Abstract
Rate capability of Li-excess Mn-based layered cathode materials is improved by treating with  . After treatment, discharge capacity of as high as 230 mAh/g can be obtained at a rate of 300 mA/g
. After treatment, discharge capacity of as high as 230 mAh/g can be obtained at a rate of 300 mA/g  . The improvement is attributed to the modification of the surface of the layered material into a spinel-like structure with the treatment, as suggested by Raman spectroscopy and electrochemical charge–discharge. X-ray diffraction results show no change in bulk lattice parameters, indicating that the structural modification is only on the surface of the active material. Chemical analyses show that both lithium and oxygen are extracted from the active material, supporting the formation of a surface spinel layer with the treatment.
. The improvement is attributed to the modification of the surface of the layered material into a spinel-like structure with the treatment, as suggested by Raman spectroscopy and electrochemical charge–discharge. X-ray diffraction results show no change in bulk lattice parameters, indicating that the structural modification is only on the surface of the active material. Chemical analyses show that both lithium and oxygen are extracted from the active material, supporting the formation of a surface spinel layer with the treatment.
Export citation and abstract BibTeX RIS
Li-excess Mn-based materials with a formula  , often written as
, often written as  where M is at least one transition metal, have demonstrated a discharge capacity of up to 270 mAh/g,1–9 higher than practical discharge capacities of common cathode materials such as
where M is at least one transition metal, have demonstrated a discharge capacity of up to 270 mAh/g,1–9 higher than practical discharge capacities of common cathode materials such as  . These materials thus have the potential to increase the overall capacity and energy density of Li-ion batteries. However, the high discharge capacity of the materials is only available at a low discharge rate (typically C/20). When the discharge current is increased, available discharge capacity of the materials is drastically reduced, with a typical discharge capacity at 1C rate below 200 mAh/g.3, 7, 10 The poor rate capability of the materials has to be overcome for practical applications.
. These materials thus have the potential to increase the overall capacity and energy density of Li-ion batteries. However, the high discharge capacity of the materials is only available at a low discharge rate (typically C/20). When the discharge current is increased, available discharge capacity of the materials is drastically reduced, with a typical discharge capacity at 1C rate below 200 mAh/g.3, 7, 10 The poor rate capability of the materials has to be overcome for practical applications.
Rate capability of a material is in general affected by bulk Li diffusion and Li transport through the surface of the particles. Reducing particle size can improve rate capability by shortening the diffusion distance within the particles. However, reducing particle size also leads to a decrease in packing density of the material, resulting in a lower volumetric energy density, which is not a desirable effect. To maintain overall particle size and energy density, improvements in rate capability of the active material by facilitating Li transport through the surface with methods such as surface treatment or coating would be preferred.
Previous papers have shown an improved rate capability of Li-excess Mn-based layered materials with surface treatment and coating such as mild acid fluorination treatment,11  coating,12
coating,12  coating,10
coating,10  coating,13 and
coating,13 and  coating.14 In most cases, the improvement in rate capability is attributed to a reduction in surface impedance or a stable surface coating that provides higher Li conductivity. Improvements are, however, not significant, with an increase in discharge capacity up to only about 200 mAh/g at 1C.13 The existence of a boundary between the active material and the surface coating may limit the effectiveness of the coating. In addition, surface coating adds an inactive layer on the surface of the active material, often resulting in a decrease in the overall capacity at low rate.15 As a result, a new approach is needed to further improve the rate capability of Li-excess Mn-based layered materials. Here, we have explored the possibility of modifying the surface of the layered material into a spinel structure, i.e., making an integrated material with a core layered structure and a surface spinel structure without a boundary layer. The reason we chose this approach is that spinel structure, such as
coating.14 In most cases, the improvement in rate capability is attributed to a reduction in surface impedance or a stable surface coating that provides higher Li conductivity. Improvements are, however, not significant, with an increase in discharge capacity up to only about 200 mAh/g at 1C.13 The existence of a boundary between the active material and the surface coating may limit the effectiveness of the coating. In addition, surface coating adds an inactive layer on the surface of the active material, often resulting in a decrease in the overall capacity at low rate.15 As a result, a new approach is needed to further improve the rate capability of Li-excess Mn-based layered materials. Here, we have explored the possibility of modifying the surface of the layered material into a spinel structure, i.e., making an integrated material with a core layered structure and a surface spinel structure without a boundary layer. The reason we chose this approach is that spinel structure, such as  ,
,  , and
