancy Ltd. (Stockport, UK). The field-ready device uses a Raspberry Pi 3 Model B + single-board computer, a custom-made (i.e., hand-soldered) potentiostat following open-source designs, 28 standard MOSFET switches as a multiplexer between the potentiostat and a card-edge connector for contacting the cartridge SPEs (Digikey S3310-ND), a resistive heating element machined from aluminium 6061, a 20,100 mAh rechargeable lithium battery with a 4.8 A USB output, a commercial GPS module (Digikey 1528–1153-ND), and a NANUK 905 protective case. A block diagram outlining how the components are laid out is detailed in Fig. S1 (available online at stacks.iop.org/JES/168/067508/mmedia).
Plasmid construction
Construction of the pFRED-As plasmid used the vector backbone of the pUC19 plasmid. 29 The pUC19 fragment (2.7 kb) was amplified via polymerase chain reaction (PCR) with the high-fidelity Q5 DNA polymerase (New England Biolabs) as outlined by the manufacturer. The 3 other fragments (0.5, 1.9 and 1.5 kb) were synthesized by Integrated DNA Technologies (gBlocks®) and encoded the E. coli arsenical resistance promoter Pars and repressor arsR (GenBank No. X16045), and the two halves of the E. coli β-galactosidase lacZ (GenBank No. AM946981), respectively. All 4 DNA fragments were assembled via the overlapping ends using a Gibson Assembly® Cloning kit (New England Biolabs; Cat. No. E5510S). Reactions were transformed into E. coli DH5α competent cells (ThermoFisher Scientific; Cat No. 18265017) using the Inoue method 30 and successful transformants were screened on Lysogeny Broth (LB) agar plates containing 100 μg l−1 CAR. Grown colonies (1–2 mm) were used to inoculate LB media and incubated overnight while shaking.
Strain and culture conditions
Routine growth of strains was carried out in LB liquid media at 37 °C with 50 μg l−1 CAR. Cultures were grown for 16 h while shaking at 300 rpm. Cultures for arsenite measurement were prepared by inoculating 10 ml of LB + CAR medium in 50 ml disposable centrifuge tubes with 100 μl of overnight culture.
Measuring response to arsenic
200 μl of the biosensor culture was mixed with 900 μl of sample and solid PAPG (final concentration 0.88 mM) and the mixture was incubated at 37°C for 1 h. In a laboratory setting, samples were diluted 5-fold using 0.2 M phosphate buffer (pH 7) and measured using a conventional 3-electrode cell (1 mm diameter glassy carbon working electrode, Pt wire counter electrode, Ag/AgCl reference electrode) with a Bio-Logic SP-50 potentiostat. In cartridge measurements, response to arsenic was measured using a battery-powered device for incubating and measuring using disposable cartridges with screen-printed electrodes (JESC, Stockport, UK).
Results
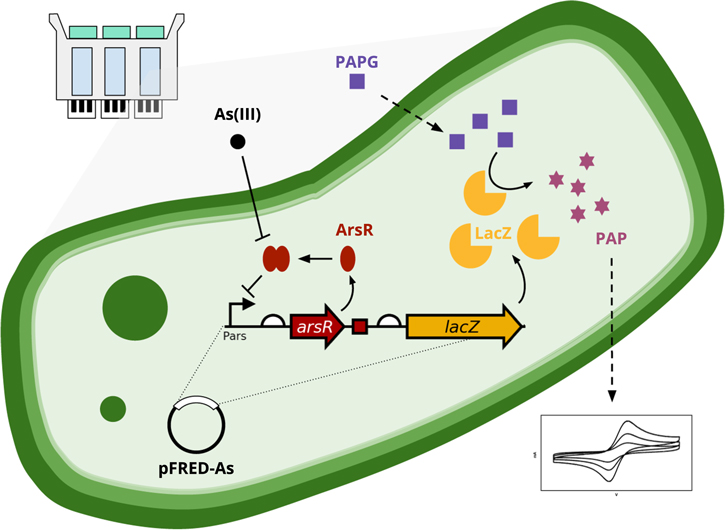
The arsenic biosensor in this system (FRED-As) is based on the genetic circuit from Wackwitz et al. 31 that controls the lacZ reporter expression through the ars operon promoter and arsenical repressor ArsR. This ars operon was originally isolated from the R773 resistance plasmid, 32,33 and shown to be inducible by arsenic when As(III) is bound by the ArsR dimer and relieves transcriptional repression. 34,35 The circuit also contains an additional ArsR-binding site upstream of the lacZ gene to reduce background expression of the reportedly "leaky" Pars promoter. 