Abstract
Lactate is one of the potential biomarkers for assessing the human condition in clinical medicine or sports application. Lactate measurement could help in alerting various emergency conditions, such as bleeding, hypoxia, respiratory failure, and sepsis. Lactate monitoring could also benefit athletes in monitoring their muscle activity to prevent injury due to excessive muscle use or fatigue. In light of this, biosensor technology has been widely explored, especially on the use of electrochemical sensors to analyze the content of biological samples through direct biological activities conversion to electronic signals. This has become imperative for the detection of lactate which offers easy, quick, and reliable measurement. Despite enzymatic sensors being the focus of many studies, the non-enzymatic sensor has started to gain attention in recent years to overcome the stability issue of enzymes. This review presents an overview of the concepts, applications, and recent advancements of different electrochemical lactate sensors. A comparison of recent studies for both enzymatic and non-enzymatic lactate sensors based on electrode modification, enzymes, enzymes immobilizer, and several performance factors, including sensitivity, linearity, detection limit, and storage stability, all of which have been performed. Towards the end, this review also highlights some recommendations for future development of lactate sensors.
Export citation and abstract BibTeX RIS
Biosensor is defined by the International Union of Pure and Applied Chemistry (IUPAC) as a self-contained integrated device that is capable of providing specific quantitative and semi-qualitative analysis using a biological recognition element that is in direct spatial contact with a transduction element. 1 It is a sensory device that comprises biological entities that measure the chemical reaction by generating signal proportional to the concentration of the analytes in the reactions. The application of biosensor plays a significant role in healthcare, sports medicine, 2–4 and food industries. 2,5,6 The emergence of biosensor technology has proven to be an innovative technique for developing quick, simple, and accurate devices for the detection of various analytes for diagnostic and monitoring purposes. 7–10 Due to its rapid and real-time capability in measuring analytes, biosensor is, therefore, deemed extremely useful for monitoring any rapid changes in biological fluids. 11–13 In addition, biosensor is often classified into two major elements, which are biological and biochemical. The biological element can specifically interact with the targeted molecules, while the transducer can convert the interaction reaction into their respective measurable signals. Meanwhile, biochemical normally reacts with isolated enzymes, tissues, or cells to detect chemical compounds through electrical, thermal, or optical signals. 14 The performance of biosensors may reflect some static (i.e., sensitivity, linearity, reproducibility) and dynamic (i.e., delay time, response time, setting time) attributes for every biosensor characteristic. 15 Figure 1 shows that a biosensor structure consists of five components, namely, analyte, bioreceptor, transducer, electronics, and display. 15–17
Figure 1. The schematic diagram of biosensor.
Download figure:
Standard image High-resolution imageHuman biofluid is a liquid within the human body that provides information upon exposure of human body towards external and internal changes of physiological activity. There are different types of biofluid that are useful for the detection of the aforementioned changes, including blood, sweat, urine, saliva, and tears. The selectivity of biofluids has become one of the vital elements in determining the performance of biosensor analysis and its specific application. For example, blood is one of the measured parameters in clinical testing for monitoring the health condition of patients. Thus, the level of lactate concentration in the blood plays a significant role to prevent incidence of ischemic condition. 18 Although previous studies showed that blood analysis is the gold standard method used to measure the electrolyte concentration, the abovementioned technique does involve invasive procedure and is unsuitable for real-time evaluation. 19–21 Hence, most researches are currently shifting towards real-time sweat sampling as an alternative biofluid due to its capability to be collected non-invasively. 22,23
Sweat is a hypotonic biofluid produced by sweat glands in the skin. 24,25 On the basis of morphology, humans have two types of sweat glands, eccrine and apocrine glands, as shown in Fig. 2. 26–28 There are three parts of the gland; first, the upper coiled duct (epidermal duct), it is a long and hollow tube of cells where sweat is produced. Then, there is the dermal duct, which transports biomarkers into sweat, attaching it to the outer skin surface. The lowest part of the gland is the secretory coil, which secretes sweat through or without pinching off the outer cell parts. The sweat is slightly acidic, with a pH range of 4–6.8 and is composed of 99% 24 water, trace of minerals (sodium, potassium, chloride), lactic acid, glucose, and urea. 29–31 Hence, apart from blood, sweat has shown to be a promising alternative for analyte detection, as it is rich in electrolyte information, which is suitable for continuous monitoring.
Figure 2. The skin anatomy.
Download figure:
Standard image High-resolution imageLactate is one of the key metabolites in the human body, which is produced in muscles due to the anaerobic metabolism of glucose. The accumulation of lactate in human muscles during training results in ache 32 is often used as a biomarker of fatigue. 33,34 Therefore, continuous monitoring of lactate is essential during exercise, particularly for athletes, to avoid cell acidosis due to the imbalance between lactate production and lactate clearance, 3,35 which may cause disruption of muscular performance. 35–37 Apart from cell acidosis, in training application, it serves as an indication to determine whether or not the acid-base (pH) balance is natural and for the evaluation of endurance performance on athletes. 38 Referring to these conditions, the blood lactate levels can lead to a drop in the pH level of the blood, causing fatigue. 39
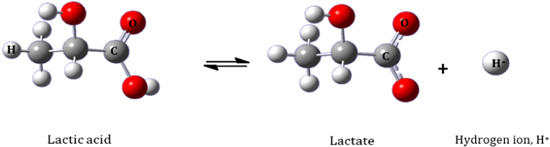
Blood contains 0.5–1.5 mM l−1 of lactate for a healthy person at rest, whereas approximately 12 mM l−1 is present during exercise 39–41 and in extreme physical activities, this level will increase up to 25 mM l−1. 42 Thus, measuring L-lactate is required to assess an athlete's performance endurance, also known as lactate threshold is needed in sports medicine. 35 Lactate threshold testing can be determined by an appropriate training intensity and monitoring the progression of an athlete's performance. Studies by Jacob and Tanaka show that lactate threshold is a key to great performance and the single best predictor in improving the athlete's endurance performance than the maximal aerobic capacity test (VO2 max). 43,44 However, the level of lactate concentration is different in other biofluids, such as tears (1–5 mM), 45,46 saliva (10–25 mM), and sweat (0.1–2.5 mM). 39,41 Monitoring of lactate level through wearable electrochemical sensors requires non-invasiveness, ideally through the analysis of sweat or interstitial body fluids. 36,47 Selectivity of metabolites is important, as the body fluids contain several other metabolites ideally through the analysis of sweat or interstitial body fluids. 36,47 Selectivity is the most important factor in a sensing application, as body fluids, such as blood and sweat, contain metabolites (i.e., glucose, urea, ethanol, ammonia) 48,49 that have almost similar structures and properties to that of lactate. Consequently, it leads to a high possibility that the sensor will interact with these biomolecules (i.e., uric acid, ascorbic acid, glucose, galactose, magnesium, and calcium ion) 35 and interfere with the detection of lactate. 50,51 Figure 3 below shows the relationship between lactate and its conjugated base of lactic acid, as one carboxy group was deproteinized and 99% of this lactic acid is reduced to lactate and hydrogen ions. This bond consists of three molecules: C (carbon), H (hydrogen), and O (oxygen).