, and  , has a three-dimensional (3D) diffusion path and would therefore theoretically improve the Li diffusivity through the surface of the particle. Spinel structure is also structurally compatible with layered structure, with the same oxygen arrangement but only differing in the arrangement of Li and transition-metal atoms. So the two structures can be integrated without having a distinct boundary. The difficulty lies in finding the right modification method to make such an integrated structure. We noticed that common spinel materials have a Li to transition metal ratio of less than 1. In addition, oxygen to transition metal ratio (O/M) of common spinel (e.g.,
, has a three-dimensional (3D) diffusion path and would therefore theoretically improve the Li diffusivity through the surface of the particle. Spinel structure is also structurally compatible with layered structure, with the same oxygen arrangement but only differing in the arrangement of Li and transition-metal atoms. So the two structures can be integrated without having a distinct boundary. The difficulty lies in finding the right modification method to make such an integrated structure. We noticed that common spinel materials have a Li to transition metal ratio of less than 1. In addition, oxygen to transition metal ratio (O/M) of common spinel (e.g.,  ) is also smaller than that of a typical Li-rich material (e.g.,
) is also smaller than that of a typical Li-rich material (e.g.,  ) (see Table I). These suggest that the formation of a surface spinel layer would most likely involve the removal of Li and O from the surface of Li-excess Mn-based layered materials. Acid treatment is one method commonly used to remove Li and O from active materials. But Kang and Thackeray16 and Thackeray et al.8 have shown that acid treatment of Li-excess Mn-based layered materials result in poorer rate capability due to damage of the surface of the particles from the preconditioning process. Instead of acids, we have set an eye on the chemical ammonium sulfate
) (see Table I). These suggest that the formation of a surface spinel layer would most likely involve the removal of Li and O from the surface of Li-excess Mn-based layered materials. Acid treatment is one method commonly used to remove Li and O from active materials. But Kang and Thackeray16 and Thackeray et al.8 have shown that acid treatment of Li-excess Mn-based layered materials result in poorer rate capability due to damage of the surface of the particles from the preconditioning process. Instead of acids, we have set an eye on the chemical ammonium sulfate  as the reagent for surface treatment because
as the reagent for surface treatment because  when decomposed would leave behind sulfate anion that could be used to extract Li from the active material. In addition,
when decomposed would leave behind sulfate anion that could be used to extract Li from the active material. In addition,  is soluble in water, so if it is formed as a by-product of the treatment, it can be removed easily from the active material.
is soluble in water, so if it is formed as a by-product of the treatment, it can be removed easily from the active material.
Table I. O/M of selected active materials.
Layered 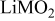
| Spinel (e.g.,  ) ) | Spinel (e.g.,  ) ) |
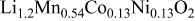
|
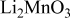
| |
|---|---|---|---|---|---|
| Oxygen/transition metal | 2 | 2 | 2.4 | 2.5 | 3 |
In this paper, cathode materials with formulas  and
and  [corresponding to
[corresponding to  and 0.4 in
and 0.4 in  ] were treated with
] were treated with  and other chemical reagents. The bulk and surface structures of the materials after treatment were studied by X-ray diffraction and Raman spectroscopy. The effects of surface treatment on the electrochemical performances of the cathode materials such as capacities and rate capabilities were also investigated. Based on the results, a reaction mechanism for the
and other chemical reagents. The bulk and surface structures of the materials after treatment were studied by X-ray diffraction and Raman spectroscopy. The effects of surface treatment on the electrochemical performances of the cathode materials such as capacities and rate capabilities were also investigated. Based on the results, a reaction mechanism for the  treatment is proposed in this paper.
treatment is proposed in this paper.
Experimental
Lithium hydroxide (LiOH) and Mn, Co, and Ni mixed hydroxide prepared by coprecipitation from Mn, Co, and Ni salts were used as the starting materials for the Li-excess Mn-based layered material. The substances were mixed in a desired stoichiometric ratio, and the mixed powder was made into pellets. The pellets were then annealed at  for 24 h to obtain positive electrode active materials with formulas
for 24 h to obtain positive electrode active materials with formulas  and
and  .
.
The active materials were then treated with  in water. The amount of
in water. The amount of  used relative to the weight of the active material was 2, 5, 10, and 20 wt %.
used relative to the weight of the active material was 2, 5, 10, and 20 wt %.  was first dissolved in distilled water. Then the active material was mixed into the solution and dried at
was first dissolved in distilled water. Then the active material was mixed into the solution and dried at  . The resulting powder was annealed at
. The resulting powder was annealed at  for 10 h to decompose
for 10 h to decompose  . The final material was washed with distilled water, filtered, and dried to remove impurities from the material. In addition to
. The final material was washed with distilled water, filtered, and dried to remove impurities from the material. In addition to  , 5 or 10 wt % ascorbic acid
, 5 or 10 wt % ascorbic acid  was also used as reagent for surface treatment. With ascorbic acid, the final materials were not washed with distilled water.
was also used as reagent for surface treatment. With ascorbic acid, the final materials were not washed with distilled water.
