Abstract
This research presents a simple method for preparing poly Schiff base ligand (L) and its metal complex (M–L, M = Al3+, Cr3+, Zn2+) as electrode materials for supercapacitors, which is derived from mixing terephthalaldehyde, m-phenylenediamine and metal nitrate in ethanol at room temperature. Compared with L, M–L combine the advantages of larger surface area, appropriate mesopore diameter, unique morphology and suitable conductivity. The electrochemical properties of the materials are assessed by cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) analysis in 6 M KOH electrolyte. The results show that the electrochemical performance of M–L significantly improve compared with L, especially when the current density is 0.5 A g−1, Al–L displays a superior specific capacitance of 608.6 F g−1. Moreover, the specific capacitance of Al–L still reaches 299.1 F g−1 after 1000 GCD cycles at 10 A g−1, which is higher than the initial capacitance of Cr–L and Zn–L. Moreover, the electrochemical resistance of Al–L is smaller than that of others. Therefore, Al–L will become an attractive material in supercapacitors, and opens the door for further research on various poly Schiff base metal complexes (poly[M(Schiff)]) as electrode materials for supercapacitors.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
With the development of time, non-renewable resources will gradually be exhausted, and energy shortage will become the biggest problem for human beings to survive on the Earth. In recent years, due to social progress and the advancement of science and technology, people are eager to find new types of clean renewable energy and energy storage materials and devices to maximize energy efficiency.1–3 Supercapacitor, as the electrochemical storage equipment, are widely used in various fields due to their high power density and long cycle life and fast charge/discharge rate as well as a wide operating temperature range.4–6 According to the classification of energy storage mechanisms, supercapacitors can be divided into electrochemical double-layer capacitors (EDLCs) are realized by ion adsorption at the electrode–electrolyte interface and pseudocapacitors via the rapidly reversible Faraday redox reaction of the electrode material and electrolyte ions surrounding the interface,7–9 which it produce much large capacitance than most EDLCs.10 As the electrode material of pseudocapacitors, conductive polymers (CPs) such as polyaniline (PANI),11–13 polypyrrole (PPy)14 and polythiophene (PTh)15,16 are widely attracted the attention of many researchers due to their low preparation cost, good temperature stability and high theoretical specific capacitance.17 However, their preparation process is affected by factors such as method, substrate, doping and template, and the electrochemical properties of the prepared electrode materials, especially the poor cycling stability, are difficult to meet the needs of practical applications.18 Therefore, in order to make up for these shortcomings and meet the needs of commercialization, the researchers combined the energy storage mechanism of EDLCs and pseudocapacitors to prepare a composite of many conductive polymers.19–22 Although these newly synthesized composite materials have a large improvement in electrochemical performance as compared with pure polymers, the high-cost and the complicated fabrication process are still great challenges for application. Therefore, it is still a focus for researchers to find new environmentally friendly and low-cost electrode materials to prepare supercapacitors.23
The poly Schiff base contain –CH=N– groups, and it presence of the lone pair electron of nitrogen (electron donor), which can form complexes with transition metals (electron acceptor).24,25 Poly Schiff base metal complexes (poly[M(Schiff)]) are widely used in many fields such as biomedicine,26,27 chemical catalysis,28–30 functional materials31–33 and analytical chemistry34,35 because of their simple preparation process, low preparation cost, numerous in variety and many other good properties. However, as one kinds of CPs, there have been few reports on the use of poly Schiff base and their metal complexes for supercapacitor electrode materials so far. Zhu et al.36 synthesized high-level N-doped microporous carbon spheres (N-MCSs) based on a very simple Schiff-base reaction in ethanol solvent, followed by a common one-step carbonization–activation process. When the solvent ethanol is 200 ml, the specific capacitance of the prepared N-MCS-200 at 1 A g−1 in the 6M KOH electrolyte reaches 292 F g−1, together with remains 86% of 10000 cycles at 10 A g−1. Zhou et al.37 reported a Schiff base Co-complex synthesized by hydrothermal method at different temperatures. The obtained complex at 180 °C performed a maximum specific capacitance of 404 F g−1 in 3M KOH electrolyte at 1 A g−1. Therefore, compared with pure poly Schiff base, the electrochemical performance of the complexes has been greatly improved. Currently, poly[Ni(salen)] is the most widely studied as the electrode material for supercapacitors in poly[M(Schiff)].38,39 The capacitance produced by poly[Ni(salen)] electrode is mainly the reversible transition of Ni2+/Ni3+. In other words, the charge carried by the ligand does not change during the reversible transformation. However, if the valence state of the central ion (e.g., Al3+, Cr3+, Zn2+) participating in the coordination is the highest value, is there a pseudocapacitance ? Unfortunately, there are no reports available in the literature about Al, Cr and Zn based Schiff base complexes for supercapacitors applications.