Figure 3. Relationship between lactate acid and it's conjugated base lactate.
Download figure:
Standard image High-resolution imageElectrochemical Sensors
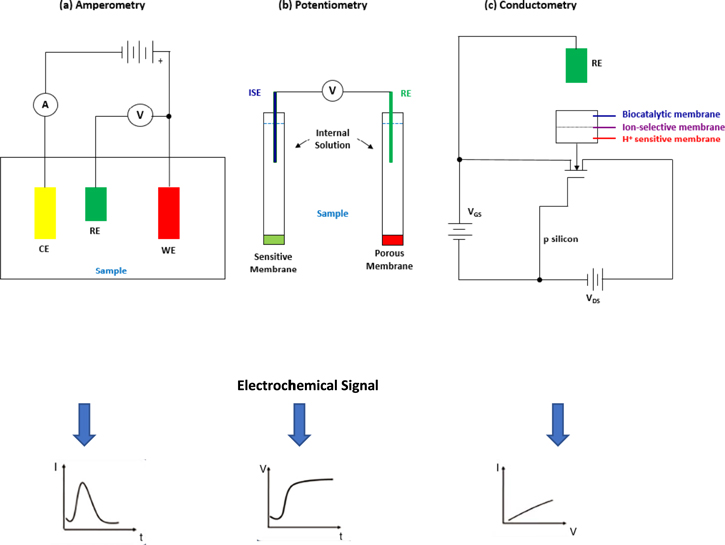
Developing highly sensitive and accurate sensor is an important role in primer industries such as sports medicine, food quality control, environmental concerns, athletes performance and nutritional sector. 52–55 The biofluid sensing principle can be divided into two types, electrochemical sensing and optical sensing (i.e., colorimetry and fluorescence). 48 Towards this sensing methods when the fluorescent materials are exposed to the particular excitation light, the relationship between analyte concentration and fluorescence intensity is directly involved. 48 However, this method needs a strong effort to reduce a measurement error that appeared despite its great in selectivity. 48 In addition, the working principle of colorimetry is determining the concentration of the analyte by color changes of the solution. The colorimetric patches are often disposable, which makes continuous detection difficult to achieve. 48 The electrochemical transducing principle possesses the enormous potential and gains attention among other transductions due to its cost-effectiveness, fast analytical time, high selectivity, instrumental simplicity, and ease of miniaturization, and portability to provide data even in remote locations. 35,52,56–59 Hence, this sensing method dominates the market owing to great performance in sweat sensing. Amperometric, potentiometric, and conductometric electrochemical sensors are established sensors often used in electrochemical detection and analysis based on their respective working principal application. 60 Conventionally, electrochemical sensors require three electrodes which are manufactured on a flexible substrate, reference electrode (RE), working electrode (WE), and counter or auxiliary electrode known as sensing or redox electrode, 17,61,62 as depicted in Fig. 4. 63
Figure 4. Illustration of sensing modalities and signals for electrochemical sensors; (a) Amperometry, (b) Potentiometry, and (c) Conductometry. 63
Download figure:
Standard image High-resolution imageGenerally, amperometric sensors can be classified based on to their field of application and structure, which include the working material electrode, biological recognition element, and the method of immobilization used. 54 The amperometric sensor has a wide range of applications in food and beverage, environmental, and healthcare industries; for example, the detection of volatile organic compounds in soils and the detection of analytes in blood. 64 The principle of amperometric sensor is based on measuring current generated by enzymatic or bioaffinity reaction at the electrode surface at a constant working potential with respect to the reference electrode. This is due to the electrochemical oxidation or the reduction process of analyte concentration and the current function as a voltage at the polarized electrode. 65,66 Commonly, Ag/AgCl with defined potentials ranging from +0.20 to +0.25 V is used for reference electrode. 67 The material of the working electrode becomes the main element in amperometric performance. Hence, various studies were carried out to invent the modification and characteristics of the electrodes for better performance. 68–71 Recently, amperometry technology shows high selectivity, sensitivity, simplicity in fabrication, ease of integration into continuous analysis system, and inexpensiveness of potentiometric and conductometric sensors. 72–74
The working principle of potentiometric sensors is measuring voltage or potential difference by comparison of an unknown voltage with a known reference voltage. 17,35,73 The emergence of technology due to the availability of well-developed ion-selective electrodes (ISEs), potentiometry method is one of the oldest electrolyte analysis technique. 23,30,75 It relies on voltage measurement and responses to proportional to the analyte concentration to the reference electrode according to Nernst equation. 65 The ion-selective electrode is an indicator capable of selectively measuring a particular ionic species between two phases. 68 The advantages of potentiometric biosensors for lactate determination is due to its robustness and simplicity of instrumentation. 76,77 In addition, the use of ion-selective electrodes (ISEs) in potentiometric method also makes it possible to develop device with adequate sensitivity and selectivity. 76,78 Previous study shows this method can be constructed using paper as a substrate for paper-based sensor fabrication. 79,80 Technically, instrumentation for potentiometric assessment is simple and affordable, requiring minimal space, thus making it an attractive choice for a low-cost structure 81 However, potentiometric sensors may suffer from poor selectivity and will contribute the faradaic current due to the components in solution, with a standard potential smaller than the operating potential. 81 In addition, it presents a low detection limit and poor stability of enzyme electrode. 73
The conductometric sensor works on the measurement of conductivity and resistivity, which involves the changes of its capacitance towards the desired analyte. 82 The changes occur due to the selectively absorbing material that acts as a dielectric layer of the capacitor. This sensor consists of source and drain electrodes with a semiconducting material in between and a capacitively coupled gate electrode. 63 Although the method has not been widely used, there is the implementation of the sensing devices, such as drug detection in human urine, microfluidic detection, and pollutant detection in environmental testing. 17,83,84 Conductometric sensors offer some advantages over different types of a transducer, which can be produced using inexpensive thin-film standard technology due to its low sensitivity towards lighting. 85 Hence, it can be miniaturized and integrated easily due to the structure of the sensor which does not require any reference electrode, 85 thereby resulting in an improved sensor sensitivity and detection speed. 17 However, since the charge carriers are less and affect the conductivity process, it is considered as an unsuitable method for use. 17,65
Application of lactate sensor
In recent years, lactate sensors have been widely used in various applications and a large array of usage can be observed particularly in medicine and healthcare, 86 sports medicine, 59 as well as in food technology and industries. 18 In food industries, for example, the concentration of lactate in dairy products, such as yogurt, cheese, and milk, could be monitored. The different level of lactate concentration provides good indication of product freshness and spoilage. Meanwhile, in sports and clinical applications, lactate is a key parameter often targeted in continuous monitoring for athlete performance and early prediction of diseases. Pressure ischemia is one of the clinical trials that use blood as a biomarker in detecting the level of lactate concentration. 54 Clinically, the blood lactate baseline level ranges from 0.5 to 1.5 mmol l−1 at rest period and increases up to 25 mmol l−1 during exercise. 75,87,88 This condition occurs when the level of lactate in blood is slightly increased; this may lead to clinical consequences, such as liver disease, heart failure, respiratory deficiency, and others. 18 Hyperlactatemia can be detected when the lactate level in the range of 2 mM l−1 to 5 mM l−. 89 This disease, which occurs due to lactic acidosis, usually develops during exercise or in individual with critical illnesses, such as kidney disease, shock, sepsis, and cardiac surgery. 90–92 The higher level of lactate concentration may evoke the risk of developing metastasis, which occurs in breast, cervical, head and neck, lung, and colorectal. 93–96 This detection of lactate concentration leads to a distinction between the regions involved. 35,97,98 Despite the emergence of lactate in clinical applications, there is still a potential development of sensor technology in sports medicine. This development which targets the evaluation of endurance performance includes the control of lactate concentration or also known as lactate threshold. Hence, lactate level can be a predictor of an athlete's physical state during an intense exercise. 39,99 This is due to the high level of blood lactate that induces fatigue when the pH level of the blood decreases. Therefore, it is important to monitor and track the analyte concentration, particularly in clinical and endurance-based physical activities.