The structure of the powders was studied by X-ray diffractometry (XRD) using a  source (50 kV, 300 mA). Morphology and particle size information were obtained from scanning electron microscopy (SEM). The surface area of the materials was determined using an AUTOSORB-1 equipment by Quantachrome: About 0.5 g of material was first dried at
source (50 kV, 300 mA). Morphology and particle size information were obtained from scanning electron microscopy (SEM). The surface area of the materials was determined using an AUTOSORB-1 equipment by Quantachrome: About 0.5 g of material was first dried at  for 1 h in vacuum and then the surface area was determined by a five-point Brunauer–Emmett–Teller (BET) method with nitrogen as the adsorbate gas. The surface structure of the active material was studied by Raman spectroscopy [Horiba Jobin Yvon (Laboratory Aramis)]. A laser with a wavelength of 532 nm and power of 20 mW was used for the measurement. Raman profiles were first calibrated with Si, which gives a peak at
for 1 h in vacuum and then the surface area was determined by a five-point Brunauer–Emmett–Teller (BET) method with nitrogen as the adsorbate gas. The surface structure of the active material was studied by Raman spectroscopy [Horiba Jobin Yvon (Laboratory Aramis)]. A laser with a wavelength of 532 nm and power of 20 mW was used for the measurement. Raman profiles were first calibrated with Si, which gives a peak at  . Li and transition-metal contents in the active materials were determined by inductively coupled plasma (ICP) atomic emission spectroscopy.
. Li and transition-metal contents in the active materials were determined by inductively coupled plasma (ICP) atomic emission spectroscopy.
For electrochemical evaluation, the surface-treated active material was mixed with acetylene black and poly(vinyl difluoride) in 1-methyl-2-pyrrolidone with a weight ratio of 80:10:10 to form a slurry. The slurry was then coated onto a roughened aluminum current collector using a doctor blade. The electrodes were rolled with a calender press to a packing density of  , with a typical thickness of
, with a typical thickness of  . These electrodes were assembled in a glove box with Ar atmosphere using Li metal as the counter and reference electrodes with a layer of separator to make flat test cells. 1 M
. These electrodes were assembled in a glove box with Ar atmosphere using Li metal as the counter and reference electrodes with a layer of separator to make flat test cells. 1 M  in ethylene carbonate/diethylcarbonate
in ethylene carbonate/diethylcarbonate  by volume was the electrolyte used in the experiments. The electrodes were tested in a pouch cell, sandwiched between glass plates on the outside to maintain contact of the electrodes. The cells were charged and discharged at room temperature
by volume was the electrolyte used in the experiments. The electrodes were tested in a pouch cell, sandwiched between glass plates on the outside to maintain contact of the electrodes. The cells were charged and discharged at room temperature  between 4.8 and 2 V vs
between 4.8 and 2 V vs  . Charge current was fixed at 20 mA/g, whereas the discharge current was varied from 10 to 300 mA/g. Rate capability was taken as the ratio of the 300 mA/g capacity to 10 mA/g capacity. Charge–discharge capacities were calculated with respect to the mass of the active material before charging. To study the effect of surface treatment, cyclic voltammograms were also taken with a scan rate of 0.03 mV/s between 4.9 and 2 V vs
. Charge current was fixed at 20 mA/g, whereas the discharge current was varied from 10 to 300 mA/g. Rate capability was taken as the ratio of the 300 mA/g capacity to 10 mA/g capacity. Charge–discharge capacities were calculated with respect to the mass of the active material before charging. To study the effect of surface treatment, cyclic voltammograms were also taken with a scan rate of 0.03 mV/s between 4.9 and 2 V vs  .
.
Results and Discussion
 material was first treated with 2, 5, 10, and 20 wt %
material was first treated with 2, 5, 10, and 20 wt %  . Because
. Because  dissolves easily in water, the active material is mixed with
dissolves easily in water, the active material is mixed with  in water so that the treatment can be more homogeneous. The heat-treatment temperature has to be high enough to decompose the reagent [
in water so that the treatment can be more homogeneous. The heat-treatment temperature has to be high enough to decompose the reagent [ for
for  ] but low enough such that long range atomic diffusion remains insignificant to limit the treatment to the surface of the particles. For this reason, a heat-treatment temperature of
] but low enough such that long range atomic diffusion remains insignificant to limit the treatment to the surface of the particles. For this reason, a heat-treatment temperature of  was chosen in this experiment.
was chosen in this experiment.
