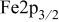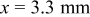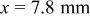Abstract
Low carbon steel substrates were covered with a γ-glycidoxypropyltrimethoxysilane (γ-GPS) modified epoxy layer. Electrochemical impedance spectroscopy and attenuated total reflection infrared spectroscopy were used to investigate the barrier properties of the coating. The resistance of the samples to ion transport processes along the epoxy/steel interface was determined by in situ scanning Kelvin probe (SKP) measurements of the interface potential. X-ray photoelectron spectroscopy studies helped to analyze the resulting ion distribution on the substrate surface. The application of γ-GPS resulted in a reduction of the interfacial water activity and temporarily stabilized the polymer/steel interface. In contrast to the experiments with the unmodified epoxy coating, interfacial ion transport processes were verifiable with the SKP. After some days of sample exposure to humid air, the stabilizing effect of γ-GPS diminished and SKP potential profiles had to be recorded in dry atmosphere to identify the electrolyte front position, zones of cation and anion separation, and areas of local corrosion damage at the interface. This approach seems to be generally promising to analyze the degradation state of polymer/oxide/metal interfaces after long-term exposures in humid air.
Export citation and abstract BibTeX RIS
In Part I of this paper, the properties of a water-borne epoxy coating were analyzed. It was focused on the effect of high water activities and on the characteristics of ion transport processes at epoxy/steel interfaces.1 Electrochemical impedance spectroscopy (EIS) indicated poor barrier properties of the polymer films. It was estimated that the  uptake level was very high and that strong wet adhesion processes occurred in humid air at the polymer/steel interface compared to substrates coated with common non-water-borne epoxy coatings.2 Oxygen reduction induced ion transport processes along the epoxy/steel interface were not verifiable with the scanning Kelvin probe (SKP) because no characteristic sigmoid potential profiles were detected. Instead, the SKP recorded a nonspecific linear profile with a small slope. It was not possible to determine an electrolyte front position unless the humidity of the surrounding atmosphere was reduced.1 Moreover, it was shown that both cations and anions of the defect electrolyte penetrated the epoxy/steel interface after the initialization of interfacial ion transport processes. With respect to previous studies,3–9 it was discussed that the unexpected and uncommon anion transport was supported by the effects of fluid dynamics at the interface rather than by ion diffusion.
uptake level was very high and that strong wet adhesion processes occurred in humid air at the polymer/steel interface compared to substrates coated with common non-water-borne epoxy coatings.2 Oxygen reduction induced ion transport processes along the epoxy/steel interface were not verifiable with the scanning Kelvin probe (SKP) because no characteristic sigmoid potential profiles were detected. Instead, the SKP recorded a nonspecific linear profile with a small slope. It was not possible to determine an electrolyte front position unless the humidity of the surrounding atmosphere was reduced.1 Moreover, it was shown that both cations and anions of the defect electrolyte penetrated the epoxy/steel interface after the initialization of interfacial ion transport processes. With respect to previous studies,3–9 it was discussed that the unexpected and uncommon anion transport was supported by the effects of fluid dynamics at the interface rather than by ion diffusion.
This part of the study complements and continues the experimental approach selected in Part I.1 It will be analyzed which effects a modification of the epoxy/steel interface with γ-glycidoxy- propyltrimethoxysilane (γ-GPS) has on the interface potentials and on the detectability of interfacial ion transport processes. Interfaces modified with adhesion promoting molecules, such as γ-GPS, are expected to be more stable in an environment of a high water activity. Covalent bonds between polymer chains, γ-GPS, and the oxide layer are less sensitive to hydrolysis and provide a major contribution to the entire adhesion forces.10–12 Consequently, attenuated total reflection infrared spectroscopy (ATR-IR) experiments are presented in this work to compare the water uptake of γ-GPS modified and nonmodified epoxy layers and to investigate the distribution of water species near the interface. Furthermore, X-ray photoelectron spectroscopy (XPS) and the SKP are utilized to analyze the degradation of γ-GPS modified epoxy/steel interfaces that occurred during the exposure to humid air for several days. Differently shaped SKP potential profiles will be discussed that were recorded on these samples. This will help to characterize complex degradation processes at the polymer/oxide/metal interfaces based on interface potentials measured with the SKP.
Experimental
For most details concerning sample preparation, sample configuration, and experimental techniques, we refer to the first part of this paper1. For the present study, however, the recipe for the epoxy coating was modified by the addition of γ-GPS. The epoxy amine mass ratio of  of epoxy component per
of epoxy component per  of amine component was maintained. Pure γ-GPS was subsequently added to this mixture until a mass fraction of 2.5% was achieved. The resulting stoichiometric ratio was