31,36 As summarized in Fig. 1, the E. coli contains the pFRED-As plasmid that encodes the Pars-arsR-lacZ operon for β-galactosidase lacZ expression as a reporter gene for arsenic concentrations. Normally, this operon is silenced by the binding of the repressor ArsR dimer to the ArsR-binding DNA sites located in the promoter Pars and in front of lacZ. When the bacteria is exposed to arsenic-containing samples, ArsR binds As(III) ions and releases the repression of the operon, triggering the production of LacZ. This enzyme then cleaves 4-aminophenyl-beta-D-galactopyranoside (PAPG) to produce the electrochemically active 4-aminophenol (PAP) that is effluxed out of the bacteria and measured at an electrode using cyclic voltammetry. The pFRED-As plasmid was propagated in the E. coli maintenance strain DH5α for several advantages. This strain allows long-term plasmid stability due to the recA1 mutation, 37 lacks inherent β-galactosidase activity with the ΔlacZ58(M15) allele, 38 and is widely considered non-pathogenic and non-toxigenic, as DH5α cannot survive or colonize in mammalian or plant hosts. 39,40 We measured growth curves to assess the impact of the high-copy number plasmid pFRED-As (>200 copies per cell from the modified pMB1 origin of replication). 41 The bacteria containing pFRED-As showed a delayed growth (Fig. S2); yet, similar doubling time to the non-modified parental strain DH5α, suggesting the delayed onset of log-phase growth is due to the metabolic burden of the plasmid. Note that the growth delay of this strain occurs in absence of carbenicillin supplementation, further indicating that the somewhat suppressed growth is from plasmid copy number and not due to the antibiotic.
Figure 1. Process schematic of the bacterial biosensor system used for arsenic detection.
Download figure:
Standard image High-resolution imageElectrochemical detection of As(III)
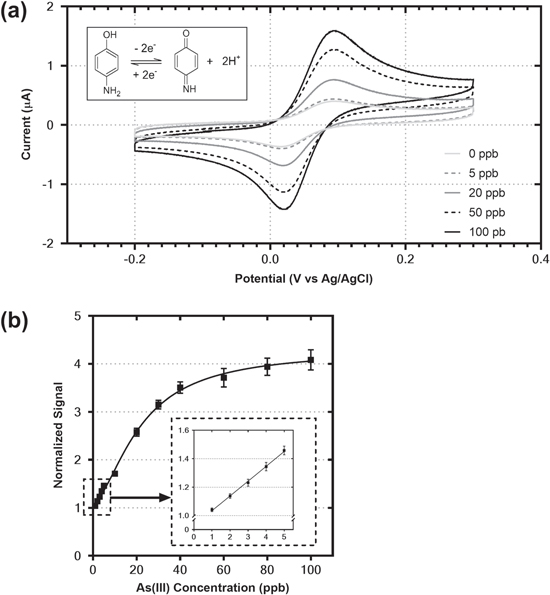
Cyclic Voltammetry (CV) was used for the detection of As(III) under optimized conditions in a laboratory setting. As previously discussed, in the presence of the bacterial biosensor, the electrochemically detectable 4-aminophenol is released upon cleavage by β-galactosidase of the substrate (PAPG) allowing for the electrochemical detection of As(III). As can be seen in Fig. 2a, all of the CVs show quasi-reversible behaviour for mass transport-controlled electrochemical reactions, with the peak currents (Ip) increasing proportionally to the arsenic concentration and a constant peak potential (Ep). The separation between the anodic and cathodic peaks (ΔEp) is greater than the predicted 29 mV for a two-electron reaction, similar to previous reports on the electrochemical behaviour of 4-aminophenol on carbon-based electrodes. 42
Figure 2. (a) Cyclic voltammograms obtained after 1 h incubation of the mixture of biosensor and sample with different concentrations of As(III). All measurements were recorded at pH 7.0 in 0.2 M phosphate buffer solution at a potential scan rate 50 mV s−1 from −0.2 V to 0.3 V vs Ag/AgCl (b) Standard curve corresponding to the normalized peak current of the oxidation of PAP at various concentrations of As(III) under the same conditions as (a).