XRD profiles of  materials before and after treating with
materials before and after treating with  at
at  (before washing) are shown in Fig. 1. Before treatment, XRD profile shows a characteristics pattern corresponding to Li-excess Mn-based material, with a superlattice peak around 21° that is due to the ordering of Li and Mn in the transition-metal layer. After heat-treatment (before washing), main XRD peaks remain unaltered, while impurity peaks corresponding to
(before washing) are shown in Fig. 1. Before treatment, XRD profile shows a characteristics pattern corresponding to Li-excess Mn-based material, with a superlattice peak around 21° that is due to the ordering of Li and Mn in the transition-metal layer. After heat-treatment (before washing), main XRD peaks remain unaltered, while impurity peaks corresponding to  is identified. The larger the
is identified. The larger the  amount, the larger the impurity peaks. Because only the active material contains Li, the result indicates that
amount, the larger the impurity peaks. Because only the active material contains Li, the result indicates that  treatment removes Li from the active material.
treatment removes Li from the active material.  is soluble in water and can be washed away with water. XRD of a 20 wt %
is soluble in water and can be washed away with water. XRD of a 20 wt %  -treated material before and after water washing is shown in Fig. 1, verifying the removal of
-treated material before and after water washing is shown in Fig. 1, verifying the removal of  impurity by the washing process. In addition, the amount of Li in the active material after treatment was measured by ICP (see Table II). The ratio of Li to transition metal (Li/M) decreases linearly with increasing
impurity by the washing process. In addition, the amount of Li in the active material after treatment was measured by ICP (see Table II). The ratio of Li to transition metal (Li/M) decreases linearly with increasing  content, confirming the removal of Li from the active material. ICP measurement of the filtrate solution shows a Li/S ratio of 2:1, consistent with the composition of
content, confirming the removal of Li from the active material. ICP measurement of the filtrate solution shows a Li/S ratio of 2:1, consistent with the composition of  . Moreover, the filtrate solution contains insignificant amount of transition metal (Co, Ni, and Mn), indicating no dissolution of the material during the treatment process.
. Moreover, the filtrate solution contains insignificant amount of transition metal (Co, Ni, and Mn), indicating no dissolution of the material during the treatment process.
Figure 1. XRD profiles of  after treating with
after treating with  at
at  .
.
Table II. Physical parameters of  treated with
treated with  at
at  (after washed). Values in brackets show the change in lattice constants with respect to pristine sample.
(after washed). Values in brackets show the change in lattice constants with respect to pristine sample.
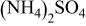 treatment treatment | A (Å) | C (Å) | BET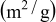
| Li/M | Estimated composition |
|---|---|---|---|---|---|
| Pristine | 2.8533 | 14.238 | 2.7 | 1.50 |
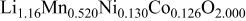
|
| 5 wt % | 2.8529 (−0.01%) | 14.245 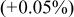
| 5.2 | 1.39 |

|
| 10 wt % | 2.8530 (−0.01%) | 14.246 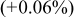
| 8.2 | 1.30 |
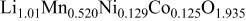
|
| 20 wt % | 2.8535 
| 14.248 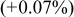
| 11.7 | 1.16 |
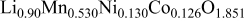
|
The main XRD peaks are expanded in Fig. 2 to see the effect of treatment on the lattice parameters of the material. XRD peak positions remained almost the same, regardless of the content of  . Lattice constants were determined by fitting the peak positions with an
. Lattice constants were determined by fitting the peak positions with an  space group and the results are shown in Table II. The changes in lattice parameters are not significant (less than 0.1%), suggesting that the treatment process does not alter the bulk structure.
space group and the results are shown in Table II. The changes in lattice parameters are not significant (less than 0.1%), suggesting that the treatment process does not alter the bulk structure.
Figure 2. XRD profiles of  treated with
treated with  (after washing).
(after washing).
Raman spectroscopy was carried out to monitor the change in surface structure of the active material with  treatment. The results are shown in Fig. 3. The pristine
treatment. The results are shown in Fig. 3. The pristine  material shows Raman peaks at 330, 368, 411, 442, 494, 567, and
material shows Raman peaks at 330, 368, 411, 442, 494, 567, and  . Comparing with Raman peaks of
. Comparing with Raman peaks of  (248, 332, 369, 413, 493, 568, and
(248, 332, 369, 413, 493, 568, and  )17 and
)17 and  (475 and
(475 and  ), Raman spectroscopy of the active material shows a profile that is a superposition of those of
), Raman spectroscopy of the active material shows a profile that is a superposition of those of  and
and  . This indicates that the active material contains local structures similar to
. This indicates that the active material contains local structures similar to  and
and  . Figure 3 shows that the Raman peak intensities are reduced with
. Figure 3 shows that the Raman peak intensities are reduced with  treatment, indicating that the treatment changes the surface structure of the material. In addition, an emergence of a peak at about
treatment, indicating that the treatment changes the surface structure of the material. In addition, an emergence of a peak at about  with increasing
with increasing  content is observed. For comparison, Raman profile of spinel
content is observed. For comparison, Raman profile of spinel  is included in Fig. 3. The high frequency Raman peak from the
is included in Fig. 3. The high frequency Raman peak from the  treatment resembles that of the spinel material, suggesting a "spinel-like" layer on the surface after treatment.
treatment resembles that of the spinel material, suggesting a "spinel-like" layer on the surface after treatment.