of amine component was maintained. Pure γ-GPS was subsequently added to this mixture until a mass fraction of 2.5% was achieved. The resulting stoichiometric ratio was  -GPS equivalents per one amine equivalent. The γ-GPS modified polymer was subsequently bar coated onto low carbon steel. The coating layer was dried in ambient atmosphere for
-GPS equivalents per one amine equivalent. The γ-GPS modified polymer was subsequently bar coated onto low carbon steel. The coating layer was dried in ambient atmosphere for  and subsequently cured at
and subsequently cured at  for
for  . The film thickness
. The film thickness  was the same as for the non-γ-GPS modified epoxy layers.1
was the same as for the non-γ-GPS modified epoxy layers.1
ATR-IR experiments were carried out with a BioRad Fourier transform infrared (FTIR) 3000 spectrometer (Digilab, Germany), equipped with a mid-infrared globar source, a deuterated triglycine sulfated detector, and an internal reflection unit of Specac Ltd. (Great Britain). 148 interferograms were recorded at room temperature with a spectral resolution of  . For the experiments n-Type silicon attenuated total reflection crystals with base trapezoidal angles of 45° C were cleaned in an aqueous solution of 30% NH3/H2O2 at 80° C, rinsed with ultrapure water, and dried. The crystals were coated with the unmodified or the γ-GPS modified epoxy. After reference spectra were recorded on the polymer coated ATR crystals, the samples were exposed to aqueous borate buffer solution. The increase of the OH-stretching vibration
. For the experiments n-Type silicon attenuated total reflection crystals with base trapezoidal angles of 45° C were cleaned in an aqueous solution of 30% NH3/H2O2 at 80° C, rinsed with ultrapure water, and dried. The crystals were coated with the unmodified or the γ-GPS modified epoxy. After reference spectra were recorded on the polymer coated ATR crystals, the samples were exposed to aqueous borate buffer solution. The increase of the OH-stretching vibration  peak intensities was evaluated as a function of time.2, 13
peak intensities was evaluated as a function of time.2, 13
Results and Discussion
Water uptake at γ-GPS modified epoxy/steel interfaces
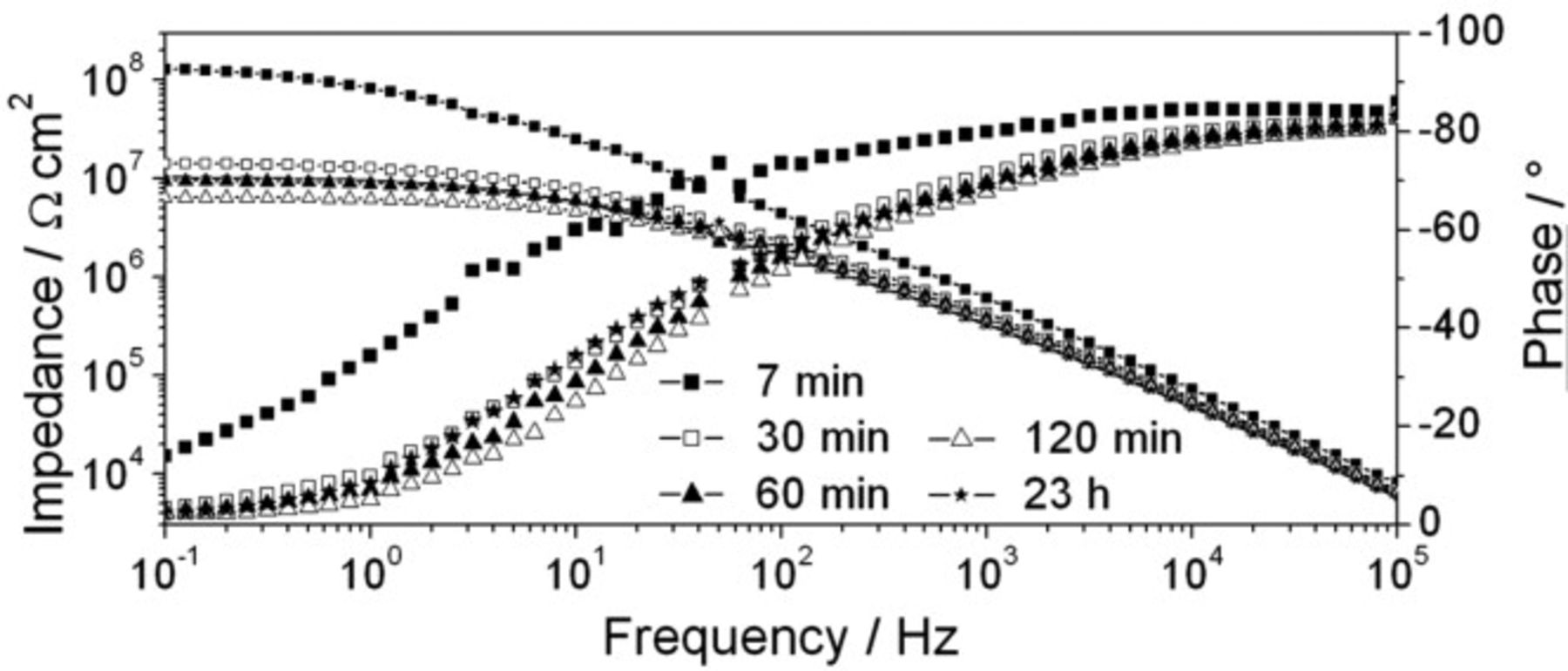
Bar rier properties of γ-GPS modified water-borne epoxy layers were investigated on low carbon steel substrates by EIS. Figure 1 shows Bode plots of a sample exposed to borate buffer solution. A decline in the impedance modulus was observed at  . After
. After  , it was approximately
, it was approximately  . The phase decreased to nearly 0° at lower frequencies due to coating pores that developed in the polymer layer.1, 14–16 In general, similar Bode plots were measured during experiments performed on steel substrates coated with the unmodified epoxy.1 Consequently, Fig. 1 does not indicate distinctly improved barrier properties for water ingress if γ-GPS is present in the polymer layer.
. The phase decreased to nearly 0° at lower frequencies due to coating pores that developed in the polymer layer.1, 14–16 In general, similar Bode plots were measured during experiments performed on steel substrates coated with the unmodified epoxy.1 Consequently, Fig. 1 does not indicate distinctly improved barrier properties for water ingress if γ-GPS is present in the polymer layer.
Figure 1. EIS study of the water uptake of the γ-GPS modified epoxy coating during its exposure to borate buffer solution. Steel was used as the substrate material. A typical development of the Bode plots with time is shown.
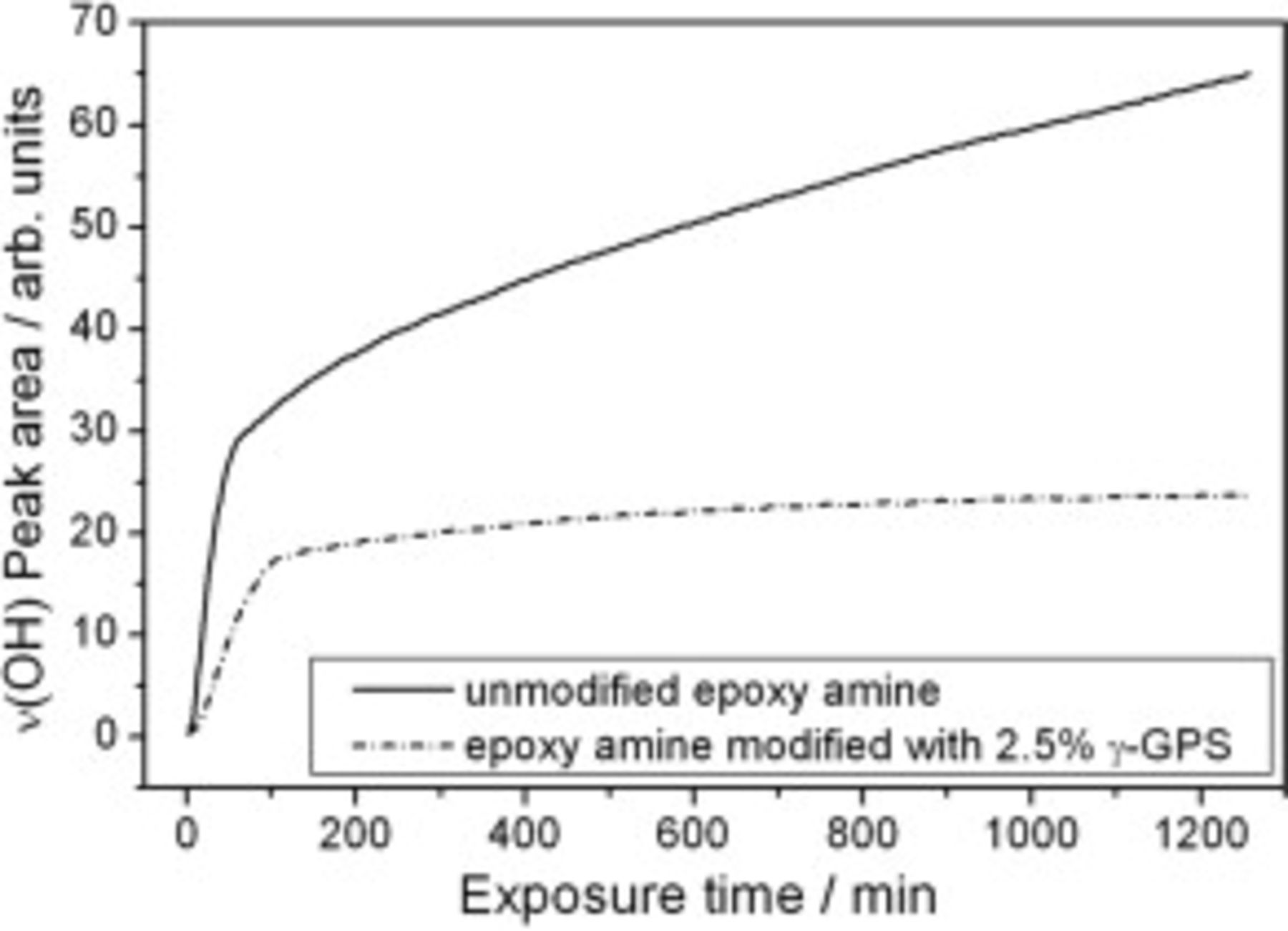
The adhesion promoting additive, however, had a strong impact on the resulting water concentration at the polymer/substrate interface, as shown by ATR-IR experiments. Silicon crystals were coated with unmodified and γ-GPS modified epoxy layers. The samples were exposed to borate buffer solution again, and the increase of the water activity at the polymer/substrate interface was detected as a function of the resulting  peak intensity changes. Figure 2 indicates a steep increase of the
peak intensity changes. Figure 2 indicates a steep increase of the  activity for the first minutes of the measurements.2, 13 During the subsequent stages of the ATR-IR experiment, a linear and continuous increase with no saturation of the water uptake was detected for the ATR crystal coated with the unmodified epoxy. This indicates wet de-adhesion processes and an expansion of free volumes at the polymer/silicon interface.13 For the γ-GPS modified epoxy these effects are less pronounced. Moreover, the graphs of Fig. 2 show that less water is present at the γ-GPS modified epoxy/Si interface at any time. It reaches a saturation level after
activity for the first minutes of the measurements.2, 13 During the subsequent stages of the ATR-IR experiment, a linear and continuous increase with no saturation of the water uptake was detected for the ATR crystal coated with the unmodified epoxy. This indicates wet de-adhesion processes and an expansion of free volumes at the polymer/silicon interface.13 For the γ-GPS modified epoxy these effects are less pronounced. Moreover, the graphs of Fig. 2 show that less water is present at the γ-GPS modified epoxy/Si interface at any time. It reaches a saturation level after  . At this stage, approximately twice as much
. At this stage, approximately twice as much  is detected at the unmodified epoxy/Si interface.
is detected at the unmodified epoxy/Si interface.
