Abstract
Ballmilling driven Li reactions are shown to be of great value to either mimic electrochemical discharge reactions in secondary Li cells or synthesize new single-phase Li-based materials. The former is illustrated by the reduction of CoO and  to Co metal plus
to Co metal plus  or
or  respectively. To demonstrate the second effect, room temperature synthesis of
respectively. To demonstrate the second effect, room temperature synthesis of  and
and  a member of a new class of promising negative electrode materials, is presented. Using Li powder as the basic ingredient, such reactions are rapid and easy to perform. © 2003 The Electrochemical Society. All rights reserved.
a member of a new class of promising negative electrode materials, is presented. Using Li powder as the basic ingredient, such reactions are rapid and easy to perform. © 2003 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
The current interest in the development of more efficient Li-ion technologies has prompted the study of several new families of either positive or negative electrode materials. On the positive side, the main focus is presently on phosphates and Cr-based layered oxides. On the negative side, in addition to the Sn-based and 3d metal oxides and intermetallics, an increasing interest is focused on metal nitrides, phosphides, or antimonides families as a possible alternative to carbon materials due to their ability to react reversibly with large amounts of Li per formula unit. For instance,  -type phases
-type phases 
 1
2 were recently reported to react reversibly with about 9 lithium atoms per formula unit. Such phases are usually synthesized at high temperatures in sealed stainless steel (SS) tubes. However, the samples obtained at high temperatures always contained second phases, regardless of attempted synthesis conditions. Thus, the search for other alternatives continued, and due to a complex solution chemistry, the ballmilling synthesis approach was utilized. Chemical reactions induced in this manner have been recognized for quite some time, and have been widely applied to various research fields as a simple and environmentally friendly alternative to high temperature synthesis or solution chemistry.
1
2 were recently reported to react reversibly with about 9 lithium atoms per formula unit. Such phases are usually synthesized at high temperatures in sealed stainless steel (SS) tubes. However, the samples obtained at high temperatures always contained second phases, regardless of attempted synthesis conditions. Thus, the search for other alternatives continued, and due to a complex solution chemistry, the ballmilling synthesis approach was utilized. Chemical reactions induced in this manner have been recognized for quite some time, and have been widely applied to various research fields as a simple and environmentally friendly alternative to high temperature synthesis or solution chemistry.
Such a process involves repeated welding fracture and rewelding of powder particles in a dry, high energy ball mill, resulting in particle and grain size reduction, together with structural defects. Energetic lattice defects, combined with short diffusion distances, are the driving forces of a faster solid-state alloying and chemical reaction at low temperatures. Surprisingly, these advantages did not capture the field of Li energy storage until the pioneering work3 of Disma et al. on the profound effect of ballmilling on the electrochemical performance of carbonaceous materials toward Li. This technique has been broadly applied to either synthesize, amongst other things, intermetallic compounds able to alloy with Li,4
5 or to modify powders morphologies to obtain, for instance, electrochemically optimized  powders operating at 3 V6 or, more recently, phosphates powders with enhanced electrochemical performances.7 Nevertheless, mechanical reactions between Li metal and various elements have until now attracted less attention, with the exception, as far as we know, of only one report dealing with the preparation of
powders operating at 3 V6 or, more recently, phosphates powders with enhanced electrochemical performances.7 Nevertheless, mechanical reactions between Li metal and various elements have until now attracted less attention, with the exception, as far as we know, of only one report dealing with the preparation of  using metallic Li powders.8
using metallic Li powders.8
We decided to pursue this route further, and we successfully used ballmilling as reported herein as:  a way to mimic displacement reactions in primary cells9 such as
a way to mimic displacement reactions in primary cells9 such as  which involves extensive bond breakage, atomic reorganization, and formation of new bonds, and
which involves extensive bond breakage, atomic reorganization, and formation of new bonds, and  as a means to easily synthesize Li-based phases at room temperature.
as a means to easily synthesize Li-based phases at room temperature.
Experimental
Attempts to perform ballmilling experiments in the presence of metallic Li did not succeed, due to its high ductility that resulted in a sticky Li deposition on the grinding vial walls. To circumvent this limitation, metallic Li powders were used as the Li precursor, together with a solvent (selected from the hydrocarbon family, and more specifically, dodecane). The CoO powder was utilized as received from Union Minière. The Sb, Ti, and P precursor powders, purchased from Aldrich chemicals, were always stored and handled in an argon glove box.  was synthesized by reacting stoichiometric mixtures of Co and Sb powders for 12 h at 750°C in sealed quartz ampules.
was synthesized by reacting stoichiometric mixtures of Co and Sb powders for 12 h at 750°C in sealed quartz ampules.