Figure 3. Raman profiles of  treated with
treated with  and
and  .
.
BET surface area of the active material after treatment was measured, and the results are listed in Table II. An increase in the BET surface area is observed, indicating that the surface of the active material is roughened during the treatment process. This is also observed in the SEM diagrams, as shown in Fig. 4. Figure 4a shows the morphology of the pristine material, with secondary particle size of about  in diameter. Figure 4b shows the material after 10 wt %
in diameter. Figure 4b shows the material after 10 wt %  treatment. The surface of the particles are roughened after surface treatment, though, the secondary particle size of the active material before and after remains about the same, indicating that the surface treatment process does not break up the secondary particles.
treatment. The surface of the particles are roughened after surface treatment, though, the secondary particle size of the active material before and after remains about the same, indicating that the surface treatment process does not break up the secondary particles.
Figure 4. SEM diagrams of  (a) without treatment and (b) with 10 wt %
(a) without treatment and (b) with 10 wt %  treatment.
treatment.
Active materials were made into electrodes for electrochemical evaluation. Charge–discharge curves during the first and second cycles of  are shown in Fig. 5a and 5b, respectively, and the capacities are listed in Table III. As shown in Fig. 5a, the first cycle charge capacity is reduced with
are shown in Fig. 5a and 5b, respectively, and the capacities are listed in Table III. As shown in Fig. 5a, the first cycle charge capacity is reduced with  treatment. The amount of charge capacity reduced is proportional to
treatment. The amount of charge capacity reduced is proportional to  content, consistent with the extraction of Li from the active material during treatment (ICP results). Despite the decrease in charge capacity, discharge capacities remain
content, consistent with the extraction of Li from the active material during treatment (ICP results). Despite the decrease in charge capacity, discharge capacities remain  (at 20 mA/g) regardless of
(at 20 mA/g) regardless of  content. Increasing
content. Increasing  content does not decrease overall capacity because the
content does not decrease overall capacity because the  by-product is prewashed away. Overall, first cycle efficiency (FCE) is increased with treatment from about 76% for the pristine material to over 95% with 20 wt %
by-product is prewashed away. Overall, first cycle efficiency (FCE) is increased with treatment from about 76% for the pristine material to over 95% with 20 wt %  treatment. The increase in FCE is advantageous for battery application, when the cathode material is coupled with anode materials such as graphite that do not contain Li to start out with.
treatment. The increase in FCE is advantageous for battery application, when the cathode material is coupled with anode materials such as graphite that do not contain Li to start out with.
Figure 5. Charge–discharge curves of  treated with
treated with  at 20 mA/g (a) first cycle and (b) second cycle.
at 20 mA/g (a) first cycle and (b) second cycle.
Table III. Electrochemical properties of  treated with
treated with  .
.
| Base material |
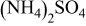 treatment treatment | First charge capacity (mAh/g) | First discharge capacity (mAh/g) | FCE (%) | 300 mA/g capacity (mAh/g) | 300 mA/g capacity over 10 mA/g capacity (%) |
|---|---|---|---|---|---|---|
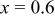
| — | 319.5 | 242.6 | 75.9 | 192.2 | 74.3 |
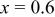
| 2 wt % | 322.7 | 261.1 | 80.9 | 203.8 | 76.7 |
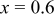
| 5 wt % | 312.1 | 262.6 | 84.1 | 212.4 | 78.9 |
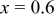
| 10 wt % | 307.2 | 267.8 | 87.2 | 222.8 | 81.4 |
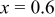
| 20 wt % | 280.1 | 270.4 | 96.5 | 228.4 | 83.5 |
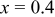
| — | 272.0 | 216.3 | 79.5 | 178.1 | 76.5 |
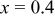
| 2 wt % | 274.7 | 226.3 | 82.4 | 187.4 | 78.4 |

| 5 wt % | 266.9 | 224.7 | 84.2 | 189.1 | 80.1 |
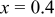
| 10 wt % | 258.5 | 226.7 | 87.7 | 191.4 | 81.4 |
A change in the profile of the discharge curves with  treatment can be seen in Fig. 5a.
treatment can be seen in Fig. 5a.  treatment leads to a higher discharge potential during the initial 50 mAh/g and lower discharge potential for the rest of the discharge curve. In addition, a plateau at 2.8 V vs
treatment leads to a higher discharge potential during the initial 50 mAh/g and lower discharge potential for the rest of the discharge curve. In addition, a plateau at 2.8 V vs  emerges with
emerges with  treatment at the end of the discharge curve. A corresponding plateau at about 2.9 V is also visible during the initial charge in the second cycle (Fig. 5b), where the length of the plateau increases with
treatment at the end of the discharge curve. A corresponding plateau at about 2.9 V is also visible during the initial charge in the second cycle (Fig. 5b), where the length of the plateau increases with  content. These features are characteristics of a spinel-like component, consistent with Raman results reported in Fig. 3.
content. These features are characteristics of a spinel-like component, consistent with Raman results reported in Fig. 3.