Enzymatic Biosensor
The first enzymatic biosensor was developed in the 1960 s by Clark et al. based on electrochemical measurement for the determination of oxygen and glucose in tissue and blood samples. 100 The use of enzymes in sensor development hugely improved its specificity, as enzymes are specific and only catalyses one type of reaction or a closely related reaction, demonstrating specificity for a particular substrate or group of substrates. Table I provides an overview of the recent reported enzymatic lactate electrochemical biosensor from 2016 until 2020. The experiments were carried out using different sensing fluids, including phosphate buffer solution (PBS), artificial sweat, human sweat, artificial interstitial fluid, human serum, and deionized water solution. Details of the summarized study were discussed in the following sections.
Table 1. Summary of recent electrochemical enzymatic-based lactate sensor.
| Working electrode | Measurement technique | Enzyme; Immobiliser | Sensing fluid | Sensitivity | Linearity | Detection Limit | References |
|---|---|---|---|---|---|---|---|
| Pd/GO Nanosheets | — | LOD; PANHS | Synthetic sweat | — | NA | 1 mM | 101 |
| Au/ZnO Nanoflakes | Amperometry | LOD | Human sweat | 11.76 μA/decade/cm2 | 10 pM to 20 mM | ∼1.26 nM | 102 |
| Cysteamine/AuNP | Amperometry | LDH | PBS/FBS | 31.40μA mM−1 cm2/73.16 μA mM−1 cm2 | 500 μM −7 mM/500 μM−5 mM | 411 μM l/- | 103 |
| Ni/TiO2/Gr | Amperometry | LOD; TiO2 Sol | PBS | — | 50 μM − 10 mM | 19 μM | 104 |
| Au/ZnO nanostructures | Chronoamperometry | LOD; DSP | Sweat buffers | — | 1–100 mM (Dynamic range) | 0.1 mM | 105 |
| Au/LOD | Amperometry | LOD; BSA | PBS/Blood plasma | 2.15 μA mM−1/0.337 μAmM−1 | 1–30 mM/5–30 mM (Dynamic range) | 1.54 mM/NA | 106 |
| SPCE/GO/NAD+/Fe (CN)6– 3 | Chronoamperometry | LDH; Diaphorase, GA and BSA | PBS | 0.14 μA mM−1 | 0.25 to 4 mM | - | 107 |
| Pt/LOD | Amperometry | LOD; GA | PBS | 440–460 nA mM−1 | 0.005–0.4 mM | 3 μM | 108 |
| Au/MWCNTs/MB | Amperometry | LOD | Artificial ISF | 1473 μA cm−2 mM−1 | 10 μM to 200 μM | 2.4 μM | 109 |
| Pt/rGO/CNTs/AuNP | Amperometry | LOD | PBS | 35.3 μA mM−1 cm−2 | 0.05–100 mM | 2.3 mM | 110 |
| SPAuE/LOD-CND | Chronoamperometry | LOD; TRIS, EGTA | Human serum + PBS | 4.98 x 10−3 μA·μM−1 | 3.0 to 500 μM | 0.9 μM | 111 |
| CE/PB | Potentiometric | LOD | Human sweat | — | — | — | 112 |
| Pt/CeO2-ZrO2 NP | Amperometry | LOD | Buffer solution | 138.9 μA mM−1 cm−2 | 0.025–0.6 mM | 1.8 μM | 113 |
| PETE/Gr/O2 plasma | Amperometry | LOD; Chitosan film, GA | Deionised water solution | — | 0.002 mM to 0.01 mM | 1.0 μM | 114 |
| Pt/LOD | Chronoamperometry | LOD; BSA, GA | Human sweat | — | 0–70 mM | 0.2 mM | 115 |
| AgNP/LOD | Amperometry | LOD; BSA, GA | Human sweat | 256 nA mM−1cm−2 | 1–25 mM | — | 116 |
| Au/LOD | Chronoamperometry | LOD | PBS | 950 nA/(cm2 mM) | — | — | 90 |
| Au/IrOx/LOD | Chronoamperometry | LOD | PBS | 9250 nA/(cm2 mM) | — | — | 90 |
NA-not-available; Pd-palladium, PANHS −1-Pyrenebutyric acid-N-hy- droxysuccinimide ester, GO-graphene oxide, ZnO-zinc oxide, DSP-dithiobis succinimidyl propionate, Ti-titanium, BSA-bovine serum albumin, SPCE-screen printed carbon electrode, SPAuE-screen printed gold electrode, CND-carbon nanotube, TRIS-tris(hydroxymethyl)aminomethane), EGTA - ethyleneglycol bis-(2-aminoethyl ether)-N,N,N,N-tetraacetic acid, ISF-interstitial fluid, MB-methylene blue, CE-carbon electrode, PB-perussian blue, PETE - polyethylene terephthalate electrodes, IrOx-iridium oxide, FBS-fetal bovine serum.