Download figure:
Standard image High-resolution imageThe relationship between the anodic peak currents and the As(III) concentration between 0 and 100 ppb can be seen in Fig. 2b. Excellent linearity is observed at low concentrations of As(III) (Fig. 2b inset), and the linearity of peak current versus As(III) concentration is sustained between 0−20 ppb As(III). This region is of special interest for the measurement of arsenic in drinking water systems, as it encompasses the 10 ppb limit recommended by the WHO. Above 20 ppb As(III) the sensor approaches saturation, beginning to plateau above 80 ppb As(III). This could be due to the previously reported toxicity of such high concentrations of As(III) to bacterial cells. 43 The biosensor can maintain a response for arsenic past the growth phase and be stored for 24 h at room temperature (Fig. S3), enabling the bacteria to be utilized outside of a laboratory setting. The biosensor still shows activity well into the stationary phase, although this response to arsenic decreases over time.
Interference studies
Studies to determine any possible interference from foreign ions that exist in drinking water supplies were then conducted. Specificity was determined by comparing the signals obtained in response to As(III) with signals obtained from 18 other metals and inorganic compounds tested individually (Ag(I), Al(III), Bi(II), Cd(II), Co(II), Cr(VI), Cu(II), Fe(II), Fe(III), Mg(II), Mn(II), Mo(VI), Ni(II), Pb(II), Zn(II) and KCN). Figure 3 demonstrates the response observed over triplicate biological replicates for all conditions after 1 h of incubation at 37 °C with 100 ppb samples of each possible interference. The results show that the sensor has high selectivity and sensitivity towards As(III) ions. It is well known that Cu(II) is the main interferent for the electrochemical detection of As(III), as the peaks observed during cathodic stripping voltammetry of Cu(II) and As(III) occur at similar potentials. 44 No cross reactivity between Cu(II) and As(III) was observed in this study.
Figure 3. Interference study of the electrochemical signal obtained from the mixture of biosensor, substrate and sample (measured under the same conditions) in the presence of various foreign ions (100 ppb concentration). The electrochemical signal obtained with different concentrations of As(III) (0–20 ppb) is given for comparison purposes.
Download figure:
Standard image High-resolution imageDesign of the single-use cartridges and field-ready detector
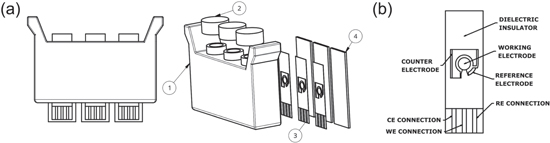
In order to account for the inherent variability of whole-cell biosensors, 45 each single-use cartridge is composed of 3 separate chambers, one for the sample and two internal calibration standards, and is sealed at the top with a self-sealing silicone cap to ensure that the samples and bacteria are always in a sealed container. The use of two internal calibration standards generates a minimal standard curve for each cartridge, allowing for the calculation of the concentration of As(III) in the sample chamber. A schematic of the cartridge design can be seen in Fig. 4 and pictures can be found in the Supplementary Information (Fig. S4). The cartridge is composed of a polyurethane body, 3 screen-printed electrodes (SPEs) and 3 heat-conducting metal backing plates. For the production of the cartridge body, the polyurethane components are thoroughly mixed and then degassed using a vacuum pump for one minute. The mixture is poured into a silicone mold and left to cure for 2 h at room temperature. After demolding, the cartridge bodies are placed in an oven at 65 °C for 4 h to finish curing. The silicone caps are made by mixing both components and pouring the silicone over the mold, and then cured for 4 h. Once the cartridge bodies are cooled to ambient temperature, the SPEs, metal plates and caps are glued to the bodies using transparent silicone. Figure S5 shows the shape and dimensions of the assembled cartridge.
Figure 4. Diagrams and exploded view of single-use cartridge (a) and JES-006 SPE (b) used for electrochemical detection of arsenic. Cartridge is composed of a polyurethane body (1), self-sealing silicone caps (2), screen-printed electrodes (3), and heat-conducting metal backing plates (4).
Download figure:
Standard image High-resolution imageSPEs have been used for a wide range of applications including in clinical, environmental, and food industry settings. 46 The SPEs used in this study utilized a three electrode system and were designed by JE Solutions Consultancy Ltd. The various SPE designs and inks that were tested in biosensor cartridges are summarized in Table SI. All SPEs with different inks and different working to counter electrode surface area ratios were tested after the sample and biosensor were in direct contact with the SPE for 1 h at 37 °C. Selected CVs with the different electrode designs can be found in the Supplementary Information (Figs. S6–S8). Optimal results were found for the JES-006 SPE, where the working and counter electrodes and contact tracks were printed with a graphene-based carbon paste (JESC-7771G) and the reference electrode with a 65:35 Ag/AgCl ink (JESC-7713Ag). The dielectric layer was printed with JESC-7775I insulation ink. A detailed engineering drawing of the JES-006 SPE, including dimensions, can be found in the Supplementary Information (Fig. S9).