The electrodes were then discharged at current rates of 10, 20, 50, 100, 200, and 300 mA/g and the corresponding rate performance is summarized in Fig. 6. An increase in discharge capacity and rate capability with  treatment is observed. Rate capability is taken as the ratio of the 300 mA/g capacity to 10 mA/g capacity, and the results are listed in Table III. Rate capability of the nontreated material is about 74%, where as that of a 20 wt %
treatment is observed. Rate capability is taken as the ratio of the 300 mA/g capacity to 10 mA/g capacity, and the results are listed in Table III. Rate capability of the nontreated material is about 74%, where as that of a 20 wt %  -treated sample is
-treated sample is  , showing a significant improvement in rate capability with
, showing a significant improvement in rate capability with  treatment. With a 20 wt %
treatment. With a 20 wt %  treatment, discharge capacity is as high as 230 mAh/g at a current rate of 300 mA/g
treatment, discharge capacity is as high as 230 mAh/g at a current rate of 300 mA/g  .
.
Figure 6. Discharge capacity of  treated with
treated with  vs current rate.
vs current rate.
A comparison of the discharge curves at a current rate of 300 mA/g when the active material is treated with different  contents is shown in Fig. 7. The result shows higher discharge capacity with higher
contents is shown in Fig. 7. The result shows higher discharge capacity with higher  content. In addition, the material treated with a larger amount of
content. In addition, the material treated with a larger amount of  shows more prominent spinel-like plateaus in the 4 and 3 V regions, suggesting that the higher rate capability is associated with this spinel-like behavior. Even though
shows more prominent spinel-like plateaus in the 4 and 3 V regions, suggesting that the higher rate capability is associated with this spinel-like behavior. Even though  treatment leads to higher rate capability, the spinel-like plateau leads to lower average potential with increasing
treatment leads to higher rate capability, the spinel-like plateau leads to lower average potential with increasing  content, so there is an optimal
content, so there is an optimal  content with respect to energy density.
content with respect to energy density.
Figure 7. Discharge curves of  treated with
treated with  at 300 mA/g.
at 300 mA/g.
Cyclic voltammetry (CV) is taken to further understand the effect of  treatment. Figure 8a and 8b shows the CV profiles of the materials during the first and second cycles with a scan rate of 0.03 mV/s. During the first cycle (Fig. 8a), the pristine sample shows an initial charge peak at around 4 and 4.556 V vs
treatment. Figure 8a and 8b shows the CV profiles of the materials during the first and second cycles with a scan rate of 0.03 mV/s. During the first cycle (Fig. 8a), the pristine sample shows an initial charge peak at around 4 and 4.556 V vs  . The 4 V peak is associated with charging of the Ni–Co–Mn component in the material, where as the peak at 4.5 V is associated with activation of the
. The 4 V peak is associated with charging of the Ni–Co–Mn component in the material, where as the peak at 4.5 V is associated with activation of the  component. After
component. After  treatment, an increase in peak height at 4.55 V is observed during first cycle (Fig. 8a), suggesting a higher Li extraction rate from the
treatment, an increase in peak height at 4.55 V is observed during first cycle (Fig. 8a), suggesting a higher Li extraction rate from the  component in the active material after treatment. In addition, a new discharge peak at 2.8 V with
component in the active material after treatment. In addition, a new discharge peak at 2.8 V with  treatment is observed. Profiles during the second cycle (Fig. 8b) show a clear redox pair at 2.8 and 2.9 V (marked by "S" in the figure), indicating change in electrochemical potential with
treatment is observed. Profiles during the second cycle (Fig. 8b) show a clear redox pair at 2.8 and 2.9 V (marked by "S" in the figure), indicating change in electrochemical potential with  treatment. The redox pair is attributed to a spinel contribution. The improvement in rate capability is attributed to a faster Li diffusion through the spinel surface layer.
treatment. The redox pair is attributed to a spinel contribution. The improvement in rate capability is attributed to a faster Li diffusion through the spinel surface layer.
Figure 8. Cyclic voltammograms of  treated with
treated with  at 0.03 mV/s (a) first cycle and (b) second cycle.
at 0.03 mV/s (a) first cycle and (b) second cycle.
To further study the reaction mechanism of  treatment, the same treatment was carried out on an active material with
treatment, the same treatment was carried out on an active material with  (containing less
(containing less  component). Results are listed in Table III. Similar to the treatment of the material with
component). Results are listed in Table III. Similar to the treatment of the material with 
 , treatment of the
, treatment of the 
 sample leads to a decrease in first cycle charge capacity, increase in FCE, and an increase in rate capability. A comparison of the increase in rate capability of the two samples is shown in Fig. 9. The increase in rate capability from
sample leads to a decrease in first cycle charge capacity, increase in FCE, and an increase in rate capability. A comparison of the increase in rate capability of the two samples is shown in Fig. 9. The increase in rate capability from  treatment is higher for the material with
treatment is higher for the material with  , suggesting that the treatment has more influence on the
, suggesting that the treatment has more influence on the  component in the material.
component in the material.