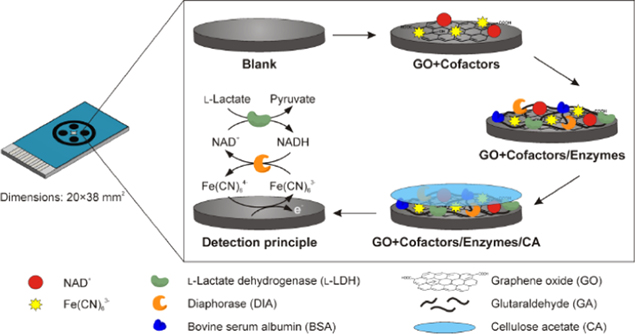
There are two enzymes that are commonly used in the fabrication of enzymatic lactate biosensor, which are lactate oxidase (LOD) and lactate dehydrogenase (LDH). Lactate oxidase is a flavin mononucleotide (FMN)-dependent alpha hydroxyl acid oxidizing enzyme, which employs FMN as a cofactor. This enzyme belongs to the oxidoreductase family that acts on single donors with oxygen as oxidant and on the incorporation of two atoms of oxygen into a substrate (oxygenase). Another type of enzyme that is commonly used for lactate determination is lactate dehydrogenase (LDH), which reduces NAD+ to NADH or NADP+ to NADPH. The presence of FMN, which acts as a cofactor in LOD, requires oxygen for the reaction to take place, hence, it can be limited by insufficient oxygen concentration. 117 However, this is not always the case during an in vitro study of lactate, since enough oxygen is usually available for the reaction to occur. Hence, the potential influence of oxygen concertation is often neglected. LDH, on the other hand, does not require oxygen, but it is essential to add NAD+ to the working buffer or immobilize this NAD+ together with the enzyme. 117 Pilas in her study modified the SPCE for the design of reagent free NAD+ dependent biosensor array. The screen-printed carbon electrode (SPCE) was modified with graphene oxide (GO) and cellulose acetate for the immobilization of LDH enzyme and cofactors (NAD+ and Fe (CN)6 3−). Figure 5 illustrates the design of the modified SPCE for multi-analyte detection. This reagentless NAD+-dependent allows for cross-talk-free simultaneous determination of L-lactate, D-lactate, ethanol, and formate. 107
Figure 5. Schematic illustration of multianalyte screen-printed electrode carbon biosensor array.
Download figure:
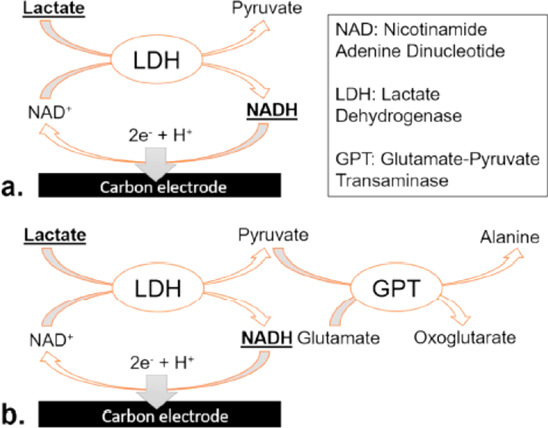
Standard image High-resolution imageFurthermore, it was learned that oxygen is needed to oxidase lactate molecules to form pyruvate and hydrogen peroxide. The level of oxygen present in the environment has a big influence on the enzymatic reaction. Any change in the level of dissolved oxygen in real samples, especially blood, can lead to fluctuation of sensor activity and associated reading errors. Therefore, high oxygen susceptibility of lactate oxidase enzyme-based amperometric sensors is an important obstacle that has to be overcome to eliminate false measurement in relation to dissolved oxygen level. Uzunoglu had developed enzymatic sensor based on CeO2-ZrO2 nanoparticles using simple chemical precipitation method and has shown no significant change in performance under O2-rich environment due to its high redox properties. This novel biosensor displayed high sensitivity of 138.9 ± 1.2 μA·mM−1·cm−2 in the range of 2.5 μM–0.6 mM. Additionally, Fig. 6 shows lactate electrochemical sensor mechanism based on LDH and a combination of LDH and Glutamate Pyruvate Transaminase (GPT). Some metabolites such as lactate and glucose are usually quantified by two enzyme families, oxidase enzymes and dehydrogenase enzymes as mentioned previously. 118,119 The oxidase enzymes will catalyze the lactate conversion to pyruvate with the production of hydrogen peroxide. Meanwhile, the dehydrogenase enzymes, will catalyze the dehydrogenation of metabolites while also reducing the NAD + cofactor to NADH. 120 In both the cases as in Figs. 6a and 6b, NADH produced by the lactate catalysis is oxidized at the electrode surface.However, with the presence of GPT (Fig. 6b), the efficiency of the catalysis is boosted by the pyruvate depletion. 120
Figure 6. Lactate electrochemical detection mechanisms based on LDH (a), and on the combined action of LDH and GPT (b). 120
Download figure:
Standard image High-resolution imageEnzyme immobilization
The successful fabrication of enzymatic biosensor would also depend on the immobilization of enzymes. Several methods and strategies for enzyme stabilization have been discovered for decades for biosensor development and industrial biocatalysts. Some of the major methods used to stabilizing the enzyme are the addition of stabilizing substances, such as sugars, polyols, sugar alcohol, polyelectrodes, chemical modification of enzyme side groups, and enzyme immobilization. 121 The immobilization of enzyme is commonly performed via physical adsorption, covalent bonding, cross-linking, and entrapment, as shown in Fig. 7. 121 Physical adsorption in Fig. 7a is attributable to the hydrophilic, hydrophobic, or ionic interaction between protein molecules and solid surface. However, this method is seldom done independently, as it has a tendency for enzyme leakage, especially when there are changes in temperature and pH. Hence, this method is often performed in combination with other methods, such as membrane entrapment (Fig. 7d) and cross-linking, to build layer-by-layer architecture. Besides that, sol–gels have been utilized to entrap enzymes by providing a similar environment to that of the enzyme solution, as illustrated in Fig. 7c. Intermolecular cross-linking (Fig. 7b) is among the widely used techniques in multiple covalent immobilizations. However, activity loss may result from intermolecular cross-linking, since it is difficult to obtain reproducible sensors because of the random mechanism and quick reaction rate of the cross-linking reaction. This can be overcome through indirect mild cross-linking with glutaraldehyde (GA), along with other immolation techniques, to increase its stability.
Figure 7. Different methods of enzyme immobilization.
