Abstract
In this article, a core–shell CuPd@UiO-66-NH2 composite material was synthesized by a double-solvent reduction method, and an N2H4 electrochemical sensor based on CuPd@UiO-66-NH2 was constructed. The relationship between the morphology, type, composition, size of the sensor interface composite material and its electrocatalytic performance and sensor response performance was studied, and a new method for detecting N2H4 was established. The surface properties and composition of the materials were studied by transmission electron microscope(TEM),energy dispersive X-ray spectroscopy(EDX) and X-ray diffraction spectroscopy(XRD). The results showed that the synthesized CuPd@UiO-66-NH2 has a regular 3D structure, particle dispersion, and uniform particle size, the particle size is about 90 nm. Electrochemical performance studies showed the sensor is made into detecting N2H4 in a linear range of 0.25 μM ∼ 1.39 mM, with a sensitivity of 386.7 μA·mM−1·cm−2, and a detection limit of 0.08 μM(S/N = 3). Compared with other electrochemical sensors based on metal nanoparticles to detect N2H4, the new sensor exhibited a wider linear range; and its sensitivity was 3 times of that obtained by the Cu-BTC/OMC/GCE. So, the sensor can be used as a potential sensing material to detect hydrazine.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
N2H4 is widely used in aerospace fuel, pharmaceutical synthesis, and material production. 1,2 But as a chemical substance that seriously harms human health health. 3 Its' detection is particularly important. Many methods for detecting N2H4 have been reported, such as spectrophotometry, 4 electrochemistry. 5 Electroanalysis has attracted much attention for its high sensitivity and low cost. 6 The slow kinetic oxidation of N2H4 on bare electrodes can easily lead to low sensitivity and serious interference. 7 Therefore, it's urgent to study suitable sensor materials to improve the analytical performance of modified electrodes.
Recently, many porous composite materials have been successfully used to construct electrochemical sensors.
8,9
Metal organic framework (MOF) is an organic- inorganic hybrid porous crystalline material assembled by the coordination of organic linking groups and metal cations.
10
And it has an extended network structure. They have attracted widespread attention mainly due to their unique advantages: large surface area, adjustable coordination space, and the inner surface of pores and cavities can be chemically modified.
11,12
They are used as catalyst supports for nanoparticles in various fields. However, the water instability and weak conductivity of some MOFs limit their sensing performance.
13
Therefore, MOF must be compounded with other materials with good conductivity to solve these problems. Metal nanoparticles (MNP)/MOF composites can be manufactured in several ways, including: used MOF as a template to generate MNP in its cavity,
14
or to encapsulate MNPs in a MOF layer,
15
MOF plays a role in protecting MNP. The pore structure of MOF restricts the migration and aggregation of MNP and promotes MNP to be evenly dispersed.
16
Combined the advantages of MNPs/MOF composites, MOF have been widely used in energy storage and conversion,
17
gas absorption,
18
catalysis.
19
MOFs have also been used to construct electrochemical sensors based on MNPs/MOF. For example, Yang et al. using UiO-66-NH2 composite AuPd alloy nanoparticles for the detection of nitrite.
20
Sharma et al. reviewed the applications of SnO2 nanomaterial and its derivative combined with metal nanoparticles and carbon materials in electrochemical gases, small biological molecules, and environmental sensors.
21
Hosseini et al. used Au-SH-SiO2NPs immobilized on Cu-MOF template to synthesize MOF-derived composite materials, and successfully constructed an electrochemical sensor for the determination of hydrazine
22
and cystein.
23
Dong and his partners loaded AuNPs on Ti-MOF to synthesize nanocomposite materials and successfully constructed a hydrazine sensor.
24
Zheng and his colleges prepared a three-dimensional Cu-MOF (Cu4.5(Btze)1.5(OH)4(SO4)(H2O)1.5 4H2O) composite material modified electrode and successfully constructed electrochemical sensor for the detection of hydrazine.
25
4H2O) composite material modified electrode and successfully constructed electrochemical sensor for the detection of hydrazine.