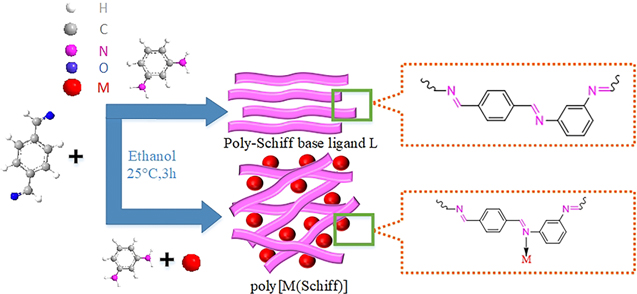
In this paper, we synthesized poly-Schiff base ligand L with m-phenylenediamine (MPDA) and terephthalaldehyde (TPAL) in ethanol solvent without any catalyst or tedious procedure, and followed synthesized several poly[M(Schiff)] (M–L, M = Al3+, Cr3+, Zn2+) and they were used as electrode materials for supercapacitors (Fig. 1). The advantages of adopting this method for synthesis include: (a) the synthesis process is very simple, does not require catalysts and other complicated conditions and the reaction time is short (3 h); (b) the reactants are inexpensive and readily available, and there are no environmentally harmful by-products in the product, all of which can be used as electrode materials and which have prospects for mass production; (c) the poly[M(Schiff)] obtained by this method exhibits many prominent characteristics such as good morphology, large specific surface area and excellent electrochemical properties including high specific capacitance, small impedance and high rate performance; (d) due to the wide variety of poly Schiff base ligands, a wide variety of poly Schiff base metal complexes with good electrochemical properties can be prepared by this method. Therefore, poly-Schiff bases and their metal complexes have great research potential as electrode materials for supercapacitors.
Figure 1. Schematic diagram of the forming of L and M–L.
Download figure:
Standard image High-resolution imageExperimental
Synthesis of ligand L and its metal complexes (M–L)
Typically, 0.02 mol of MDPA (2.1628 g) and TPAL (2.6826 g) were weighed separately in a beaker containing 30 ml of ethanol at room temperature under magnetic stirring, and designated as solutions A and B. The solid was dissolved to obtain a clear and transparent solution, and the B solution was slowly poured into the solution A and the mixture was stirred for 3 h at room temperature. Then, the ligand L was obtained by filtration, washed with ethanol for 2 to 3 times, and dried in a 60 °C for 12 h. The synthesis of M–L is similar to ligand L. First, 0.02 mol of hydrated nitrate (Al(NO3)3·9H2O, 7.5026 g) or (Zn(NO3)2·6H2O, 5.9489 g) or (Cr(NO3)3·9H2O, 8.0030 g) was dissolved in ethanol, the solution was named C solution. Then, the C solution was slowly poured into the mixture solutions (A + B) before the ligand L precipitation was appeared. After that, the mixture was stirred for 3 h at room temperature. Finally, Al–L, Zn–L, Cr–L is obtained by filtration, washing and drying.