Download figure:
Standard image High-resolution imageTur-Garcia et al. in her study optimized two highly porous polycarbonate membranes to support the immobilization of lactate oxidase enzyme via covalent cross-linking. 115 An enzyme-crosslinking solution was created by rapidly mixing the enzyme/bovine serum albumin solution with glutaraldehyde (GA), followed by incubation process to form an enzyme laminate. 115 Different parameters including incubation time and GA concentration ratio to LOD/BSA play important roles to achieving optimum sensor operational stability, reproducibility, and linearity response. Kucherenko et al. in his study also used GA cross-linker for covalent immobilization of enzyme. In the aforementioned study, the enzyme and BSA solution were mixed with GA and added with glycerol to stabilize the enzyme during immobilization and improve membrane adhesion to the transducer surface. 108
Non-Enzymatic Sensor
The development of enzymatic biosensor has always been the preferred selection in the last decades due to its high selectivity and sensitive capability. However, researchers now have started to explore the possibility of non-enzymatic biosensor development due to several limitations on enzymatic-based sensors, such as complicated enzyme immobilization process, poor chemical, thermal stability, and high cost of fabrication methods. Stability is one of the major issues in enzyme-based sensors, as enzymes can become denatured after the immobilization process, hence, affecting their performance and shelf-life. Moreover, enzymes can easily be affected by the changes in environment, such as temperature, pH, toxic chemicals, and humidity. 122 Hence, more research are now focused on non-enzymatic-based sensors, which provides several advantages in terms of cost, stability, simple fabrication methods, and reproducibility. 123
Recent demonstrations of non-enzymatic sensors indicate that synthetic receptors can provide the basis for sensing features that rival enzyme-based sensors in ways that can overcome many of the abovementioned drawbacks. One of the approaches used in non-enzymatic sensor development leverage on the excellent catalytic and modular features of metal-organic frameworks (MOFs). 124 Among the large family of functionalized nanomaterials, MOFs represent an interesting type of solid crystallize materials that can easily self-assemble via coordination of metal ions/clusters with organic linkers. 125–128 Wang et al. in his study presented a novel modular approach to synthesize MOF electrode by facile two-dimensional (2D) oriented assembly of Cu3(btc)2 nanocubes on amino-functionalized graphene paper. The acquired electrode was able to detect human sweat lactate sensitively. The efficient modular strategy made it possible for high loading of Cu3(btc)2 nanocubes and orientation of monolayer arrangements to form paper electrode, which significantly improved the conductivity and catalytic activity of Cu3(btc)2. 50 However, MOF-based material fabrication still requires extensive development in its synthesis, including the adaptation of micro- and nanofabrication process, in which there is still a lack of literature regarding the abovementioned matter. 124
Furthermore, another non-enzymatic path that uses molecularly imprinted polymers (MIPs) consists of poly (3-aminophenylboronic acid) templates for the identification of sweat lactate. MIPs are functional porous materials with high affinity binding sites that complement the analyte of interest in terms of size, dimension, and functionality. 129,130 They have been used as biomimetic recognition components in sensing platforms for a wide variety of targets, from small to large macromolecules. Historically, the technology of MIPs began in the 1930 s by Polyakov, 131 which was further progressed in the 1970 s by Wulff. 132 The are several advantages of MIPs, including its low cost, less complex preparation, improved stability and shelf life, reusability, and excellent chemical and thermal stability within various experimental conditions permitting specific biding of the analyte in a harsh chemical media. 133 Recent MIP studies were reported by Zhang et al., which showed that the developed AgNWs network coated on SPCE offered excellent electric conductivity and stability, which was then tested on human sweat during mount cycling exercise. 134 However, one of the major challenges in adopting the MIP techniques is in adapting the polymers to work in an aqueous environment, since water can interfere with the non-covalent bonding between a monomer and a template. 135
Meanwhile, metal oxides are highly dense semiconducting solids that offer substantial electrochemical and sensing properties, which have been exploited as key recognition elements in chemical and gas sensors. 136 It offers several advantages, including low cost, high chemical and thermal stability, and excellent mechanical and electrical properties. Hussain et al. exploited metal oxide in the development of non-enzymatic enzyme through modification of GCE with CuO·MWCNT NCs. This modification provides larger surface area and better exposure to enhance the effectiveness of L-lactic acid (L-LA) adsorption into permeable nanocomposite surfaces. 137 Table II provides the recently reported non-enzymatic electrochemical lactate sensors. Experiments were performed in a variety of sensing fluids, including blood plasma, lactic acid solution, human sweat, PBS, and urine.
Table 2. Summary of recent electrochemical non-enzymatic lactate sensor.
| Working electrode | Measurement technique | Sensing fluid | Sensitivity | Linearity | Detection limit | References |
|---|---|---|---|---|---|---|
| Ag NPs/NiO NPs/PU | Amperometry | Blood plasma | 8.86 nA mM−1 mm−2 | 0.6–2.2 nM | 0.38 mM | 138 |
| GCE/CuO.MWCNT NCs/Nafion matrix | Current-voltage | PBS | 633.0 pAμM−1cm−2 | 100.0 pM ~ 10.0 mM | ≈ 88.5pM | 137 |
| pANI/PBA-pNIPAM @CNC/CNT | Amperometry | Sweat | — | 1−25 mM | 0.10 ± 0.04 mM | 139 |
| GCE/3-ABPA/rGO/Au NPs | Amperometry | PBS | 1.9 × 105 μAM−1 | 0.1–15.0 nM | 0.0089 nM | 140 |
| SPCE/MIPs/AgNWs | Amperometry | PBS | - | - | 0.22 μM | 134 |
| HS-NiS/NiF | Amperometry | Human urine | 0.65 μμA μM−1 cm−2 | 0.5 to 88.5 μM | 0.023 μM | 141 |
| NiS-NC@NiS-MS/NiF | Amperometry | Human urine | 0.39 μA μM−1 | 0.5 μM to 85.5 μM | 0.5 μM | 142 |
| GCE/Co3O4 | Voltametric determination Amperometric response | PBS | — | 0.05–3 mM | 0.006 mM | 143 |
| GrE/PPy;LAC | Potentiometry | Lactate solution | — | 0.1 and 10.0 mmol l−1 | 81 μmol l−1 | 76 |
| GCE/NiO NPs | Amperometry | PBS | — | 0.01–5 mM | 5.7 μM | 18 |
| GCE/NiO | Amperometry | Lactate solution | 62.35 μA mM (cm2) | NA | 27 μM | 40 |
| SPCE/PdCu | Amperometry | PBS | 396.7 μA mM−1 cm2 | 0.5 to 11 mM | 0.7 μM | 144 |
| GCE/NiO | Amperometric | PBS | 9.08 μA mM-1 cm2 | 0.01–32.76 mM | 0.53 mM | 145 |
| GCE/Ni (OH)2 | Amperometry | PBS | 35.76 μAmM−1 cm2 | — | 0.59 mM | 145 |
| SPCE/3-ABPA | Amperometry | Human sweat | — | 3 −100 mM | 1.5 mM | 146 |
| SPCE/Fe (III)/NaCl | Potentiometry | Human sweat | — | 1−180 mM | — | 147 |
| Gr/CU3(btc)2 | Amperometry | PBS | 0.029 mA cm−2 mM−1 | 0.05−22.6 mM | 5 μM | 50 |
NA-not-available; GCE-glassy carbon electrode, NiO-nickel oxide, PU-polyurethane, CuO-cooper oxide, pANI/PBA - polyaniline/phenylboronic acid, pNIPAM - poly (N-isopropylacrylamide), 3-ABPA-3-aminophenulboronic acid, NiS-NC - nickel sulfide nanoclusters, NiS-MS - nickel sulfide microsphere, NiF-nickel foam, HS-NiS - hollow sphere structured nickel sulfide,, PPy-polypyrrole, Pd-palladium, CU3(btc)2-MOF-1.