25
In this study, core–shell CuPd@UiO-66-NH2 was synthesized by double-solvent reduction method, where UiO-66-NH2 acted support matrix. Then, using CuPd@UiO-66-NH2 modified glassy carbon electrode (GCE), a new method of hydrazine electrochemical sensing was established.
Experimental
Reagents and instruments
ZrCl4, CuCl2, PdCl2 and 2-aminoterephthalic acid (H2N-BDC), were purchased from Aladdin Chemical Co., Ltd. (Shanghai, China). Acetic acid (HAc), NaBH4, DMF, n-hexane and ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. ultrapure water was used in the experiment.
The product was characterized by a transmission electron microscope (TEM, Talos F200X). The surface element composition of the synthesized sample was characterized by energy dispersive X-ray spectrometer (EDX). The structure and morphology of the samples were marked by X-ray diffraction spectroscopy (XRD, Bruker D8-ADVANCE). Electrochemical measurements were carried out in a conventional three-electrode electroanalysis system controlled by CHI660D electrochemical workstation (Shanghai CH Instrument Co. Ltd., China). A conventional three-electrode cell was used, including a glassy carbon electrode (GCE, geometric area = 0.07 cm2) as the working electrode, a saturated calomel electrode as the reference electrode and platinum foil as the counter electrode. All potentials given in this work were referred to the saturated calomel electrode.
Synthesis of UiO-66-NH2
Synthesized UiO-66-NH2 according to the method reported in literature. 26 (0.107 g, 0.45 mmol)ZrCl4 and CH3COOH (750 μl) were added in DMF (17 ml) solution. The mixture was stirred for 10 min, then, added (0.083 g, 0.45 mmol) NH2-BDC and DMF solution into the mixture solution and stirred continued for 10 min. And then transferred into a 50 ml Teflon-lined autoclave and heated at 120 °C, 24 h. After that, the orange-red solid product was separated from the solution by centrifugation and washed 3 times with DMF and MeOH.
Synthesis of CuPd@UiO-66-NH2
100 mg of activated NH2-UiO-66(Zr) was suspended in 25 ml of hydrophobic n-hexane. In order to obtain CuPd@UiO-66-NH2 nanocomposite with 5%wt metal nanoparticles, the resulting suspension was sonicated for 0.5 h and stirred for 1 h, and then the CuCl2 (0.53 mg, 12.5 μl) and PdCl2 (0.42 mg, 12.5 μl) (Cu:Pd = 1:1) solution were added dropwise to the above suspension with vigorous stirring.After stirring for 1.5 h, NaBH4 solution was added and the suspension was stirred for another 1.5 h to ensure a complete reduction of the metal particles inside the pore channels of NH2-UiO-66(Zr). Centrifugation to obtain a brown-black solid product, which was washed with ethanol and dried under 60 °C.
Preparation of nanocomposite-modified electrode
The glassy carbon electrode (GCE) is prepared by a simple method. Before use, first polish GCE with 0.3 μm alumina powder to remove dirt and scratches, and rinse with distilled water to obtain a mirror-like surface, and then sequentially ultrasonically treat it in distilled water. Then, wipe the GCE dry for later use. The prepared nanocomposite material (1 mg) was ultrasonically dispersed in a chitosan (1 ml, 0.5%) solution for 30 min. Then drop the obtained suspension (6.5 μl) on the GCE indicator and dry it naturally in the air. The prepared electrode is expressed as CuPd@UiO-66-NH2/GCE. And then a UiO-66-NH2/GCE was prepared by the same method as the former.