Figure 2. Development of the  peak intensity with time, detected by ATR-FTIR (between 3600 and
peak intensity with time, detected by ATR-FTIR (between 3600 and  ). An epoxy layer was applied on silicon ATR-crystals and exposed to borate buffer solution. Black curve: Graph of the γ-GPS modified epoxy. Dashed line: Graph of the unmodified epoxy.
). An epoxy layer was applied on silicon ATR-crystals and exposed to borate buffer solution. Black curve: Graph of the γ-GPS modified epoxy. Dashed line: Graph of the unmodified epoxy.
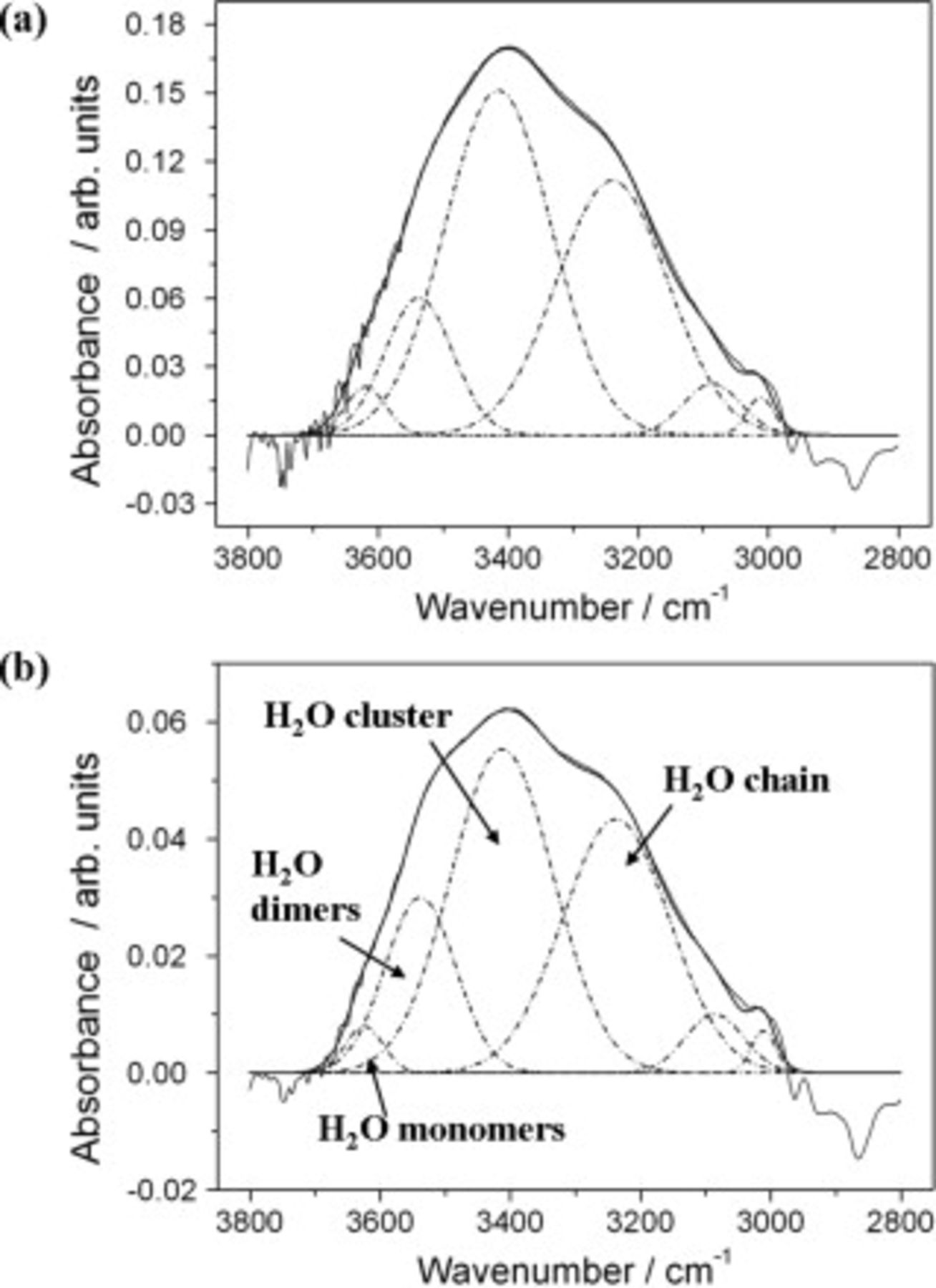
For a more detailed analysis, Fig. 3a and 3b illustrate the increase of the  peak intensities for both the unmodified and γ-GPS modified interfaces after
peak intensities for both the unmodified and γ-GPS modified interfaces after  of exposure of the samples to borate buffer solution (see Fig. 2). The
of exposure of the samples to borate buffer solution (see Fig. 2). The  peak shape of the signal was fitted according to different aggregation states of water.2, 17–20 Such states can be assigned to non-H-bound
peak shape of the signal was fitted according to different aggregation states of water.2, 17–20 Such states can be assigned to non-H-bound  monomers at
monomers at  , weakly H-bound dimers at
, weakly H-bound dimers at  , larger water clusters at
, larger water clusters at  as well as to water molecules firmly bound to specific sites of the polymer network at
as well as to water molecules firmly bound to specific sites of the polymer network at  and lower wavenumbers.2, 17 The peak at
and lower wavenumbers.2, 17 The peak at  is also expected to reflect associated
is also expected to reflect associated  chains and water clusters with more bulklike properties, formed e.g., by capillary condensation in film micropores.20 Figure 3b indicates that a slightly larger amount of water dimers seem to be detected for the γ-GPS modified epoxy as compared to the unmodified polymer. Apart from this, peak shapes and fitted peaks of Fig. 3a and 3b are similar. The distribution of water species and their agglomerates in the polymer matrix near the adhesive/substrate interface were not significantly influenced by the presence of γ-GPS. Although Fig. 3b does not indicate the formation of a macroscopic
chains and water clusters with more bulklike properties, formed e.g., by capillary condensation in film micropores.20 Figure 3b indicates that a slightly larger amount of water dimers seem to be detected for the γ-GPS modified epoxy as compared to the unmodified polymer. Apart from this, peak shapes and fitted peaks of Fig. 3a and 3b are similar. The distribution of water species and their agglomerates in the polymer matrix near the adhesive/substrate interface were not significantly influenced by the presence of γ-GPS. Although Fig. 3b does not indicate the formation of a macroscopic  layer at the unmodified polymer/Si interface,2 the detected peak intensities suggest that the interfacial water concentration is higher compared to that of the γ-GPS modified sample (see Fig. 3a). Different
layer at the unmodified polymer/Si interface,2 the detected peak intensities suggest that the interfacial water concentration is higher compared to that of the γ-GPS modified sample (see Fig. 3a). Different  activities had a strong impact on the interface potential of the unmodified epoxy/steel interface.1 Consequently, it can be expected that a modification of the polymer with γ-GPS will influence the detectability of interfacial ion transport processes with the SKP.
activities had a strong impact on the interface potential of the unmodified epoxy/steel interface.1 Consequently, it can be expected that a modification of the polymer with γ-GPS will influence the detectability of interfacial ion transport processes with the SKP.