Working Electrode Materials
Electrode material with a large surface-to-volume ratio that employs nanomaterials has led to a new era of biosensor research for producing highly sensitive and selective sensing capability. Nanocomposite material provides a significant contribution to the development of sensors due to their properties of multiphase solid material with a variety of dimensions in one of the phases. The characteristics of a nanocomposite allow the creation of new materials with improved flexibility and physical properties. There has been a growing demand for the use of doped nanocomposite materials in non-enzymatic biosensor development. The combination of metal oxide and nanocomposites (NCs) is often studied for their high porosity, permeability, porosity, and huge dynamic exterior area. 148,149 Chang. A. S et al. exploited the unique features of cobalt oxide nanostructures that exhibit the nanowire, lump, bundle of nanowire, and flower-like morphologies in the presence of counter ions or anions, which improved the sensitivity and selectivity of the sensor. 143 The integration of nanomaterial into biosensor technology have demonstrated significant advantage over the conventional bulk metal electrode.
Graphene (Gr) and carbon nanotubes (CNTs) are other commonly used nanomaterials in electrochemical sensor development owing to their distinct properties, such as high surface-to-volume ratio, high electrocatalytic performance and electron transfer stability, and low cost, 150–152 The high surface area and atomic thickness of graphene layers concentrate the entire carbon atoms to be in direct contact with the analytes, thus providing better sensitivity. 153 Gr also has an ultrathin thickness and good mechanical flexibility that allows for intimate contact with the skin in the case of sweat lactate analysis or wearable sensors. These properties would also help in acquiring high quality signals with reduced motion artefacts, contamination, or irritation 154 Chen et al. in his study developed the oxygen-plasma technique to improve the wettability of the flexible PET electrode modified with graphene nanowalls (GNWs) and LOD. 114 The GNW film was produced with interlaced three-dimensional (3D) conductive network and treated with oxygen plasma. These properties, along with their large surface area of GNWs, has allowed for the development of flexible sensor with good sensitivity, specificity, and stability. 114
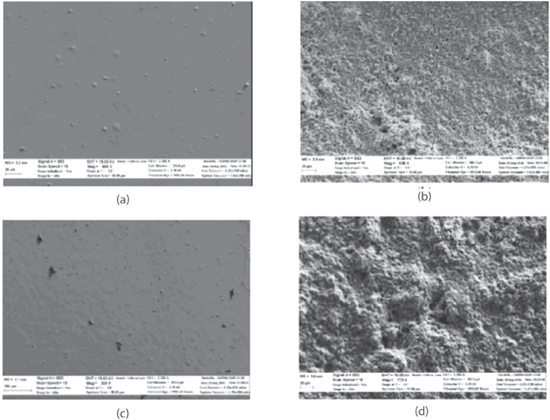
The use of AuNP provides advantages in terms of its stability due to their excellent biocompatibility and large surface area. Fabrication of microwave electrode modified with AuNP-cysteamine-LDH by Narayanan et al. showed excellent behavior towards lactic acid, with very high sensitivity of 31.40 μA mM−1 cm−2 in various lactic acid solutions and sensitivity of 73.16 μA mM−1·cm−2 in fetal bovine sample. 155 Yang. X. et al. fabricated a dual electrode lactate sensor on flexible substrates modified with LOD. 90 The dual electrode sensor based on LOD was fabricated using Au and iridium oxide (IrOx). Result from the study shows that the presence of IrOx had greatly improved the sensor performance due to its high roughness factor, which allows them to accumulate more enzyme protein for catalytic reaction. 90 The comparison between Au electrode and IrOx electrode under scanning electron microscopy (SEM) before and after enzyme loading are shown in Fig. 8. 90 Figure 8c shows that the IrOx surface roughness is higher than the Au in Fig. 8a before enzyme loading, which allowed for more enzymes to be deposited, as seen in Fig. 8d, compared with the electrode in Fig. 8b. Image from IrOx has shown to increase the surface area with normalized sensitivity by 9.17 times compared with Au electrode alone. Moreover, the inert properties of IrOx electrode also enabled better biocompatibility for animal and human use. 90
Figure 8. Surface characterization of iridium oxide and gold electrode (a) gold electrode surface area before enzyme deposition, (b) gold electrode surface area with enzyme deposition, (c) iridium oxide electrode surface area without enzyme, (d) iridium oxide electrode surface area after enzyme deposition. 90
Download figure:
Standard image High-resolution imageFurthermore, semiconducting metal oxides and their nanomaterials have also been optimized in many lactate sensing developments to achieve sensitivity, selectivity, portability, simplicity, miniaturization, and low cost, all of which are crucial for commercialization. 102 One of the recent studies by Alam et al. had successfully constructed a linker-free enzymatic sensor based on zinc oxide nanoflakes (NF) for non-invasive L-lactate sensing in sweat. The ZnO-NF electrode was functionalized by immobilizing LOD via electrostatic physical absorption. ZnO-NF has high isoelectric point, which enhances the immobilization of enzymes and the difference in isoelectric point (IEP) between LOD and ZnO-NF, which allows for the elimination of intermediary thin film as a linker. 156 These factors result in the formation of steady and shorter pathway for improved electron transportation, hence, increasing the electron transfer rate. This biosensor displayed a wide range of linearity with sensitivity of 11.76 μA/decade cm−2 and detection limit of 1.26 nM.