Characterizations
The morphology of the prepared samples was observed with a Hitachi S-4800 field emission scanning electron microscope (FE-SEM) equipped with an INCA-350 energy dispersive spectrometer (EDS). Elemental analysis was conducted using a PerkinElmer 2400 Series II elemental analyzer. The crystal structure of the sample was analyzed by a Bruker D8 Advance X-ray diffractometer (XRD) under Cu-Kα radiation (0.15418 nm). Fourier transform infrared spectrum (FT-IR) was characterized on a Bruker Tensor II in the spectral range of 4000–400 cm−1 using a platinum ATR module. UV–vis spectroscopy was performed using WFZ-26A in the range of 200–800 nm. Nitrogen adsorption isotherms were measured at 77.3 K with a Tristar II3020 analyzer. The pore volume and pore size distribution were obtained from the isotherm by the Barrett–Joyner–Halenda (BJH) method. Thermogravimetric analysis (TGA) was performed using an SDT Q600 analyzer (N2, 10 °C min−1).
Electrochemical measurement
To evaluate the electrochemical performance of the prepared samples, all electrochemical tests were conducted on a CS310 electrochemical workstation (Wuhan Corrtest Instruments Co., Ltd., China) that was tested at room temperature. In a typical three-electrode system, the counter and reference electrodes are Pt wire and Hg/HgO, respectively. The working electrode was prepared by mixing 80 wt% of the active material with 10 wt% acetylene black and 10 wt% PVDF and then adding N-Methyl pyrrolidone (NMP) to form a slurry, which was then pressed into the foamed nickel (1 * 1 cm2) as a current collector and dried at 60 °C for 12 h. Herein, the active material loaded for electrochemical testing on the working electrode is about 1 ∼ 2 mg. The electrochemical performance of as-prepared L and M–L as an active electrode material for supercapacitor was evaluated by Cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) measurements in an electrolyte of 6M KOH and used the same counter and reference electrodes.
Results and Discussion
The elemental composition of L and M–L was determined by elemental analysis. As shown in Table I, the experimental quantification of L obtained was C = 79.63%, H = 4.787%, and N = 13.05%. Theoretically, the carbon, hydrogen, and nitrogen contents of the L framework are C = 81.55%, H = 4.85%, and N = 13.59%, which have some deviations with the experimental data, a reasonable explanation is the polymer L without further purification. There is an interesting phenomenon that C content of M–L decrease in comparison with L, indicating that metal ions coordination with the lone pair electron of nitrogen. Simultaneously, N content has tiny increase in polymer (Al–L, Cr–L), which illustrate that nitrate ions (NO3−) also enter the skeleton of poly[M(Schiff)] as counter anions.
Table I. Content of (C, H and N) element in L and M–L.
| Sample | C (%) | H (%) | N (%) |
|---|---|---|---|
| L | 79.63 | 4.787 | 13.05 |
| Al–L | 54.27 | 4.805 | 13.18 |
| Cr–L | 54.01 | 4.292 | 13.17 |
| Zn–L | 62.42 | 3.873 | 12.71 |
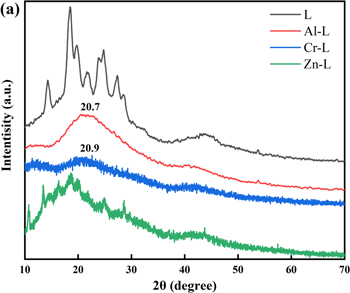
The crystalline characteristics of L and M–L were characterized by X-ray diffraction (XRD) analysis with a 2θ angle ranging from 10° to 70°. As shown in Fig. 2, some diffraction peaks are measured in L, which are ascribed to the intermolecular π-π stacking structure of the layered architecture.40,41 With metal ion coordination and counter anions (NO3−) entering the molecular chain, hinder effect lead to the intermolecular π-π stacking weakening, and the diffraction intensity of M–L decrease because the disorder stacking tendency increase in molecular frameworks. A board peak (about 20.7°) is observed, which indicates the amorphous nature of Al–L and Cr–L. The low crystallinity of the complex can be attributed to metal ion coordination and counter anions doping.