Figure 3. FTIR-ATR spectra of the  peak region measured during the water uptake of epoxy polymers.
peak region measured during the water uptake of epoxy polymers.  peak shapes are fitted according to different aggregate states of water.18–21 (a) Peak region shown for the non-γ-GPS modified epoxy/steel interface. It reflects the black graph of Fig. 2 at
peak shapes are fitted according to different aggregate states of water.18–21 (a) Peak region shown for the non-γ-GPS modified epoxy/steel interface. It reflects the black graph of Fig. 2 at  . (b) Peak region shown for the γ-GPS modified epoxy/steel interface. It reflects the dashed graph of Fig. 2 at
. (b) Peak region shown for the γ-GPS modified epoxy/steel interface. It reflects the dashed graph of Fig. 2 at  .
.
Shape of the SKP potential profiles
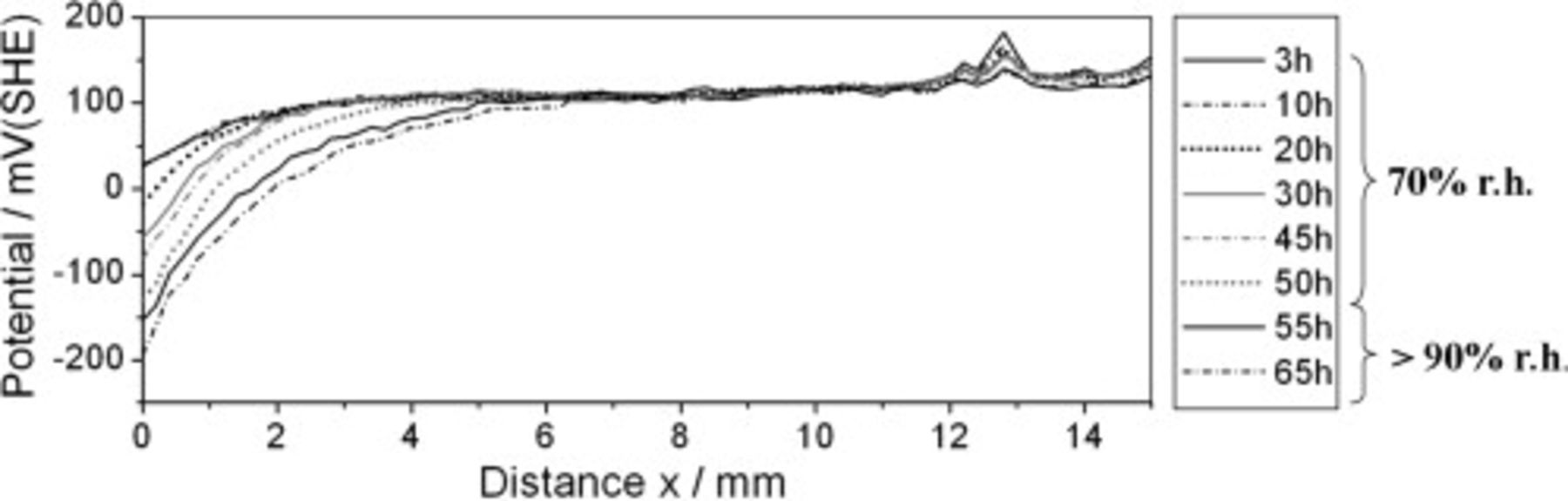
Ion transport processes that proceeded along steel surfaces covered with the unmodified water-borne epoxy could not be investigated with the SKP while the samples were exposed to humid air.1 The SKP approach was continued to determine whether the same applies to γ-GPS modified polymer coated steel. A defect in the epoxy layer was covered with  KBr solution, and the sample was exposed to humid air. Figure 4 presents the recorded SKP potential profiles. The experiment started at a relative atmospheric humidity of approximately 70%. Areas affected by interfacial ion transport and intact interface sections can be easily distinguished in Fig. 4. The ingress of electrolyte resulted in a reduction of the interface potential near the defect. The graphs exhibit a transition width of several millimeters between already degraded and intact interface sections rather than a sharp potential drop. A similar shape of SKP potential profiles was reported for cathodic delamination processes along polymer coated iron samples that were protected against corrosion by an additional
KBr solution, and the sample was exposed to humid air. Figure 4 presents the recorded SKP potential profiles. The experiment started at a relative atmospheric humidity of approximately 70%. Areas affected by interfacial ion transport and intact interface sections can be easily distinguished in Fig. 4. The ingress of electrolyte resulted in a reduction of the interface potential near the defect. The graphs exhibit a transition width of several millimeters between already degraded and intact interface sections rather than a sharp potential drop. A similar shape of SKP potential profiles was reported for cathodic delamination processes along polymer coated iron samples that were protected against corrosion by an additional  plasma polymer layer or an adhesion promoting layer of 3-(trimethoxysilyl)-propylamine (γ-APS).21
plasma polymer layer or an adhesion promoting layer of 3-(trimethoxysilyl)-propylamine (γ-APS).21
Figure 4. SKP study of ion transport processes along the γ-GPS modified epoxy/steel interface. Potential profiles were first recorded in ambient air of around 70% relative humidity. After  , the humidity was increased to
, the humidity was increased to  r.h. A defect in the epoxy layer (located at
r.h. A defect in the epoxy layer (located at  ) was covered with
) was covered with  KBr solution.
KBr solution.
For the experiment shown in Fig. 4, the relative humidity (r.h.) of the surrounding air was increased to  after
after  . A uniform potential decrease was detected on steel coated with the unmodified epoxy under these conditions.1 This was assigned to the interaction of hydrolysis effects, wet de-adhesion processes, and an adjustment of dipole moments at the interface.1, 22–25 No such effects occur after the addition of 2.5% γ-GPS to the epoxy mixture because the geometry of the potential profiles presented in Fig. 4 does not change at
. A uniform potential decrease was detected on steel coated with the unmodified epoxy under these conditions.1 This was assigned to the interaction of hydrolysis effects, wet de-adhesion processes, and an adjustment of dipole moments at the interface.1, 22–25 No such effects occur after the addition of 2.5% γ-GPS to the epoxy mixture because the geometry of the potential profiles presented in Fig. 4 does not change at  r.h. Moreover, larger adhesion forces were detected at the γ-GPS modified polymer/substrate sample system. The unmodified epoxy was easily removed from the substrate in humid air. In contrast, peeling of the γ-GPS modified coating from substrate areas unaffected by interfacial ion transport processes often resulted in a rupture within the polymer film and not in de-adhesion at or near the polymer/substrate interface. Consequently, the detectability of interfacial ion transport processes by SKP potential profiles presented in Fig. 4 indicates an increased stability of the γ-GPS modified sample. This is connected to reduced wet de-adhesion processes at the epoxy/steel interface. Figures 2 and 3 show that a decreased tendency of the coating to undergo wet de-adhesion is indeed determined by a reduction of the water concentration in the polymer matrix near the substrate surface. However, it is not dependent on a change of the macroscopic distribution of water species and agglomerates at and near the γ-GPS modified polymer/substrate interface. This shows that it was not the formation of a bulk water layer that inhibited an identification of interfacial ion transport processes along the non-γ-GPS modified interface by the SKP.1