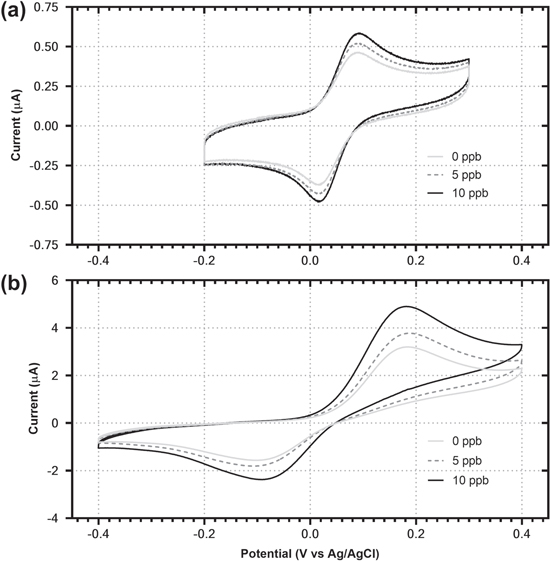
Figure 5 shows CVs for the biosensor responding to 0, 5 and 10 ppb As(III) when measured with a glassy carbon electrode (Fig. 5a) or with a JES-006 SPE after incubating for 1 h at 37 °C with the bacteria in contact with the SPE surface (Fig. 5b). The peak currents are approximately eight times larger with the SPEs due to the larger surface area when compared to the glassy carbon electrode (∼7 mm2 vs ∼0.8 mm2, respectively). The ΔEp after the 1 h incubation is significantly greater than what was observed for the reaction when measured with the glassy carbon electrode, approaching 300 mV. This is likely due to the bacteria partially fouling the electrode surface, 47 resulting in hindered electron transfer kinetics and the observed electrochemical irreversibility of the reaction. Importantly, the peak potentials remain constant between the As(III) concentrations, enabling comparisons between their peak currents and the interpolation of As(III) concentrations within the generated calibration curves. This combined incubation and measurement also simplifies the overall process and reduces the potential for error to be introduced by field testing personnel through eliminating a transfer step if the incubation and measurement were performed separately.
Figure 5. Cyclic voltammograms of bacterial biosensor using a glassy carbon electrode (a) and JES-006 SPE (b) for the detection of As(III). All measurements were recorded at pH 7.0 in 0.2 M phosphate buffer solution at a potential scan rate 50 mV s−1. The JES-006 SPE was incubated with the bacterial biosensor for 1 h prior to measurement.
Download figure:
Standard image High-resolution imageThe field device is equipped with an aluminum resistive heating block for the incubation of the samples, as well as a potentiostat and multiplexer in order to allow for the simultaneous testing of 4 samples (Fig. S1). The potentiostat is designed for a three electrode system, and allows for 4 different full-scale current ranges (1, 10, 100 μA, and 1 mA), as well as for a voltage ramp between −10 V and +10 V. The user interface includes multiple LEDs on a custom nylon computerized numerical control (CNC) machined top panel to convey basic information (Fig. S10), as well as a custom firmware package that enables the user to change the test parameters related to the incubation and electrochemical measurement. The device is automated to minimize the user's actions: once the cartridge is inserted in the device, it will go through all the steps of the test (1 h incubation at 37 °C and measurement of the sample and two internal calibration standards in each cartridge) and produce a file at the end that contains the calculated concentration of As(III), raw CV data, date, time and GPS location of the test. The device is powered by a rechargeable 20,100 mAh lithium-ion battery, providing power for 8 consecutive rounds of testing to occur. The heater provides a temperature precision of 0.1 °C and requires 12 min. for the device to reach the incubation temperature of 37 °C (Fig. S11).