Figure 2. XRD pattern of L and M–L.
Download figure:
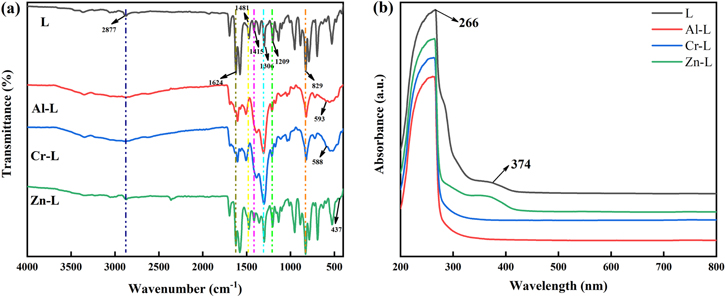
Standard image High-resolution imageIn Fig. 3a, FT-IR spectra of L and M–L show similar absorption peaks at different wavelengths, which indicates that the metal ions coordination did not cause large-scale changes in the original L framework. The peak value of the out-of-plane deformation vibration of the para-disubstituted C–H in the benzene ring appeared at 829 cm−1.42 The peaks at 1306, 1415 and 1481 cm−1 are respectively due to stretching vibration of the C–N, C–C and C=C bonds.36 The band at 2877 cm−1 is assigned to the C–H stretching vibration.8 In addition, the characteristic peaks of C=N bonds in L and M–L can be found at 1624 and 1209 cm−1.43,44 Meanwhile, a new small absorption peak was found at Al–L, Cr–L and Zn–L at 593 cm−1, 588 cm−1 and 437 cm−1, respectively. This can be attributed to the stretching vibration of M–N, which is another evidence of coordination bond formation between L and metal ions.28,38,45
Figure 3. (a) FT-IR spectra of L and M–L, (b) UV–vis absorption spectra of L and M–L.
Download figure:
Standard image High-resolution imageUV–vis absorption spectrum was used to further study the conjugated network structure of L and M–L, as shown in Fig. 3b. The observed absorption peaks are around 266 and 374 nm, which can be attributed to the π-π* transition of the benzene ring and the n-π transition of C=N.38 In addition, it was found that the metal complex formed by the added metal forming a coordination bond with N has a significant blue shift at the n-π transition of C=N.
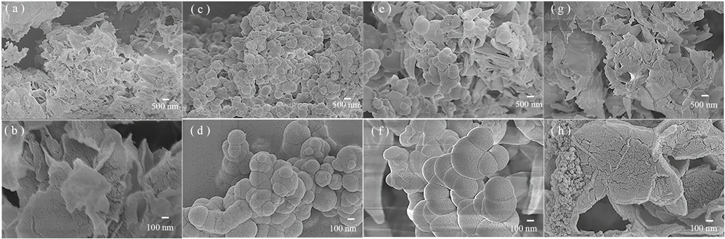
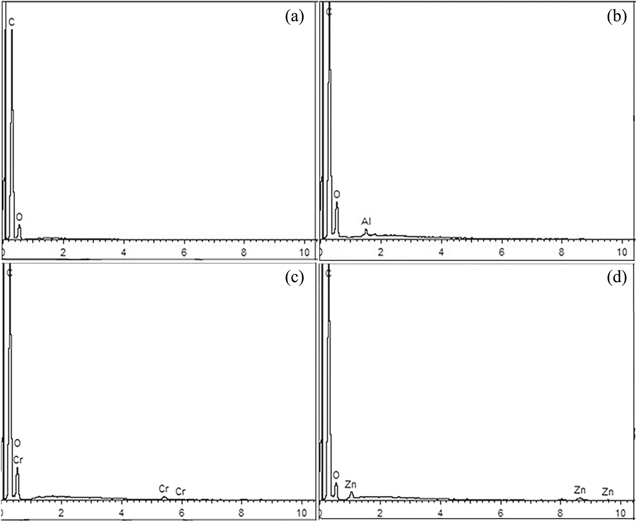
L (Figs. 4a and 4b) and Zn–L (Figs. 4g and 4h) have similar SEM images, both of which are layered morphologies composed of sheets with the lateral sizes of about several microns. However, some aggregated small particles appear on the Zn–L layer due to the coordination effect of Zn2+. The images in Figs. 4c and 4d show that Al–L has the morphology of agglomerated spheres with a diameter of about 100∼200 nm. As shown in Figs. 4e and 4f, the image is similar to Al–L, but Cr–L is not as regular and uniform as Al–L. In addition, the corresponding EDS spectra of L and M–L are shown in Fig. 5. Among them, the corresponding metal elements were detected in the M–L spectrum, which is a supplementary explanation of elemental analysis. As for the undetected N element, it may be due to the overlapping with element C signal in the EDS measurement.