r.h. Moreover, larger adhesion forces were detected at the γ-GPS modified polymer/substrate sample system. The unmodified epoxy was easily removed from the substrate in humid air. In contrast, peeling of the γ-GPS modified coating from substrate areas unaffected by interfacial ion transport processes often resulted in a rupture within the polymer film and not in de-adhesion at or near the polymer/substrate interface. Consequently, the detectability of interfacial ion transport processes by SKP potential profiles presented in Fig. 4 indicates an increased stability of the γ-GPS modified sample. This is connected to reduced wet de-adhesion processes at the epoxy/steel interface. Figures 2 and 3 show that a decreased tendency of the coating to undergo wet de-adhesion is indeed determined by a reduction of the water concentration in the polymer matrix near the substrate surface. However, it is not dependent on a change of the macroscopic distribution of water species and agglomerates at and near the γ-GPS modified polymer/substrate interface. This shows that it was not the formation of a bulk water layer that inhibited an identification of interfacial ion transport processes along the non-γ-GPS modified interface by the SKP.1
The degradation of γ-GPS modified epoxy/steel interfaces
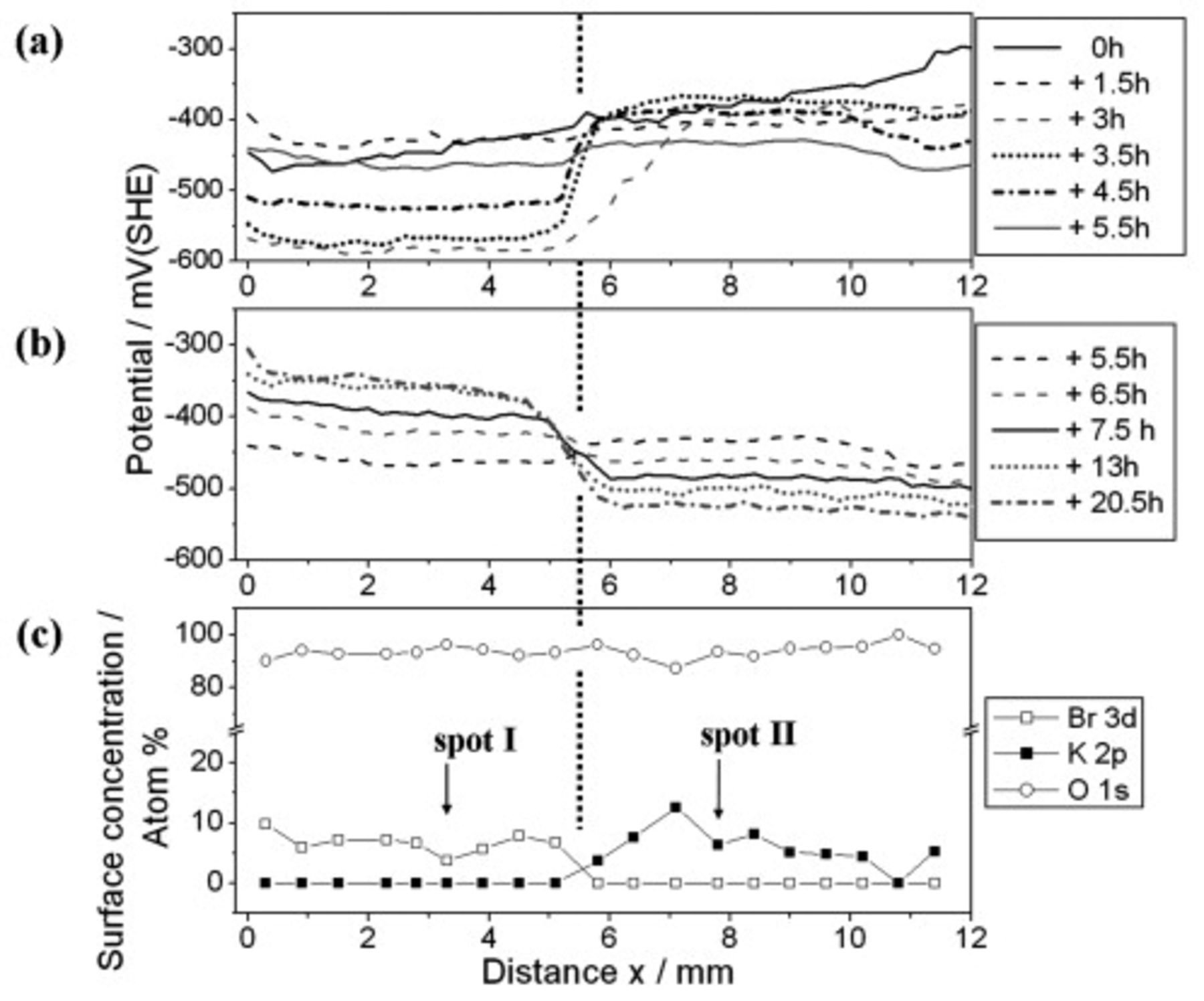
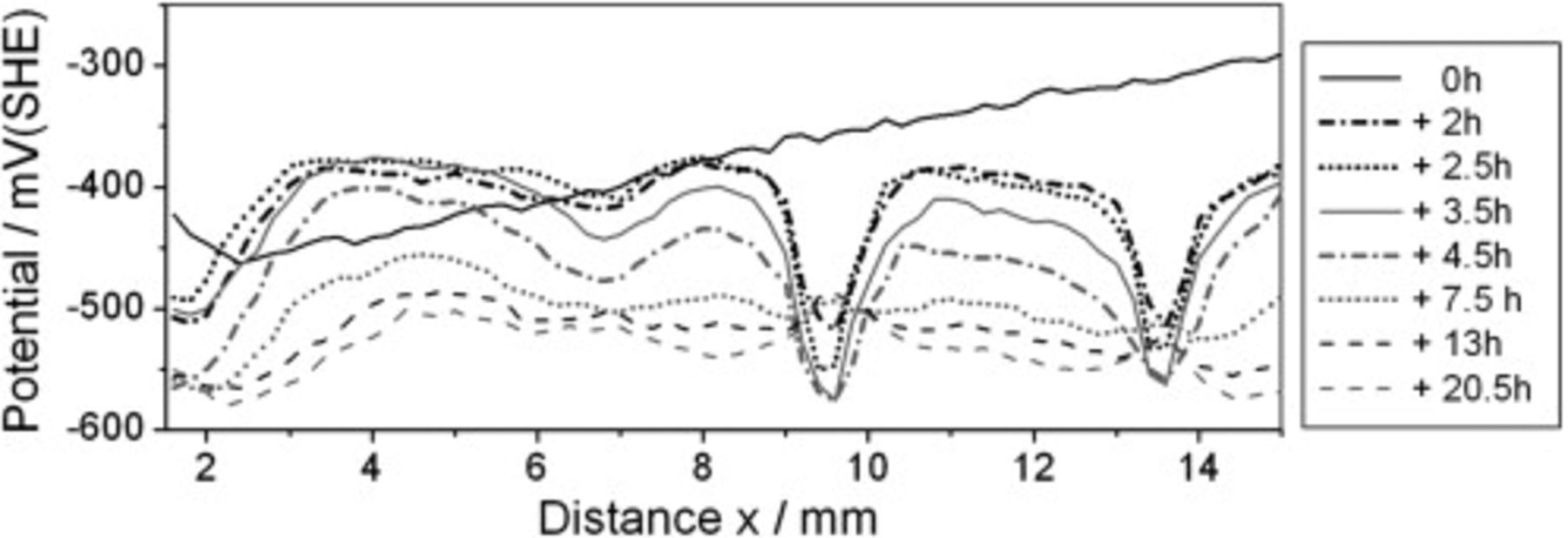
It is of interest to determine whether the interface stabilizing effect of γ-GPS is enduring for the analyzed water-borne epoxy film. Therefore, the ion transport experiment of Fig. 4 was repeated. Again, a coating defect was covered with a  KBr solution. But this time the sample was exposed to air with a relative humidity of
KBr solution. But this time the sample was exposed to air with a relative humidity of  for
for  prior to the SKP measurement. Figure 5 presents the recorded potential profiles. With rising distance to the defect the initial "
prior to the SKP measurement. Figure 5 presents the recorded potential profiles. With rising distance to the defect the initial " "-graph exhibits a continuous and nearly linear increase of the potential. No characteristic steep potential step indicates the position of the electrolyte front. The geometry of the potential profiles changes to a sigmoid shape after the humidity of the surrounding atmosphere was reduced and reached approximately 50% r.h. at around
"-graph exhibits a continuous and nearly linear increase of the potential. No characteristic steep potential step indicates the position of the electrolyte front. The geometry of the potential profiles changes to a sigmoid shape after the humidity of the surrounding atmosphere was reduced and reached approximately 50% r.h. at around  . At this time, the electrolyte front position at the γ-GPS modified epoxy/steel interface can be identified.1 It is located at
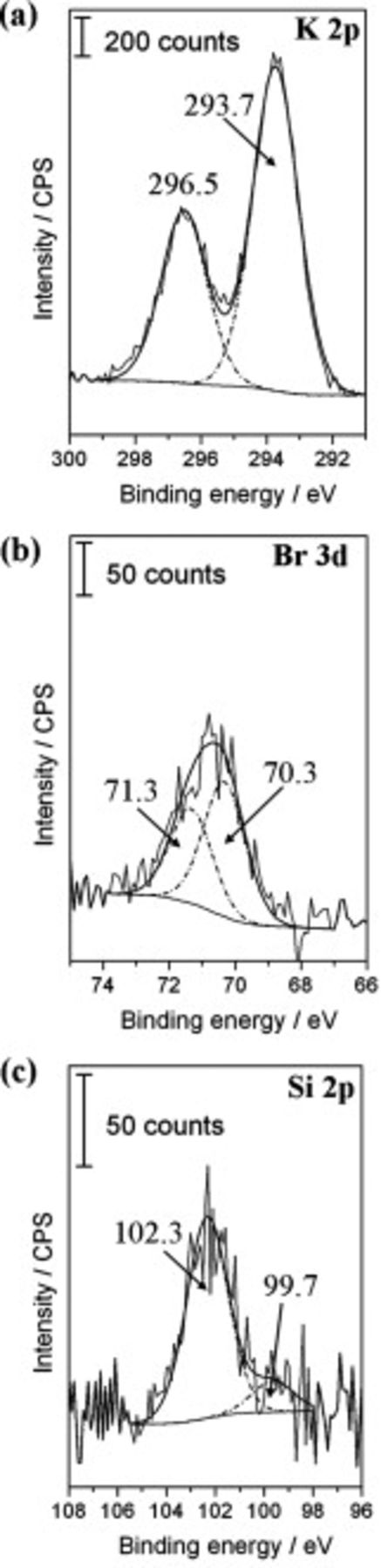
. At this time, the electrolyte front position at the γ-GPS modified epoxy/steel interface can be identified.1 It is located at  . Figure 6 shows XPS detail spectra measured on the steel surface after the polymer layer was removed. They were recorded at
. Figure 6 shows XPS detail spectra measured on the steel surface after the polymer layer was removed. They were recorded at  in the area of electrolyte ingress and low interface potentials. Figures 6a and 6b confirm that potassium ions as well as bromide species were transported along the polymer/steel interface. The K2p signal intensity is higher compared to the Br3d signal. It indicates a surplus of
in the area of electrolyte ingress and low interface potentials. Figures 6a and 6b confirm that potassium ions as well as bromide species were transported along the polymer/steel interface. The K2p signal intensity is higher compared to the Br3d signal. It indicates a surplus of  at the interface. Similar ion distributions were detected after ion transport processes proceeded along the non-γ-GPS modified epoxy/steel interface.1 The same applies to the observed change of the geometry of SKP potential profiles from linear graphs to sigmoid shaped profiles during drying of the non-γ-GPS modified samples.