Results and Discussion
Study of the structure and morphology of CuPd@UiO-66-NH2
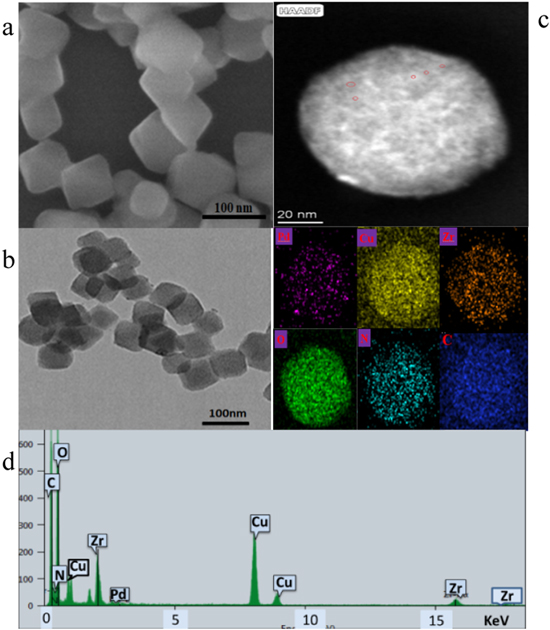
Figure 1A shows the powder XRD spectrum of NH2-UiO-66(Zr) framework. As shown, The UiO-66-NH2 crystal shows sharp diffraction peaks in the figure, indicating that its crystallinity is excellent, and the positions of all its characteristic diffraction peaks (curve a) are consistent with the peak positions reported in the literature. 27 After composite metal nanoparticles, the peak position of CuPd@UiO-66-NH2 is roughly the same as UiO-66-NH2, but the peak intensity is significantly reduced. 25 From the research of Farzaneh 28 et al., we can know that one possible reason for the decrease of the peak intensity of the XRD diffraction peak is that when depositing CuPd nanoparticles, the hydrophilic Pd2+ and Cu2+ enter the porous channel of UiO-66-NH2 under the action of hydrophobic and capillary. However, since metal nanoparticles enter the pore channel, their particle size is larger than that of the cavity channel, which may damage the adjacent cavities and cause small regular changes in the pore structure frame. 29 Another reason may be that high voltage makes the shape of UIO-66-NH2 irregular when we are doing the mapping. Certain interactions between the amino groups on the MOF and the Pd particles will affect the effect to a certain extent. 30 Curve a shows the successful synthesis of UiO-66-NH2, an ultra-broad peak emerged near 41.8° for the CuPd@UiO-66-NH2 (curve b), which located between those of pure Pd (111) crystal plane and Cu (111) crystal plane, suggesting the formation of CuPd alloy rather than the simple mixture of monometallic Cu and Pd. The FTIR spectrum (Fig. 1B) obtained by the KBr tablet method shows that the symmetric and asymmetric N-H tensile vibration peaks of the amino group in UiO-66-NH2 are located at 3500 cm-1 and 3426 cm-1, respectively, and the bending vibration of N-H is 1573 cm-1,and 1346 cm-1 is the shear stretching vibration of the C-N structure in arylamine. 31 Figure 2d is the chemical composition diagram of CuPd@UiO-66-NH2 nanocomposite characterized by EDS. It can be seen that Cu and Pd metal nanoparticles successfully entered the pore structure of UiO-66-NH2, further indicating that the obtained sample was CuPd@UiO-66-NH2 nanocomposite.
Figure 1. (A) XRD patterns of (a) UiO-66-NH2, (b) CuPd@UiO-66-NH2. (B) FT-IR spectra of (a) UiO-66-NH2, (b) CuPd@UiO-66-NH2.
Download figure:
Standard image High-resolution imageFigure 2. UiO-66-NH2 SEM image (a), CuPd@UiO-66-NH2 TEM image (b), HAADF of map data (c). EDS spectrum of CuPd@UiO-66-NH2 nanocomposite (d).