Results in single-use cartridges with the field-ready device
To test the effectiveness of the combined device and cartridge systems, 48 samples with varying As(III) concentrations (0−20 ppb) were measured using cartridges in the field device and are summarized in Table I and Fig. 6. In each cartridge, the sample and two standard solutions were mixed with the biosensor and substrate via shaking and then inserted into the device to be incubated for 1 h. at 37 °C. The anodic peak current of the oxidation of 4-aminophenol, of both the sample and standards, was then measured by the field device. The peak current from the standard solutions are used to obtain the relationship between the signal and the concentration of arsenite in the sample matrix, which is then used for the determination of As(III) concentration of the sample.
Table I. Results in single-use cartridges (mean ± SD) of determination of As(III).
| Sample Concentration As(III) (ppb) | Measured As(III)(ppb) | Recovery (%) |
|---|---|---|
| 0 | 0.7 ± 1.2 | — |
| 5 | 4.7 ± 1.2 | 94.0 |
| 10 | 10.0 ± 1.3 | 100.0 |
| 15 | 16.9 ± 2.5 | 112.6 |
| 20 | 21.8 ± 2.4 | 109.0 |
Figure 6. Scatter plot showing the FRED-As response upon exposure of the sensor to samples with different concentrations of As(III). The area between the dashed lines represents the 95% confidence interval of the measurement.
Download figure:
Standard image High-resolution imageThe results show high accuracy in the detection of arsenite, especially when compared to commercially available field kits, with a standard deviation of approximately 1 ppb between 0−10 ppb. The Method Detection Limit (MDL, 95% confidence) based on the measured samples is 2.2 ppb As(III), well under the 10 ppb WHO recommended limit for drinking water.
Discussion
This system is designed for ease of use, with no sample preparation required and only a small quantity of water (0.9 ml) needed for the test. After the sample and both standards are inserted into the appropriate chamber of the cartridge, 0.2 ml of the biosensor is inserted into each of the chambers and the cartridge is shaken by hand for 1 min. The cartridge is then inserted into the device and no further involvement is needed from the end-user. The incubation and measurement takes place automatically after the cartridge has been inserted into the device. When the sample has been analyzed, the used cartridge can be removed from the detector and the results are automatically stored on a USB, producing the possibility of near real-time monitoring of arsenic in the field. The simplicity of the steps makes this system ideal for field testing requiring no technical training. Due to the demonstrated low-range accuracy for As(III), this system could be used for detection of arsenic in the range of the limit established by the WHO for drinking water (10 ppb), while using safe and non-hazardous reagents. Table II summarizes the differences between the FRED-As system and other common technologies used for detection of arsenic in field kits.
Table II. Comparison of different technologies used for field detection of arsenic. 1,3
| Method | MDL (ppb) | Benefits | Drawbacks |
|---|---|---|---|
| FRED-As | 2.2 | Accurate and sensitive | Arsenite-only detection |
| Easy to use | |||
| Potential for measuring a wide spectrum of metals | |||
| Colorimetric assays | 1−30 | Cheap | Typically generates arsine gas and mercury waste |
| More accurate readings can be obtained with an absorption device | Prone to both false positive and false negative readings | ||
| Inaccurate | |||
| Portable XRF devices | 50 | Allow for arsenic speciation | Interference for lead |
| Sample preparation required | |||
| ESTCP testing of ASV field ready device | 1 | High sensitivity | Requires technically trained operator |
| Interference from copper, mercury and zinc |
Conclusions
The FRED-As system, using a bio-electrochemical detection method, is designed for measuring the reduced form of arsenic in water. Arsenic field test kits are widely used to measure arsenic levels in drinking water sources, but previous studies have shown that the kits are difficult to use or show poor results across a broad range of sample matrices and concentrations. This biosensor, coupled with single-use cartridges and a field measurement device, is a sensitive and accurate method for arsenic detection. The bacterial strain has been approved for field use in Canada (NSN No. 19393) and the USA (MCAN No. J-18–0041), making it one of the only known genetically engineered systems approved for field deployments. This easy-to-use system opens the door for the possibility of implementation of other biosensors that could detect additional contaminants using this electrochemical output system. By using different genetically modified bacteria and other electrochemically detectable substrates, there is the possibility of creating a platform where the same sample could be analyzed in a quick and reliable manner for different contaminants at the same time.
Acknowledgments
The authors would like to acknowledge Dr. Chimoné Dalton for her experimental work on the storage of the biosensor, as well as Christopher Wintersinger and Ali Honarmand for technical support and helpful discussions. The authors also acknowledge funding support from the National Research Council's Industrial Research Assistance Program (NRC IRAP) and the Refined Manufacturing Acceleration Process Network (ReMAP), as well as ECO Canada for the support of KLB.