at the interface. Similar ion distributions were detected after ion transport processes proceeded along the non-γ-GPS modified epoxy/steel interface.1 The same applies to the observed change of the geometry of SKP potential profiles from linear graphs to sigmoid shaped profiles during drying of the non-γ-GPS modified samples.
Figure 5. SKP study of ion transport processes along the γ-GPS modified epoxy/steel interface. A defect in the epoxy layer (located at  ) was covered with
) was covered with  KBr solution. The sample was exposed to humid air of
KBr solution. The sample was exposed to humid air of  r.h. for
r.h. for  prior to the SKP measurement. After the initial SKP line scan was recorded, the atmospheric humidity has been continuously reduced, fell below approximately 50% r.h. after
prior to the SKP measurement. After the initial SKP line scan was recorded, the atmospheric humidity has been continuously reduced, fell below approximately 50% r.h. after  and reached around 13% r.h. after
and reached around 13% r.h. after  .
.
Figure 6. XPS study of the local surface chemistry for the area of interfacial ion transport. The detailed spectra were recorded at  on the sample presented in Fig. 5 after termination of the SKP measurement and removal of the coating. (a) K2p spectrum, (b) Br3d spectrum, and (c) Si2p peaks.
on the sample presented in Fig. 5 after termination of the SKP measurement and removal of the coating. (a) K2p spectrum, (b) Br3d spectrum, and (c) Si2p peaks.
These correlations mean that the interface stabilizing effect of γ-GPS diminishes if steel substrates coated with the γ-GPS modified water-borne epoxy are exposed to air of high humidity for longer times. This is not due to a complete hydrolysis or de-adhesion of the γ-GPS molecules, because Fig. 6c also shows that  (peak at
(peak at  ) and
) and  species (peak at
species (peak at  ) were detected in the area of interfacial ion transport. Moreover, the absence of γ-GPS molecules or fragments is not a requirement to detect nonspecific linear potential profiles at epoxy coated steel samples in humid air.
) were detected in the area of interfacial ion transport. Moreover, the absence of γ-GPS molecules or fragments is not a requirement to detect nonspecific linear potential profiles at epoxy coated steel samples in humid air.
The results show that it can be necessary to record SKP potential profiles in humid as well as in dry atmosphere to interpret the degradation state of polymer/oxide/metal interfaces. Otherwise, the progress of interfacial ion transport processes may be misjudged if solely analyzed in humid air. In this context, the slope of the initial " "-graph of Fig. 5 could be misleadingly interpreted as
"-graph of Fig. 5 could be misleadingly interpreted as  drop (
drop ( : current,
: current,  : resistance), indicating the area of interfacial ion transport and cathodic delamination processes.3–6 This will result in an incorrect localization of the electrolyte front position at
: resistance), indicating the area of interfacial ion transport and cathodic delamination processes.3–6 This will result in an incorrect localization of the electrolyte front position at  . Conversely, it is worth scanning the polymer/substrate interface in humid air to check whether nonspecific potential profiles are recorded. Such profiles will not allow an investigation of interfacial ion transport kinetics, but provide information about swelling and wet de-adhesion of the organic coating at the polymer/substrate interface.1 The SKP is a powerful and one of the rare analytical tools suited to perform such nondestructive studies in situ.
. Conversely, it is worth scanning the polymer/substrate interface in humid air to check whether nonspecific potential profiles are recorded. Such profiles will not allow an investigation of interfacial ion transport kinetics, but provide information about swelling and wet de-adhesion of the organic coating at the polymer/substrate interface.1 The SKP is a powerful and one of the rare analytical tools suited to perform such nondestructive studies in situ.
If polymer/oxide/metal interfaces are exposed to humid air and affected by interfacial ion transport processes for longer times, the inflection point in sigmoid SKP potential profiles, however, does not necessarily indicate the electrolyte front position. This is in particular relevant for coatings such as the water-borne epoxy investigated in the present study1 and substrates such as Zn,26–28 because they enable a transport of cations as well as anions of the defect electrolyte along the interface. Additional analysis may be necessary to clarify the ion distribution at the substrate surface of these samples. This has to be kept in mind if interfacial ion transport kinetics is intended to be used as a parameter to predict the corrosion resistance of polymer coated metals during long-term exposures.