Download figure:
Standard image High-resolution imageFigure 2a is the SEM image of NH2-UiO-66(Zr), which shows the successful synthesis of MOF with a particle size of about 90 nm. From Fig. 2b, it can be seen that there are only very few metal nanoparticles on the outer surface of CuPd@NH2-UiO-66 (Zr), and these metal nanoparticles do not enter the MOF structure and gather near the outer surface. Figure 2c is an enlarged image of (b), and its structure shows a little irregularity, which may be caused by the entry of bimetallic nanoparticles into the pore size. By the high-angle circular dark-field scanning TEM and energy-dispersive X-ray spectroscopy (EDX) mapping image [Fig. 2d] further proves the formation of CuPd nanoclusters in the UiO-66-NH2 (Zr) cavity. It can be seen that the CuPd alloy nanoparticles successfully enter the UiO-66-NH2 channel, and the CuPd alloy nanoparticles are extremely small in size. Generally, due to the high surface energy of nanoclusters, they aggregate during the preparation process, so it is difficult to obtain such small size nanoclusters, however, according to Iglesia 32 and Luo 33 research team's report, ethylenediamine was used to introduce amino groups in MIL-101-Cr. The amino group stabilizes Pb2+ through coordination and enhances the adsorption capacity of MOF to Pb2+.The study by Wang 34 et al. also pointed out that the amino group in UiO-66-NH2 and Ag+ enter the MOF channel under the coordination effect to stabilize metal ions. Through the research of the Ning 35 research group, we can also know that there is a certain interaction between the amino group and the palladium nanoparticles, which all indicate that the amino group can stabilize the metal precursor to a certain extent. The pore channel can act as a nanoreactor, limiting the growth of these nanoparticles and promoting the preparation of CuPd nanoclusters in the MOF cavity. This shows that the double-solvent method and different reduction processes can growth of small-sized nanoparticles in the MOF cavity.
Investigation on electrochemical properties of CuPd@UiO-66-NH2/GCE
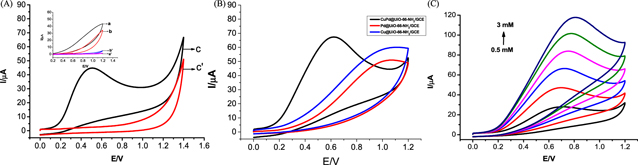
The electrochemical characteristics of various modified electrodes were studied. Figure 3A shows that in the presence of (a, b, c) 1.0 mM N2H4 and the absence of (a, b, c) 1.0 mM N2H4 in 0.1 M PBS (pH = 7.4) bare/GCE, UiO-66-NH2/GCE and CuPd@UiO-66-NH2/GCE Cyclic Voltammetry (CV), at this time the rate is 100 mVs−1. Figure 3A shows that in the presence of (a, b, c) 1.0 mM N2H4 and the absence of (a', b', c') 1.0 mM N2H4 in 0.1 M PBS (pH = 7.4) solution, the Cyclic Voltammetry (CV) of bare/GCE, UiO-66-NH2/GCE and CuPd@UiO-66-NH2/GCE, at this time with a rate of 100 mVs−1. It can be easily seen that the bare GCE (a'), UiO-66-NH2/GCE (b') and CuPd@UiO-66-NH2/GCE (c') in the absence of N2H4 have almost no electrochemical response. When added 1.0 mM N2H4 in solution, no obvious oxidation peak appeared at bare/GCE (a), which indicated that the oxidation kinetics of N2H4 on the bare/GCE surface was very slowly. The sluggish reaction increased oxidation over potential. UiO-66-NH2/GCE (b) shows a lower current response than bare/GCE due to the poor conductivity of the metal organic framework. And we can obvious seen that CuPd@UiO-66-NH2/GCE (c) showed a higher oxidation peak response at 0.6 V, the intensity is about 45 μA, indicating that CuPd@UiO-66-NH2 has excellent electrocatalytic activity for N2H4. The reaction process of N2H4 on the electrode surface may be as follows: 36

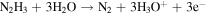
And which should be attributed to the synergistic effect of Cu and Pd. 37 Figure 3B is the electrochemical sensing experiment of adding Pd@UiO-66-NH2/GCE and Cu@UiO-66-NH2/GCE to 0.1 M PBS (pH = 7.4) solution containing 1.0 mM N2H4. It is found that copper and palladium nanometer under the coexistence of particles, CuPd@UiO-66-NH2/GCE has higher catalytic activity at lower potential, indicating that there is a synergistic effect between Cu and Pd.