Figure 4. SEM images of L, Al–L, Cr–L and Zn–L at low magnification ((a) (c) (e) (g)) and high ((b) (d) (f) (h)) magnification.
Download figure:
Standard image High-resolution imageFigure 5. (a)–(d) are EDS spectra of L, Al–L, Cr–L, and Zn–L, respectively.
Download figure:
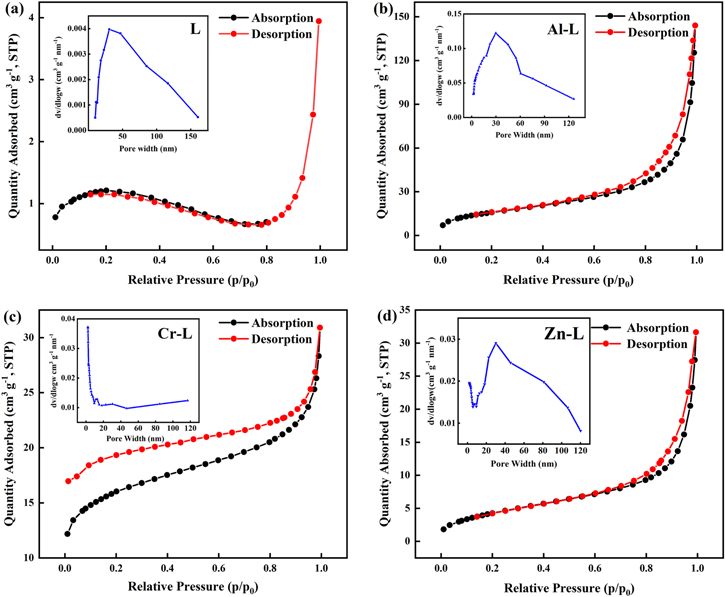
Standard image High-resolution imageFigure 6 exhibits the N2 adsorption-desorption isotherms of L and M–L and the corresponding Barrette-Joynere-Halenda (BJH) PSD (inset) performed at 77.3 K. The isotherm of L can be approximated as a type II isotherm, which indicates that it is non-porous and the corresponding BET surface area is only the 3.58 m2 g−1. It can be observed that M–L is a typical type IV isotherm with H3 hysteresis loop, indicating that the sample has a typical mesoporous structure.46 The difference between the L and M–L isotherms may be due to the introduction of metal ions, which will cause the original L framework to loosen, resulting in a transition from a nonporous to a mesoporous structure. It can be seen from the L and M–L PSD curves that there are almost no micropores, but the pore distribution in the mesopore and macropore regions is good. The presence of mesopores and macropores in the active material promotes the diffusion of electrolyte ions to the active site and can be used to maximize charge storage.20 As shown in Table II, the BET specific surface areas of Al–L (57.91 m2 g−1), Cr–L (50.95 m2 g−1), and Zn–L (16.32 m2 g−1) are much higher than L (3.58 m2 g−1). Correspondingly, the specific capacitance of M–L is greater than L at a current density of 0.5 A g−1, which indicates that the surface area of the electrode material is an important factor affecting electrochemical performance. The larger pores generally provide lower resistance to ion insertion and extraction within the electrode material, and a shorter diffusion path.47 Herein, high specific surface area and large pore structure of Al–L determine its excellent electrochemical performance compared with other M–L materials.