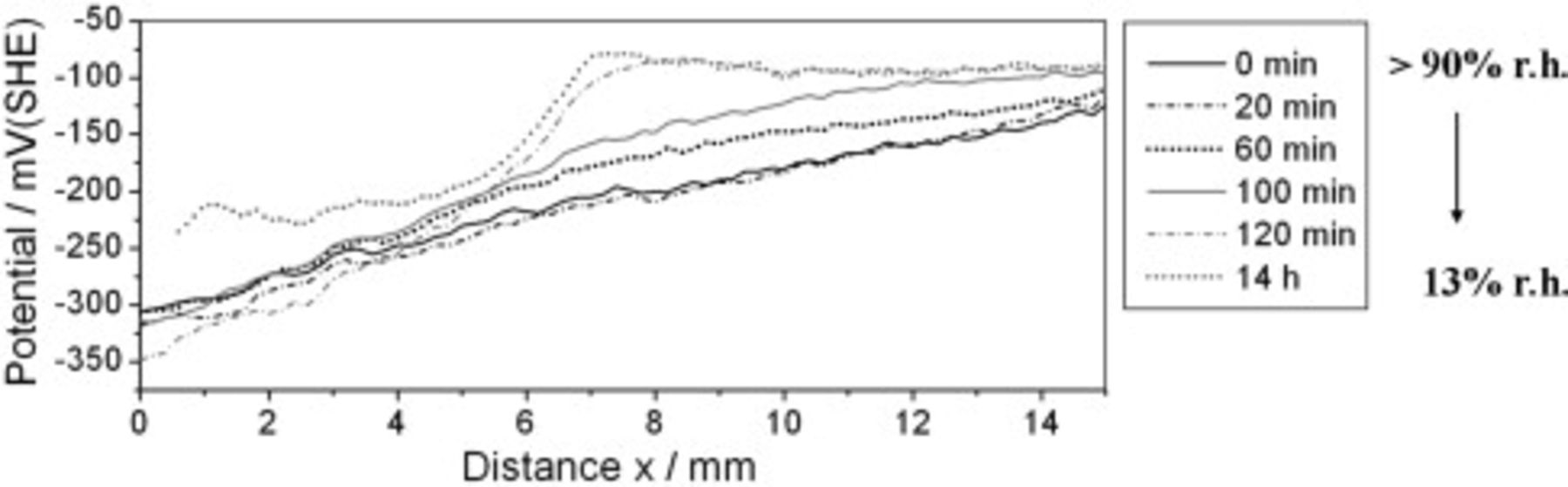
As an example, Fig. 7 presents an SKP study performed on a sample coated with the γ-GPS modified epoxy and exposed to air of  r.h. for
r.h. for  prior. The initial SKP potential profile of Fig. 7a and the profile recorded at
prior. The initial SKP potential profile of Fig. 7a and the profile recorded at  again exhibit a continuous linear slope with an increasing potential at increasing distance to the defect area. After the atmospheric humidity was reduced a sigmoid shape of the profile was detected with low potentials at
again exhibit a continuous linear slope with an increasing potential at increasing distance to the defect area. After the atmospheric humidity was reduced a sigmoid shape of the profile was detected with low potentials at  and high potentials at
and high potentials at  (see the "
(see the " -plot" in Fig. 7a). Between
-plot" in Fig. 7a). Between  and
and  , these potential levels equalized. When the atmospheric humidity was further reduced, an inversion of the potential levels was recorded (see Fig. 7b). After
, these potential levels equalized. When the atmospheric humidity was further reduced, an inversion of the potential levels was recorded (see Fig. 7b). After  no characteristic change of the geometry of the potential profiles was observed, but the potential levels still shifted up to
no characteristic change of the geometry of the potential profiles was observed, but the potential levels still shifted up to  in the area at
in the area at  and to
and to  and lower for
and lower for  at
at  .
.
Figure 7. SKP and XPS study of ion transport processes along the γ-GPS modified epoxy/steel interface. A defect in the epoxy layer (located at  ) was covered with
) was covered with  KBr solution. The sample was exposed to humid air of
KBr solution. The sample was exposed to humid air of  r.h. for
r.h. for  prior to the SKP measurement. After the initial SKP line scan was recorded, the atmospheric humidity has been continuously reduced, decreased below approximately 50% r.h. after
prior to the SKP measurement. After the initial SKP line scan was recorded, the atmospheric humidity has been continuously reduced, decreased below approximately 50% r.h. after  and reached around 13% r.h. after
and reached around 13% r.h. after  . (a) Potential profiles of the first
. (a) Potential profiles of the first  of the SKP experiment. (b) SKP potential profiles measured between
of the SKP experiment. (b) SKP potential profiles measured between  and
and  . (c) XPS line scan of the Br3d, K2p, and O1s signals after termination of the SKP measurement and removal of the coating.
. (c) XPS line scan of the Br3d, K2p, and O1s signals after termination of the SKP measurement and removal of the coating.
Figure 7c presents the distribution of  and
and  on the steel surface after the removal of the epoxy layer. It shows that the inflection point observed at around
on the steel surface after the removal of the epoxy layer. It shows that the inflection point observed at around  in the potential profiles of Fig. 7a and 7b does not indicate the transition between interface sections affected and unaffected by interfacial ion transport processes. Instead, it indicates the borderline between the area of anion and cation presence because bromide ions were only verifiable in the area at
in the potential profiles of Fig. 7a and 7b does not indicate the transition between interface sections affected and unaffected by interfacial ion transport processes. Instead, it indicates the borderline between the area of anion and cation presence because bromide ions were only verifiable in the area at  and potassium cations were only detected for
and potassium cations were only detected for  . A separation of
. A separation of  and
and  , however, must have already occurred during the
, however, must have already occurred during the  exposure of the sample to an atmosphere of
exposure of the sample to an atmosphere of  r.h. The ions were immobilized once the relative atmospheric humidity decreased below approximately 50% around
r.h. The ions were immobilized once the relative atmospheric humidity decreased below approximately 50% around  after the SKP measurement was started. Moreover, the relative humidity of the air was already low enough between
after the SKP measurement was started. Moreover, the relative humidity of the air was already low enough between  and
and  that interfacial ion transport processes are unlikely to bridge distances in the range of centimeters or several millimeters.13, 29 The distribution of
that interfacial ion transport processes are unlikely to bridge distances in the range of centimeters or several millimeters.13, 29 The distribution of  and
and  on the steel surface indicates an electrochemically driven separation of cations and anions. In this context, the SKP detected the transition between local electrodes of a galvanic element after the water activity at the epoxy/steel interface was initially reduced. Further drying of the interface resulted in an inhibition of the galvanic cell and an adjustment of the different local interface chemistry in the potassium and bromide area, for example, due to a precipitation of dissolved ion species and other corrosion products. This is expected to have caused the interface potential shifts observed in Fig. 7a and 7b after
on the steel surface indicates an electrochemically driven separation of cations and anions. In this context, the SKP detected the transition between local electrodes of a galvanic element after the water activity at the epoxy/steel interface was initially reduced. Further drying of the interface resulted in an inhibition of the galvanic cell and an adjustment of the different local interface chemistry in the potassium and bromide area, for example, due to a precipitation of dissolved ion species and other corrosion products. This is expected to have caused the interface potential shifts observed in Fig. 7a and 7b after  .
.