Figure 3. (A) Cyclic voltammograms obtained by bare GCE (a), (a)', UiO-66-NH2/GCE (b), (b)' and. CuPd@UiO-66-NH2/GCE (c), (c') in pH 7.4 PBS in the absence (a)', (b)', (c)' and presence (a)–(c) of 1.0 mM N2H4 at a scan rate of 100 m V s−1. (B) Cyclic voltammograms obtained by Cu@UiO-66-NH2/GCE,Pd@UiO-66-NH2/GCE and CuPd@UiO-66-NH2/GCE in pH 7.4 PBS in 1.0 mM N2H4 at a scan rate of 100 m V s−1. (C) Cyclic voltammograms obtained at CuPd@UiO-66-NH2/GCE in pH 7.4 PBS in the presence of N2H4 with different concentrations (from 0.5 to 3 mM) at a scan rate of 100 mVs−1.
Download figure:
Standard image High-resolution imageFigure 3C studied the electrocatalytic activity of CuPd@UiO-66-NH2/GCE on N2H4. After adding N2H4 to 0.10 M PBS (Ph = 7.4), it can be seen, with increasing concentration of N2H4, a significant increase in the oxidation current, the oxidation peak potential increased correspondingly. Figure 4A studied the effect of scanning rate on CuPd@UiO-66-NH2/GCE electrocatalytic oxidation of 1.0 mM N2H4. As the scan rate increases in the range of 10∼200 mVs−1, the peak current also increases with a certain rule, and the peak potential moves in the positive direction. In addition, when plotting the relationship between the peak current and the square root of the scan rate, good linearity was obtained, which indicates that the oxidation of N2H4 is a diffusion-controlled process [Fig. 4B].
Figure 4. (A) Cyclic voltammograms obtained at CuPd@UiO-66-NH2/GCE in pH 7.4 PBS containing 1 mM N2H4 at different scan rates (from 20 to 200 mVs−1). (B) Linear fitting program of N2H4 vs the square root of scan rate.
Download figure:
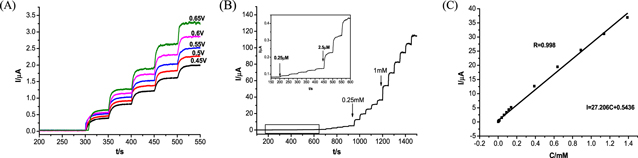
Standard image High-resolution imageThe sensing characteristics of CuPd@UiO-66-NH2/GCE were further studied by current-time electrochemical technology. Figure 5A shows the ampere response of CuPd@UiO-66-NH2/GCE when a 0.1 M PBS (pH = 7.4) solution containing 0.01 mM N2H4 is continuously stirred under a gradient potential. As shown in Fig. 5A, the current response increases as the operating voltage increases, the highest current response occurs at 0.65 V. However, when the working voltage is 0.65 V, the interfering substance is activated and generates a lot of noise. Therefore, in order to ensure excellent current response, it is beneficial to choose 0.6 V with low noise and less interference as the working potential. Figure 5B records the ampere response of CuPd@ UiO-66-NH2/GCE when N2H4 is continuously added to continuously stirred 0.1 M PBS (pH = 7.4) under the working potential of 0.6 V, which shows that after adding N2H4 the response increases rapidly. When N2H4 diffuses from the solution surface to the electrode/solution interface, the current response increases rapidly, and N2H4 will rapidly oxidized. However, when a high concentration of N2H4 is added, the response time is longer and the current response is slower. This may be the electrode surface cannot provide enough space for the high concentration of N2H4 reaction. Figure 5C shows the linear correlation between the current response and the N2H4 concentration, thereby obtaining a wide linear range from 0.25 μM to 1.38875 mM. The linear regression equation can be expressed as Ip (μA) = 0.544 + 27.206 C (mM). When using the formula LOD = 3SB/N to calculate, the lowest detection limit obtained is 0.08 μM (where SB represents the standard deviation of the blank solution and b represents the slope of the calibration curve), when the slope of the standard curve is 27.206, and the GCE surface area is 0.07cm2, the sensitivity of the sensor is estimated to be 386.7 μA·mM-1 ·cm-2. Table I is the this sensor compared with other electrocatalytic N2H4 sensors,CuPd@UiO-66-NH2/GCE shows excellent N2H4 detection performance. Owing to the three-dimensional porous structure of UiO-66-NH2 provides a large number of N2H4 reactive sites for CuPd alloy and the synergy of copper and palladium, which is beneficial to enhance the current response. The detection limit of this sensor is low and the sensitivity is high.