Figure 9. Comparison of rate capability with  treatment.
treatment.
Surface treatments with  indicate the removal of Li from the active material. However, charge–discharge profiles suggest that there are spinel-like regions in the material. One remaining question is whether oxygen is also removed from the active material during treatment. We were not able to directly measure oxygen content in the active material. But instead, we estimated oxygen content from the ICP results assuming that oxygen accounts for the remaining mass in the measurement. For the calculation, oxygen content of the pristine sample is normalized to 2, and the total amount of transition metal after treatment is set to be the same (no transition-metal dissolution measured from the treatment). The estimated compositions of the active materials are listed in Table II. The estimated composition is slightly different from the targeted composition, which could be due to impurities in the material that are not accounted for in the calculation. Because the same active material is used for all the treatments, impurity contents only change the absolute amount of oxygen but do not affect the trend between Li and oxygen contents from the treatment. Figure 10 shows the relationship between Li and oxygen contents within the active material after treatment; removal of Li from the active material is accompanied by the removal of oxygen from the lattice. The slope of the curve in Fig. 10 is about 2, suggesting an overall extraction of
indicate the removal of Li from the active material. However, charge–discharge profiles suggest that there are spinel-like regions in the material. One remaining question is whether oxygen is also removed from the active material during treatment. We were not able to directly measure oxygen content in the active material. But instead, we estimated oxygen content from the ICP results assuming that oxygen accounts for the remaining mass in the measurement. For the calculation, oxygen content of the pristine sample is normalized to 2, and the total amount of transition metal after treatment is set to be the same (no transition-metal dissolution measured from the treatment). The estimated compositions of the active materials are listed in Table II. The estimated composition is slightly different from the targeted composition, which could be due to impurities in the material that are not accounted for in the calculation. Because the same active material is used for all the treatments, impurity contents only change the absolute amount of oxygen but do not affect the trend between Li and oxygen contents from the treatment. Figure 10 shows the relationship between Li and oxygen contents within the active material after treatment; removal of Li from the active material is accompanied by the removal of oxygen from the lattice. The slope of the curve in Fig. 10 is about 2, suggesting an overall extraction of  from the active material from the treatment, consistent with the formation of spinel structure. We suspect that
from the active material from the treatment, consistent with the formation of spinel structure. We suspect that  is extracted from the
is extracted from the  component of the active material because the effect of the treatment is greater for material with larger
component of the active material because the effect of the treatment is greater for material with larger  . Thus, the reaction mechanism could be written as
. Thus, the reaction mechanism could be written as
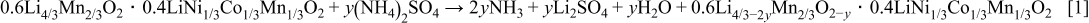
We proposed a schematic diagram for the reaction mechanism, as shown in Fig. 11. Reagent is first coated on the surface of Li-excess Mn-based material. During heat-treatment, decomposition of the reagent extracts oxygen atoms and Li atoms from the active material and atomic rearrangements on the surface lead to a spinel-like region in the material. The spinel-like region is expected to be only on the surface of the active material due to the low treatment temperature of  . Because both layered and spinel structures share the same oxygen arrangement, only differing in the position of the transitional metal and Li atoms, we expect a continuous transition from the bulk layered material to spinel-like region on the surface without a distinct boundary in between. Impurities from the by-product of the reaction such as
. Because both layered and spinel structures share the same oxygen arrangement, only differing in the position of the transitional metal and Li atoms, we expect a continuous transition from the bulk layered material to spinel-like region on the surface without a distinct boundary in between. Impurities from the by-product of the reaction such as  can be removed from the active material by a washing process. The treatment is different from acid treatment because no
can be removed from the active material by a washing process. The treatment is different from acid treatment because no  exchange takes place in the process. We suspect the spinel-like region facilitate Li diffusion (3D diffusion path) through the surface of the particles, improving rate capability of the active material.
exchange takes place in the process. We suspect the spinel-like region facilitate Li diffusion (3D diffusion path) through the surface of the particles, improving rate capability of the active material.
Figure 10. Variation of Li and O contents in the active material with  treatment.
treatment.
Figure 11. Schematics of surface treatment of Li-excess Mn-based cathode with  .
.
We further investigated whether similar spinel-like behaviors could be obtained by just removing oxygen from the active material without removing Li atoms. Treatment with ascorbic acid  was attempted. Ascorbic acid is chosen because it is a reducing agent, which may be able to remove oxygen from the active material during annealing. In addition, it presumably does not extract Li from the active material during decomposition.
was attempted. Ascorbic acid is chosen because it is a reducing agent, which may be able to remove oxygen from the active material during annealing. In addition, it presumably does not extract Li from the active material during decomposition.