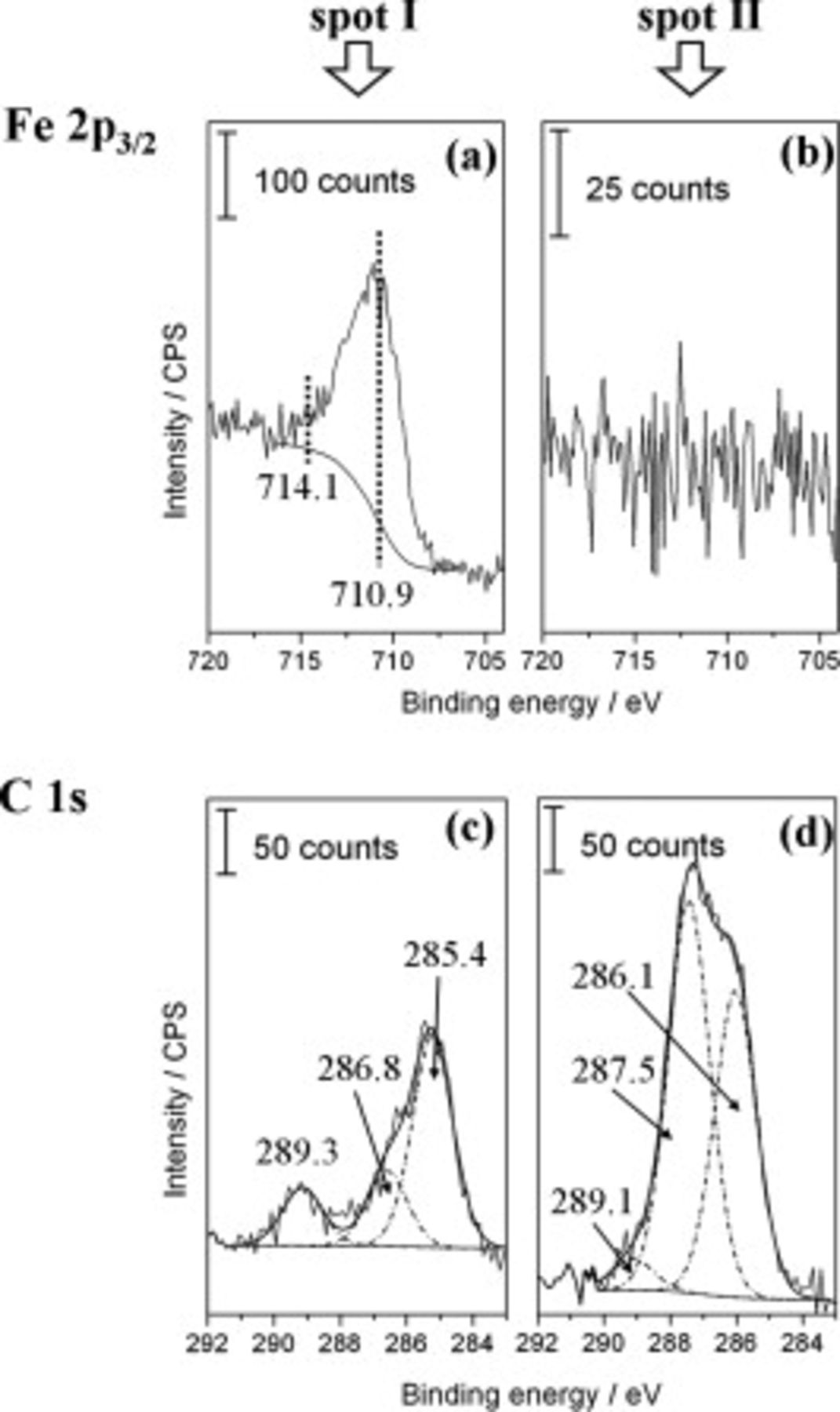
XPS measurements confirm that the deterioration state of the interface differed between the two zones. Iron  XPS signals were always recordable in the area where
XPS signals were always recordable in the area where  was detected (spot I, see Fig. 8a), but were of very low intensity or even absent in the area of
was detected (spot I, see Fig. 8a), but were of very low intensity or even absent in the area of  presence (spot II, see Fig. 8b). This corresponds to a lower C1s intensity detected at spot I (see Fig. 8c) compared to the C1s signal recorded at spot II (see Fig. 8d). The data show that peeling of the epoxy film from the steel surface resulted in a rather cohesive rupture of the polymer in the potassium zone and a rupture closer to the polymer/substrate interface in the bromide area. More organic residues consequently cover the steel surface at
presence (spot II, see Fig. 8b). This corresponds to a lower C1s intensity detected at spot I (see Fig. 8c) compared to the C1s signal recorded at spot II (see Fig. 8d). The data show that peeling of the epoxy film from the steel surface resulted in a rather cohesive rupture of the polymer in the potassium zone and a rupture closer to the polymer/substrate interface in the bromide area. More organic residues consequently cover the steel surface at  . This indicates that higher adhesion forces were maintained at the epoxy/steel interface in this area during the exposure of the sample to humid air.
. This indicates that higher adhesion forces were maintained at the epoxy/steel interface in this area during the exposure of the sample to humid air.
It should be noted that the SKP potential profiles recorded in dry air are not only suited to determine electrolyte front positions and zones of cation and anion separation at buried interfaces. Figure 9 presents an interface potential study performed on the sample that was already analyzed in Fig. 7. The SKP line scan was measured on a different sample area that was close but parallel to the edge of the epoxy coated steel surface. The initial " "' potential profile of Fig. 9 was recorded after
"' potential profile of Fig. 9 was recorded after  of sample exposure to humid air. Its geometry is nonspecific and resembles the "
of sample exposure to humid air. Its geometry is nonspecific and resembles the " "-graph of Fig. 7a. Once the relative humidity of the air decreased, local minima of the interface potential were observed at
"-graph of Fig. 7a. Once the relative humidity of the air decreased, local minima of the interface potential were observed at  ,
,  , and
, and  . They are assigned to corrosion damage22 that started at the nearby edge of the polymer coated steel surface. Such local damage will not be detectable if the γ-GPS modified epoxy coated sample area is only scanned in humid air. This again emphasizes the necessity to investigate interface potentials at different relative humidity of the atmosphere in certain cases.
. They are assigned to corrosion damage22 that started at the nearby edge of the polymer coated steel surface. Such local damage will not be detectable if the γ-GPS modified epoxy coated sample area is only scanned in humid air. This again emphasizes the necessity to investigate interface potentials at different relative humidity of the atmosphere in certain cases.
Figure 9. Interface potential study on steel coated with γ-GPS modified epoxy. It was performed on the same sample that was investigated in Fig. 7 (meaning that the sample was exposed to humid air of  r.h. for
r.h. for  before). The SKP the line scan was measured on a different sample area that was close but parallel to the edge of the epoxy coated steel surface. After the initial SKP line scan was recorded, the humidity of the air was continuously reduced, decreased below approximately 50% r.h. after
before). The SKP the line scan was measured on a different sample area that was close but parallel to the edge of the epoxy coated steel surface. After the initial SKP line scan was recorded, the humidity of the air was continuously reduced, decreased below approximately 50% r.h. after  and reached around 13% r.h. after
and reached around 13% r.h. after  .
.
Conclusions
The corrosion stability of low carbon steel sheets coated with a γ-GPS modified water-borne epoxy film was investigated and compared to the properties of non-γ-GPS modified samples. EIS Bode plots did not indicate distinctly improved barrier properties of the coating if γ-GPS was present in the polymer matrix, but ATR-IR measurements confirmed a decreased water uptake near the substrate surface in this case. Although the macroscopic distribution of water species and their agglomerates at and near the epoxy/substrate interface was very similar, wet de-adhesion processes were reduced for the γ-GPS modified samples. Due to the increased interface stability, SKP potential profiles allowed an identification of ion transport processes that proceeded along the γ-GPS modified epoxy/steel interface. The stabilizing effect of γ-GPS diminished after the epoxy film coated steel samples were exposed to humid air for several days. This was not due to a complete hydrolysis or de-adhesion of the γ-GPS molecules from the steel surface. Both cations and anions of the defect electrolyte penetrated the interface, but no ion ingress was detectable by the SKP unless the humidity of the atmosphere was reduced for these measurements. This showed that it is necessary to record SKP potential profiles in humid as well as in dry air to distinguish wet de-adhesion processes, ion transport kinetics, zones of cation and anion separation, and zones of local corrosion damage at degraded γ-GPS modified epoxy/steel interfaces. Such an approach will also help to analyze interface potentials measured on other polymer coated samples that were affected by ion (in particular both cation and anion) transport along the interface and/or a high water uptake in particular during long-term exposures in humid air.
Acknowledgments
The financial support of Cognis GmbH, Düsseldorf, is gratefully acknowledged.
The Max-Planck Institut für Eisenforschung GmbH assisted in meeting the publication costs of this article.