Fabrication Techniques
Basic microfabrication technique that is generally used for biosensor developments are deposition, patterning, doping, and etching, which are similar to those used for fabricating an integrated circuit (IC). The combination of these techniques is used to develop the devices layer-by-layer. Chemical vapor deposition (CVD) was performed at low pressure, atmospheric pressure or plasma enhanced, and physical vapor deposition (PVD), which encompassed electron beam (EB) evaporation and sputtering, the two most common thin-film deposition techniques in microfabrication. 157 These methods are able to form a thin film layer with a thickness of tens of nanometers up to a few micrometers. Alam et al. in her study used e-beam evaporation technique to deposit a thin gold layer (60 nm) on PET substrate with a thin adhesive layer of titanium (20 nm). 102 Meanwhile, Bhide et al. performed a two-step deposition process, which began with the deposition of ~150 nm gold electrode on the substrate, followed by sputtering of ZnO thin films, thereby enabling the formation of film with the thickness ranging between ~100–120 nm measured using profilometer. 105 Chemical vapor deposition techniques were used by Nagamine et al. to form a 150-nm thick parylene gate dielectric layer for the development of LOD/PB-modified electrode. 158 Besides that, other techniques such as electroplating of metal films and spin or spray coating of polymeric films are also capable of yielding film thickness from less than 1 μm to several hundred micrometers. 157
Pattern can be produced using a standard process of photolithography, which works by transferring the designed pattern onto a certain material, which allows a mask with the desired pattern to be created. The patterned photoresist can also be used as a mask for subsequent implantation of ions. Yang et al. performed a double lithography process in the development of enzymatic based gold electrode lactate sensor. Firstly, photolithography and wet etching were performed to pattern the probe with varying electrode sizes, followed by a second photolithography for the formation of insulation and protection layer. 90
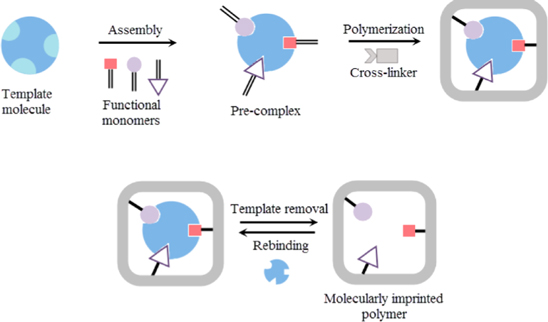
Electropolymerization is the most common method used in the generation of polymer films for the development of MIPs sensor due to the nature of the support materials, such as metal-based electrodes, semi-conductor, and carbon. 159 Electropolymerization has numerous advantages over conventional preparation methods, such as superior adherence to the surface of the transducer, preparation speed, aqueous preparation capacity, as well as layer thickness and morphology control. 160,161 The thickness of the polymer film can be regulated by controlling the voltage range during sweeping process. 162 The fabrication process of MIPs sensor via electropolymerization involves three main steps, including the assembly of functional monomer and template, polymerization of monomer-template with cross-linkers, and removal of template to reveal the binding cavities that are specific to the template. 163 Figure 9 illustrates the process involved in fabrication of MIPs based sensor via electropolymerization. 164 Recent study by Zarayanov et al. performed eletropolymerization of 3-aminophenylboronic acid (3-APBA) onto the glassy carbon electrode and results showed good correlation with the values obtained by the highly specific LOD lactate biosensor. 146 Another study performed on the electroplymerization of 3-ABPA on Ag nanowires (AgNWs) working electrode revealed high sensitivity and specificity of lactate detection. 134 However, there are certain areas in which advances must be made in electropolymerization. These include the removal of imprinted templates and the inability to optimize both imprinting and re-binding conditions simultaneously, such as the solubility and minimization of interaction between the solvent, target, and monomer. 162
Figure 9. Fabrication process of molecular imprinted polymer based sensor via electropolymerization techniques. 164
Download figure:
Standard image High-resolution imageThe relevance of imprinting polymer as compared to non-imprinted polymer was explained in a study by Baggiani. He hypothesised that the presence of template molecules in pre-polymerization mixture acts to enhance binding properties that already pre-exist in a non-imprinted polymer. 165 This means that if the non-imprinted polymer shows poor binding properties towards target analyte, the corresponding MIP will also show a weak imprinting effect. He then performed a study to validate his hypothesis by studying the relationship of binding properties between MIP and NIP. Result shows that the hypothesis is true while MIP also shown better selectivity between enantiomers pairs or structurally related molecules as a results of imprinting process. 165
Performance Analysis
Several factors have been used to compare the performance of lactate sensors among different studies, as shown in Tables I and II. Classification was made based on enzymatic and non-enzymatic-based lactate sensors. The parameters used to evaluate the performance of the sensors are sensitivity, linearity, and detection limit, which are compiled along with the type of lactate sensing fluid.
Sensitivity of the electrode is defined as the slope of the output characteristic curve and is generally described as the minimum input of physical parameters that will create detectable output change. There are multiple factors that determine the sensitivity of the sensors developed including the size and surface area of particles, as well as the surface area of electrode, which allows for higher loading of enzymes in the deposition site. The electrode surface area can be modified to accommodate a larger surface area by utilizing nanomaterials such as carbon nanotubes (CNT), 137,139 gold nanoparticle, 110 silver nanoparticle, 116 platinum nanoparticle, 166 metal oxide nanoparticles such as graphene oxide, 110,114,101 and semiconductor material-based nanoparticles such as zinc oxide. 102,105 Hashemzadeh et al. used platinum electrode modified with a composite made up of reduced graphene oxide (rGO), carbon nanotubes (CNTs), and gold nanoparticles. This triple mixture showed a synergistic effect on the electrocatalytic activity and electron transfer abilities, which is also reported in another study on different analyte. 167
Linearity is one of the attributes that determine the accuracy of the measured analyte. The measured response to a straight line for a from a set of measurements with different analyte concentration can be represented mathematically as a linear equation of y = mc, where c is the concentrations of the analyte, y is the output signal, and m is the sensitivity of the biosensor. 168 This linearity is also associated with the resolution of the biosensor, which is defined as the smallest change of analyte concentration required to bring change in the response of the biosensor. Good resolution is important for the measurement of analyte over a wide working range. Linear range is another term associated with linearity, which is defined as the range of analyte concentration for which biosensor response changes linearly with concentration. 168 An enzymatic L-lactate biosensor by Bravo et al. displayed excellent linearity in the range of 3 to 500 m. This study reports on the use of carbon nanodots (CND) in the development of reagentless oxidoreductase-based biosensors. 111
Besides that, the performance of the sensor can also be determined based on the detection limit, which can be defined as the lowest concentration of the analyte being measured that determines the minimum detectable difference signal. The determination of detection limit can be calculated based on multiple approaches, including from standard deviation at low concertation, calibration curve, and the instrument resolution limit. A lower limit of detection can be achieved by improving the affinity of the binding affinity of biological molecules. Arivazhagan et al. reported a non-enzymatic lactic acid biosensor that is based on hollow sphere structured nickel sulfide (HS-NiS) nanomaterials for lactic acid detection in human urine. 141 This study displayed the lowest detection limit among all reviewed articles with LOD value of 0.023 μM, which can be attributed to the excellent electrocatalytic aptitude of hollow sphere NiS nanomaterials due to its high electronic conductivity, rich redox chemistry, different anatomic configurations, and crystalline structure. 169,170
Storage stability or lifetime assessment is also an important factor that can contribute to the need of the industry for commercialization of the sensors. Based on the summary of storage stability lactate biosensor in Table III, it can be observed that non-enzymatic lactate sensor shows the longest storage stability up to seven months, as compared with enzymatic-based sensors which last approximately between four and five weeks maximum. The lack of stability in enzymatic sensor is due to the loss of weakly-bonded enzymes, inactivation of enzyme, or even direct damage to the sensing film surface. 90 Meanwhile, non-enzymatic lactate sensor developed by Zaryanov et al. showed that the sensitivity of the sensors remained unchanged even after six months of storage in a Petri plate at room temperature in a dry state. 146 The study also compared their sensor with the LOD based biosensors, which is made based on the improved enzyme immobilization protocol, where the results obtained showed that the enzyme-based biosensors had lost almost half of their sensitivity within the first 10 days. One of the crucial criteria for applications of biosensor that were previously mentioned is an inherent instability of biomolecules due to environmental conditions, which affects the storage stability and leads to commercialization issues. Hence, avoiding the use of enzyme as a biomolecule would significantly improve the shelf life of the sensors.