Figure 6. Nitrogen adsorption-desorption isotherms and pore size distribution curves (PSD, inset) for L and M–L.
Download figure:
Standard image High-resolution imageTable II. The pore structure parameters and specific capacitance of the L and M–L samples at a current density of 0.5 A g−1.
| Sample | Specific capacitance (F g−1) | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Micropore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|---|---|
| L | 66.8 | 3.58 | 0.00551 | 0.000302 | 3.90 |
| Al–L | 608.6 | 57.91 | 0.22475 | — | 9.76 |
| Cr–L | 448.0 | 50.95 | 0.03183 | 0.011484 | 3.07 |
| Zn–L | 125.8 | 16.32 | 0.05080 | — | 7.77 |
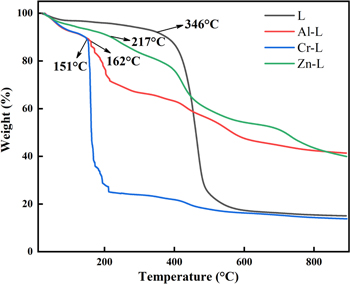
To evaluate the thermal stability of the samples, they were subjected to thermogravimetric analysis (TGA) under a nitrogen atmosphere. As shown in Fig. 7, the thermal decomposition of L can be divided into three steps. The first decomposition is about 7.08% weight loss in the temperature from 50 °C to 346 °C, which is due to the physical adsorption of molecular water on the material surface and unreacted monomer.16,48 The second sharp major weight loss is the decomposition of the long chain of ligand L in the temperature range of 346 °C to 453 °C. Finally, the weight loss of more than 453 °C can be attributed to the carbonization of the organic residues after chain decomposition. The TGA curves of M–L are very similar to that of L. It is worth noting that in the second stage, the polymer long-chain decomposition temperatures of Al–L, Cr–L, and Zn–L are 162 °C, 151 °C, and 217 °C, respectively, which is significantly lower than L (346 °C). M–L formation via metal ions coordination and counter anions entering into the long-chain skeleton of L cause instability of the overall structure, which is consistent with previous XRD and nitrogen adsorption measurements.
Figure 7. TGA curves of prepared L and M–L samples.