Figure 5. (A) Amperometric response obtained by CuPd@UiO-66-NH2/GCE on successive addition of 0.01 mM N2H4 into 0.1 M PBS (Ph = 7.4) at various applied potentials. (B) Amperometric curve obtained by CuPd@UiO-66-NH2/GCE with addition of N2H4 in pH 7.4 PBS at 0.6 V. (C) Calibration curve of N2H4.
Download figure:
Standard image High-resolution imageTable I. Comparison of non-enzymatic N2H4 sensors.
| Sensors | Linear range(μM) | Detection limit (μM) | Sensitivity(μA·mM−1·cm−2) | References |
|---|---|---|---|---|
| Pd/CNF | 10–4000 | 1.2 | 2.9 | 38 |
| Ag/Zn-MOF | 6–5000 | 1.6 | — | 39 |
| Cu3P@NH2-MIL-125(Ti)/GCE | 5–7500 | 0.08 | 10.9 | 40 |
| CuS-rGO/GCE | 1–1000 | 0.3 | 111.3 | 41 |
| Cu-BTC/OMC/GCE | 0.5–710 | 0.4 | 154 | 42 |
| Ni(OH)2/Au/glass | 0.01–0.12 | 0.01 | 1.7 | 43 |
| ZnO nanosheets/SiO2/Si | 0.005–0.11 | 0.0012 | 12.2 | 44 |
| CuPd@UiO-66-NH2/GCE | 0.25–1388.8 | 0.08 | 386.7 | This work |
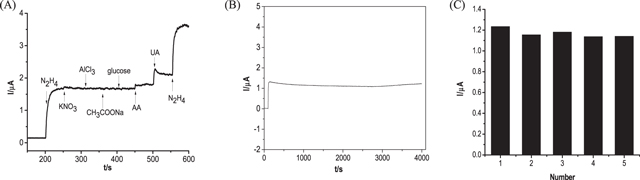
As shown in Fig. 6A, the influence of some common interfering substances was studied. The addition of N2H4 leads to an enhanced current response, which indicates that CuPd@UiO-66-NH2/GCE has electrocatalytic activity for N2H4 oxidation. However, the current response does not change much after the addition of 10 times the concentration of inorganic interferences, and the current response is extremely low when the 2 times the concentration of organic interferences AA and UA are added, which means that these interfering substances have almost no effect on the detection of N2H4. Figure 6B shows that the initial current response of CuPd@UiO-66-NH2/GCE at 4000 s basically maintains a straight line, indicating acceptable stability, and Fig. 6C shows that five CuPd@UiO-66-NH2/GCE prepared to detect 0.01 mM N2H4, and the relative standard deviation (RSD) obtained was 4.0%.
Figure 6. (A) Amperometric response obtained by CuPd@UiO-66-NH2/GCE on successive additions of 0.01 mM N2H4, 0.1 mM KNO3, AlCl3, CH3COONa, glucose, 0.02 mM ascorbic acid (AA) and uric acid (UA) into 0.1 M PBS (pH 7.4) at 0.6 V under constant stirring. (B) Amperometric response of 0.01 mM N2H4 obtained by CuPd@UiO-66-NH2/GCE in 4000 s measurement. (C) The measurement results obtained by 5 CuPd@UiO-66-NH2/GCE.
Download figure:
Standard image High-resolution imageConclusions
In conclusion, CuPd@UiO-66-NH2 nanocomposite was successfully synthesized, and the N2H4 electrochemical sensing method based on the CuPd@UiO-66-NH2 was successfully constructed. The nanocomposite research shows that the preparation method is simple, and the nanocomposite has a regular three-dimensional structure and uniform particle size. Electrochemical performance studies showed that compared with other electrochemical sensors, the new sensor exhibited a wider linear range, and its sensitivity was 3 times of that obtained by the Cu-BTC/OMC/GCE. Considering the facile preparation route and excellent experimental results, CuPd@UiO-66-NH2 nanocomposite can be applied as a promising type of sensing material.