Table 3. Storage stability for enzymatic and non-enzymatic lactate sensor.
| Type | Working electrode | Storage stability | Storage condition; Testing condition | References |
|---|---|---|---|---|
| Enzymatic | AuNP-cysteamine-LDH | Stable up to 18 days | Physiological conditions | 103 |
| Enzymatic | Pt/rGO/CNTs/AuNP | Stable up to 30 days | −18 °C; pH 7.4 | 108 |
| Enzymatic | Au/MWCNTs/MB | Sensitivity less 10% at 30 days | — | 109 |
| Enzymatic | Pt/CeO2-ZrO2 NP | Sensitivity less 30% at 15 days | –pH 7.4 | 113 |
| Enzymatic | Au/IrOx/LOD | Reduce sensitivity at 5th week | Room temperature;- | 90 |
| Non-enzymatic | SPCE/MIPs/AgNWs | 7 months | Room temperature, unsealed plastic box wrapped in tinfoil; pH 7.4 | 134 |
| Non-enzymatic | SPCE/PdCu | Up to 35 days | 4 °C; pH 7.4 | 144 |
| Non-enzymatic | SPCE/3-ABPA | Up to 6 months | Room temperature, petri plate, dry; pH 6.0 | 146 |
| Non-enzymatic | Gr/CU3(btc)2 electrode | More than 50 days | — | 50 |
Based on the studies on both enzymatic and non-enzymatic sensors, the enzymatic sensor still has the edge in term on their catalytic performance due to high selectivity of LOD and LDH provided that it has excellent enzyme immobilization. Enzymes have been known to have higher activity as compared to metal catalyst and higher conversion can be achieved without significant loss in selectivity. 171 However, non-enzymatic sensor that optimised metal oxide and nanomaterials compound have also successfully exhibit improved catalytic performance and stability and have attracted considerable attention. This can be attributed to the properties nano-composites that have large surface area that support large numbers of electroactive substances and thus significantly improving proton and electron transfer. 172 The molecular imprinted polymer would also bring additional sensitivity and selectivity to the non-enzymatic sensor.
Challenges and Future Recommendation
Although enzymatic-based sensors suffer from several limitations, such as environmental effect and storage stability, the potential use of an enzyme as a detecting agent still provides big advantages due to its specificity of detection method. However, very few studies perform stability testing especially for enzymatic sensors. Besides, many studies that performed stability testing unfortunately did not fully described their methods of assessment. According to a technical report produced by IUPAC, although some biosensors have been reported to be usable under laboratory conditions for more than a year, their on-shelf or practical lifetime is usually limited, especially when they are incorporated into industrial processes or into biological tissue, such as implanted glucose biosensors. Standardized storage stability assessment should be applied in all studies, which consists of significant parameters such as the stage of storage, atmospheric composition, pH, buffer composition, and presence of additives. In addition, robust biosensing includes stable biosensor activity in varying sweat pH microenvironments, and a performance that is invariant to fluctuating sweat pH. From the perspective of translating the established platform into a wearable diagnostic, it is important to maintain the biosensing output measurements in all pH conditions. 105
Most papers show promising results of lactate detection in lab measurements. However, testing of developed sensors in actual biofluids could be challenging. One of the studies tested their biosensing capability in different media, including PBS, human plasma, and whole blood subsequently, which shows that electrochemical signals produced by the same biosensor differed although the same concentration of lactate was used in all three media. Further investigation showed that the difference in electrochemical signals produced can be explained by the different viscosities and conductivities of the three media. The reduction of current is shown to be inversely proportional to the square root of the viscosity of the electrolyte and solution. 173 Higher electrolyte conductivity results in lower resistive losses and more effective charge mitigation, thus the higher current. 174 Hence, it is important to test the reliability of the developed biosensor on actual biofluids.
Besides, another great challenge in experimenting with biofluids such as sweat is the reliability of the collected samples. Sweat sampling is not a straight forward process as there are many factors that need to be considered such as the volume, flow rate, location of sampling, collecting tube, and time of sampling. Although sweat offers non-invasive biofluid access, there are several challenges exist alongside such as contamination at skin surface, mixture of old and new sweat, very low samples production rates, large pH changes, and presence of active analyte channels in eccrine gland that might skew the analyte concentration as seen in blood. 175 A standard guideline for sweat collection methods should be establish, hence a more reliable sample could be obtained for the analysis.
Biofluids may present in varying viscosity due to certain medical condition or changes in biological condition. For example, the viscosity of sweat is significantly elevated in patient with cystic fibrosis of pancreas (CFP). 176 This can be explained due the increased concentration of salt in sweat of CFP patients. Similarly to the viscosity of human saliva which often varies between individuals and easily affected by the changes in biological environement. 177 According to our review, we couldn't find any studies that report the effect of analyte solution viscosity towards the reliability of electrochemical responses particularly for lactate sensing. However, one of the closest study in glucose sensor reported that the increase in blood viscosity slows the diffusion of all components and reduces the current in the amperometric sensors. 178 Therefore, future research should consider studying these effects on other biofluids for lactate analyte.
The excellent stability of non-enzymatic based sensor especially on molecular imprinting technology using a functional monomer should be explored and optimized in future research. MIP can be categorized as one of the advanced synthetic methods for designing robust recognition materials that mimic biomolecules. This promising technology must be enhanced to be able to bring this technology in the marketplace against other conventional electrochemical sensors.
Conclusions
Lactate is an important metabolic biomarker that is present in blood, sweat, and other biofluids that can be used in clinical practice, sports monitoring, and others. In this review, the recent publication of electrochemical lactate sensor for both enzymatic and non-enzymatic in the last five years was discussed and summarized. Numerous researches on the development of high-performing ultrasensitive biosensors on lactate measurement provided an insight on the importance of lactate in various applications. Although enzyme-based electrochemical lactate sensors are still predominant due to their sensitivity, a growing number of researches has moved towards non-enzymatic-based sensors for improved performance. The development of non-enzymatic sensors has attracted huge interest as a fascinating alternative to overcoming the inherent limitation of enzyme-based sensors and is expected to provide a solution to the stability problem, as well as to the complex and irreproducible process for mass production. As a result, there are growing numbers of publications in recent years focusing on enzyme-free sensors fueled by the advancement of nanotechnology and nanomaterial field over the past decade. Research in this field needs different novel materials that could offer new possibilities for improved sensing electrode. With the trending of wearable sensors, we believed that the non-enzymatic sensor would bring an added advantage as it provide greater stability and selectivity especially in molecular imprinted polymer sensor. Besides, more efforts can also be aligned to investigate problems hampering reliable operation in biofluid samples.
Acknowledgments
Authors would like to express gratitude to the Ministry of Science, Technology and Innovation (MOSTI) and Ministry of Higher Education Malaysia (MOHE) with grant number 5F364 and 07G22 for supporting this research.