Download figure:
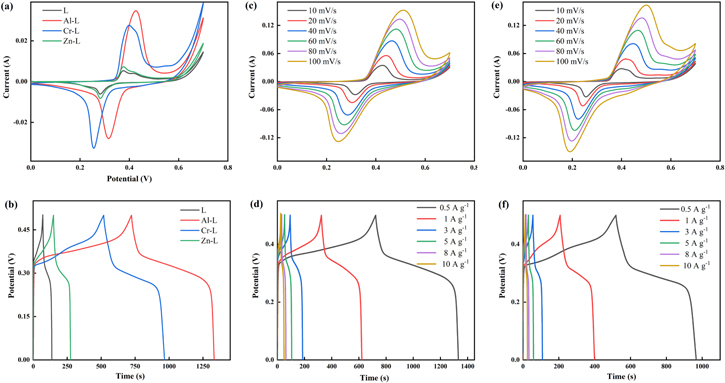
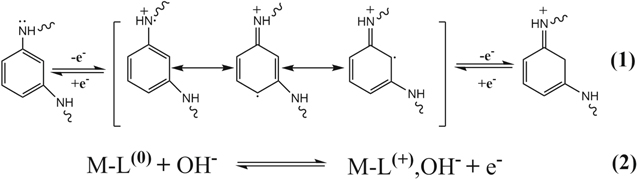
Standard image High-resolution imageIn order to understand the macroscopic electrochemical reaction of the supercapacitor electrode surface during charging and discharging, the as-prepared L and M–L electrode materials were tested for CV and GCD in a 6M KOH three-electrode system. Figure 8a shows the CV curves of the L and M–L with a scan rate of 10 mV s−1 at a potential window of 0–0.7 V. The CV curve shows a distinct symmetrical redox peak indicating the presence of a quasi-reversible redox process, indicating the characteristics of the pseudocapacitor. Yan et al.49 reported that there are two models for the charge transfer mechanism of poly[M(Schiff)]. In the first model, when the conjugated π-bonds are present in the ligand, the charge is exchanged between adjacent central metal atoms, that is, the central metal undergoes a redox reaction. In the second model, the central metal does not participate in charge transfer and the charge carried does not change, mainly because the ligand experiences a rapidly reversible redox reaction. Combining previous reports by Senthilkumar et al.,50 Song et al.51 and Li et al.,40 we believe that the second model is more suitable for explaining the redox mechanisms of L and M–L. The possibility of a redox mechanism is explained in Scheme
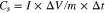
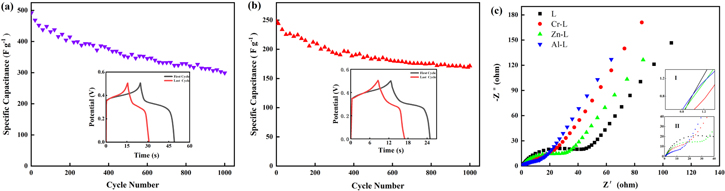
where I is the discharge current (A), Δt is the discharge time (s), ΔV is the potential window (V) and m is the mass of the active materials (g). The nonlinearity of the charge-discharge curve illustrates the characteristics of the pseudocapacitor of active materials L and M–L due to the faradic reaction occurring on the electrode surface, which is consistent with the results of the CV measurement. The specific capacitances of L, Al–L, Cr–L and Zn–L at 0.5 A g−1were calculated by the Eq. 1 to be 66.8, 608.6, 448 and 125.8 F g−1, respectively. The specific capacitance of M–L is much higher than the specific capacitance of L, because the introduction of metal ions destroys the dense spatial structure of original L and forms a microspherical surface with a large specific surface area, and thus providing more active sites for the intercalation and deintercalation of OH−. The GCD curves of Al–L and Cr–L from 0.5 to 10 A g−1 are shown in Figs. 8d and 8f, respectively. The specific capacitance values of Al–L and Cr–L decrease with increasing current density, which may be due to the limitation of ions entering the pores and the weak redox kinetics of the material at high current densities. When the current density is increased to 10 A g−1, the capacitances of Al–L and Cr–L are 492 and 248 F g−1, and the specific capacitance retention rates are 80.8% and 55.4%, respectively, which indicates that Al–L has a stronger endurance at a large current and a great rate capability.
Figure 8. (a) CV curves of the L and M–L at a scan rate of 10 mV s−1 in 6 M KOH, (b) GCD curves of L and M–L at a current density of 0.5 A g−1, (C) and (e) are the CV curves of Al–L and Cr–L at different scan rates of 10 to 100 mV s−1, (d) and (f) are the GCD curves of Al–L and Cr–L at different current densities ranging from 0.5 to 10 A g−1.
Download figure:
Standard image High-resolution imageScheme 1. Redox mechanism of the MPDA units for L and M–L.