Similar to the  treatment, 5 or 10 wt % of the
treatment, 5 or 10 wt % of the  reagent was dissolved in water and the active material
reagent was dissolved in water and the active material  was added to the solution. The solution was dried and annealed at
was added to the solution. The solution was dried and annealed at  . A final wash and filter process was not carried out for these samples.
. A final wash and filter process was not carried out for these samples.
XRD profiles of the samples after  treatment shows no impurity peaks (not shown here). First and second cycle charge–discharge curves of the treated active materials are shown in Fig. 12a and 12b, respectively. There is no effective change in the first charge profiles for both treated materials in Fig. 12a, indicating that Li is not extracted from the active material. But spinel-like features are seen in the discharge profiles. The plateau at around 3 V can again be seen in the charge and discharge curves during the second cycle (Fig. 12b). This suggests that the spinel-like behavior is most likely due to oxygen removal from the active material, rather than Li removal from the active material. Slight increase in rate capability (300 over 10 mA/g capacity) to about 76.5 with 5% ascorbic acid is observed. Rate capability can be improved without Li extraction, also confirming that oxygen removal is an important part of the reaction.
treatment shows no impurity peaks (not shown here). First and second cycle charge–discharge curves of the treated active materials are shown in Fig. 12a and 12b, respectively. There is no effective change in the first charge profiles for both treated materials in Fig. 12a, indicating that Li is not extracted from the active material. But spinel-like features are seen in the discharge profiles. The plateau at around 3 V can again be seen in the charge and discharge curves during the second cycle (Fig. 12b). This suggests that the spinel-like behavior is most likely due to oxygen removal from the active material, rather than Li removal from the active material. Slight increase in rate capability (300 over 10 mA/g capacity) to about 76.5 with 5% ascorbic acid is observed. Rate capability can be improved without Li extraction, also confirming that oxygen removal is an important part of the reaction.
Figure 12. Charge–discharge curves of  treated with ascorbic acid: (a) first cycle and (b) second cycle.
treated with ascorbic acid: (a) first cycle and (b) second cycle.
Preliminary cycle tests of the electrode with and without surface treatment were performed and the results are shown in Fig. 13. The first three cycles were taken as a discharge rate of 20 mA/g, followed by 40, 100, 200, and 300 mA/g in the next four cycles. Cycle 8 and 9 were done at 20 mA/g again. Subsequent cycle tests were carried out at a discharge rate of 100 mA/g (with the 19th and 39th cycle at 20 mA/g). The pristine material shows a gradual decrease in discharge capacity, which is attributed to the dissolution of transition metal from the active material during cycling. The cycle performance of the material after surface treatment is almost the same. This indicates that surface treatment does not significantly affect cycle performance because the surface treatment method does not insulate the active material from direct contact with the electrolyte. Additional surface coating may be required to improve cycle performance of the surface-treated or nontreated Li-rich materials.
Figure 13. Cycle performance of  with or without
with or without  treatment.
treatment.
In general, we expect the treatment to also work on other Li-excess Mn-based oxide materials with a formula  . Future work is in progress to study the effect of the surface modification on Li-ion batteries when the modified cathode is combined with anode materials such as graphite.
. Future work is in progress to study the effect of the surface modification on Li-ion batteries when the modified cathode is combined with anode materials such as graphite.
Conclusions
Significant enhancement in the rate capability of Li-excess Mn-based material is observed by surface treating the active material with  reagent. After treatment, active material can provide both high capacity and good rate capability (260 mAh/g at rate of 10 mA/g and 230 mAh/g at a rate of 300 mA/g). Treatment process removes oxygen and lithium atoms from the surface of the active material, resulting in a spinel-like surface layer. The existence of a spinel-like behavior is confirmed by CV and Raman spectroscopy. The improved rate capability is attributed to the spinel layer that facilitates Li diffusion into the particle.
reagent. After treatment, active material can provide both high capacity and good rate capability (260 mAh/g at rate of 10 mA/g and 230 mAh/g at a rate of 300 mA/g). Treatment process removes oxygen and lithium atoms from the surface of the active material, resulting in a spinel-like surface layer. The existence of a spinel-like behavior is confirmed by CV and Raman spectroscopy. The improved rate capability is attributed to the spinel layer that facilitates Li diffusion into the particle.
These results suggest that electrochemical performances of Li-excess Mn-based active materials are surface-sensitive and further work is in progress to improve material stability through surface treatments. The ability to improve rate capability of Li-excess Mn-based material is an important step toward the goal of utilizing these materials in batteries for high energy applications.
SANYO Electric Co., Ltd. assisted in meeting the publication costs of this article.