Download figure:
Standard image High-resolution imageLong cycle life is a critical parameter for active materials as supercapacitor electrode materials. In order to evaluate the cycle stability of Al–L and Cr–L, we performed 1000 GCD cycles at a current density of 10 A g−1 in a potential range of 0 to 0.5 V (Figs. 9a and 9b). The results show that the capacitance retention rates of Al–L and Cr–L are 60.8% (299.1 F g−1) and 69.2% (171.5 F g−1), respectively. Meanwhile, the reason for the decrease in the specific capacitance of Al–L and Cr–L after the cycle may be the same as that of other CPs. At high current densities, long-term and rapidly reversible redox reactions of structural units in polymers will cause swelling and shrinking of their structural units, ultimately destroying their structure and conductivity.54
Figure 9. (a) and (b) are the cycle stability of Al–L and Cr–L electrodes at 1000 A cycles at 10 A g−1, respectively (c) Nyquist plots of the L and M–L.
Download figure:
Standard image High-resolution imageEIS measurements were performed for further examine the charge-transfer and ion transport process of the L and M–L electrodes in the range of 0.01 Hz to 100 KHz with an amplitude of 5 mV. The corresponding Nyquist plots are shown in Fig. 9c. All of the plots present a similar shape, which is a semicircle in the mid-high frequency regions and a straight line with a slope of 45° in the low frequency region. The equivalent series resistance (Rs) composed of the resistance of the electrolyte and the internal active material can be known from the intersection of the semicircle and the X-axis.55 It can be clearly seen from the inset I that the Rs values of L and M–L are both less than 1 Ω, reflecting their good charge/discharge performance. The charge transfer resistance (Rct) caused by the charge transfer process of the Faraday reaction can be obtained by the diameter of a semicircle.56 Inset II shows that the Rct values of L, Cr–L, Zn–L and Al–L are 42.8, 16.8, 28.6 and 12.4 Ω, respectively. Rct of M–L is less than the value of L, indicating that defects in the M–L spatial structure due to the introduction of metal ions and counter anions lead to an increase in specific surface area, thereby providing more active sites for charge transfer, which ultimately resulting in a decrease in Rct. The Warburg impedance (W) represents the interfacial diffusion resistance of electrolyte ions, which can be acquired by the slope of a straight line in the low frequency region.57,58 Except for Zn–L, the slopes of Cr–L and Al–L are greater than L, illustrating that they have less resistance to ion diffusion and better conductivity, which is consistent with the above CV and GCD measurement results.
Conclusions
In summary, we have designed a new type of ligand (L) and its complexes (Al–L, Cr–L, Zn–L), which can be easily synthesized by one-step reaction of MDPA, TPAL and metal nitrates in ethanol at room temperature. M–L has many excellent characteristics relative to L, such as large specific surface area, suitable mesopore diameter and excellent conductivity. The electrochemical properties of the prepared L and M–L materials were studied for the first time. In 6M KOH, Al–L shows a specific capacitance of 608.6 F g−1 at a current density of 0.5 A g−1. More importantly, at the current density of 10 A g−1, the specific capacitance of Al–L still reaches 299.1 F g−1 after 1000 GCD cycles, which is higher than the initial capacitance of Cr–L and Zn–L. The different coordination abilities of metal ions and L will cause the porosity of the π-conjugated stack in M–L to vary widely, and eventually lead to different electrochemical properties. This study demonstrates that Al–L is a promising candidate for supercapacitors and provides new ideas for further research on various poly[M(Schiff)] as electrode materials for supercapacitors or other energy storage devices.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 51678059), the Key Research and Development Program of Shaanxi Province (grant no. 2019GY-179), the Fundamental Research Funds for the Central Universities (grant no. 300102298202), the Open Foundation of the Laboratory of Degraded and Unused Land Consolidation Engineering, the Ministry of Land Resources (grant no. SXDJ2017-1) and the Open Foundation of Key Laboratory of Shaanxi Provincial Land Rehabilitation (grant no. 2018-JC10).