Ballmilling experiments (denoted hereafter as BM) were performed using a Spex 8000 mixer mill that generates normal mechanical strain. The grinding vials were loaded and sealed in an argon dry box, using a weight ratio of SS ball to powder of 8:1. Unless otherwise specified,  of dodecane was added to 400 mg of precursor mixtures, and grinding times ranged from 2 to 48 h.
of dodecane was added to 400 mg of precursor mixtures, and grinding times ranged from 2 to 48 h.
Powder purity and crystallinity were examined by X-ray diffraction (XRD), with a Scintag diffractometer operating in Bragg-Brentano geometry with a Cu Kα radiation or, unless otherwise specified, by a Siemens D8 diffractometer using Co Kα radiation, and equipped with a power spectral density detector. Because of the moisture sensitivity of the Li-based reduced samples, a hermetically closed sample holder was used. The powders morphology and composition were investigated by scanning electron microscopy with a Philips XL 30 field emission gun, coupled to an Oxford Link instrument for energy-dispersive X-ray spectroscopy (EDS). Transmission electron microscopy (TEM) was carried out using a Philips CM12 microscope equipped with an EDS analyzer.
The ballmilled samples were tested in Swagelok-type cells assembled in an argon-filled dry box, using Li metal as the negative electrode, and a Whatman glass filter, size D (GF/D) borosilicate glass fiber sheet, saturated with 1 M  in ethylene carbonate (EC), dimethyl carbonate (DMC) (1:1 in weight) as the electrolyte. The positive electrodes consisted of a few milligrams of powder directly deposited on the swagelok plunger. The electrochemical lithium reactivity was monitored with a VMP potentiostat/galvanostat (Biologic SA, Claix, France), operating either in galvanostatic, galvanostatic intermittent titration technique (GITT) or potentiostatic intermittent titration technique (PITT) mode.
in ethylene carbonate (EC), dimethyl carbonate (DMC) (1:1 in weight) as the electrolyte. The positive electrodes consisted of a few milligrams of powder directly deposited on the swagelok plunger. The electrochemical lithium reactivity was monitored with a VMP potentiostat/galvanostat (Biologic SA, Claix, France), operating either in galvanostatic, galvanostatic intermittent titration technique (GITT) or potentiostatic intermittent titration technique (PITT) mode.
Results and Discussion
By ballmilling Li powders (5% in excess) with either CoO or  a first attempt was made to simulate the electrochemical reduction of CoO and
a first attempt was made to simulate the electrochemical reduction of CoO and  by Li, which was shown to lead to the formation of
by Li, which was shown to lead to the formation of  10 and
10 and  11 composites, respectively. From a study of various grinding times coupled with XRD and SAED analyses, we concluded that the ballmilling-driven Li reduction reactions were complete after 10 h for the CoO system and about 12 h for the
11 composites, respectively. From a study of various grinding times coupled with XRD and SAED analyses, we concluded that the ballmilling-driven Li reduction reactions were complete after 10 h for the CoO system and about 12 h for the  samples. The XRD pattern of the 10 h milled CoO sample was featureless with the exception of weak, broad peaks corresponding to Co, while its SAED pattern (Fig. 1a) showed well-defined rings corresponding to the
samples. The XRD pattern of the 10 h milled CoO sample was featureless with the exception of weak, broad peaks corresponding to Co, while its SAED pattern (Fig. 1a) showed well-defined rings corresponding to the  and Co phases. There was no evidence for traces of residual CoO. Regarding the 12 h milled
and Co phases. There was no evidence for traces of residual CoO. Regarding the 12 h milled  sample, its XRD pattern (Fig. 2) revealed the presence of a single face-centered cubic fcc phase
sample, its XRD pattern (Fig. 2) revealed the presence of a single face-centered cubic fcc phase 
 with lattice parameter
with lattice parameter  in agreement with the reported literature value of 6.57 Å (JCPDF 040791). Unlike the CoO sample, XRD showed no evidence of Co metal after ballmilling. The SAED patterns (not shown here) confirmed the formation of
in agreement with the reported literature value of 6.57 Å (JCPDF 040791). Unlike the CoO sample, XRD showed no evidence of Co metal after ballmilling. The SAED patterns (not shown here) confirmed the formation of  with no evidence of residual
with no evidence of residual  or Co metal, as reported for the electrochemical reduction of
or Co metal, as reported for the electrochemical reduction of  powders.12
powders.12
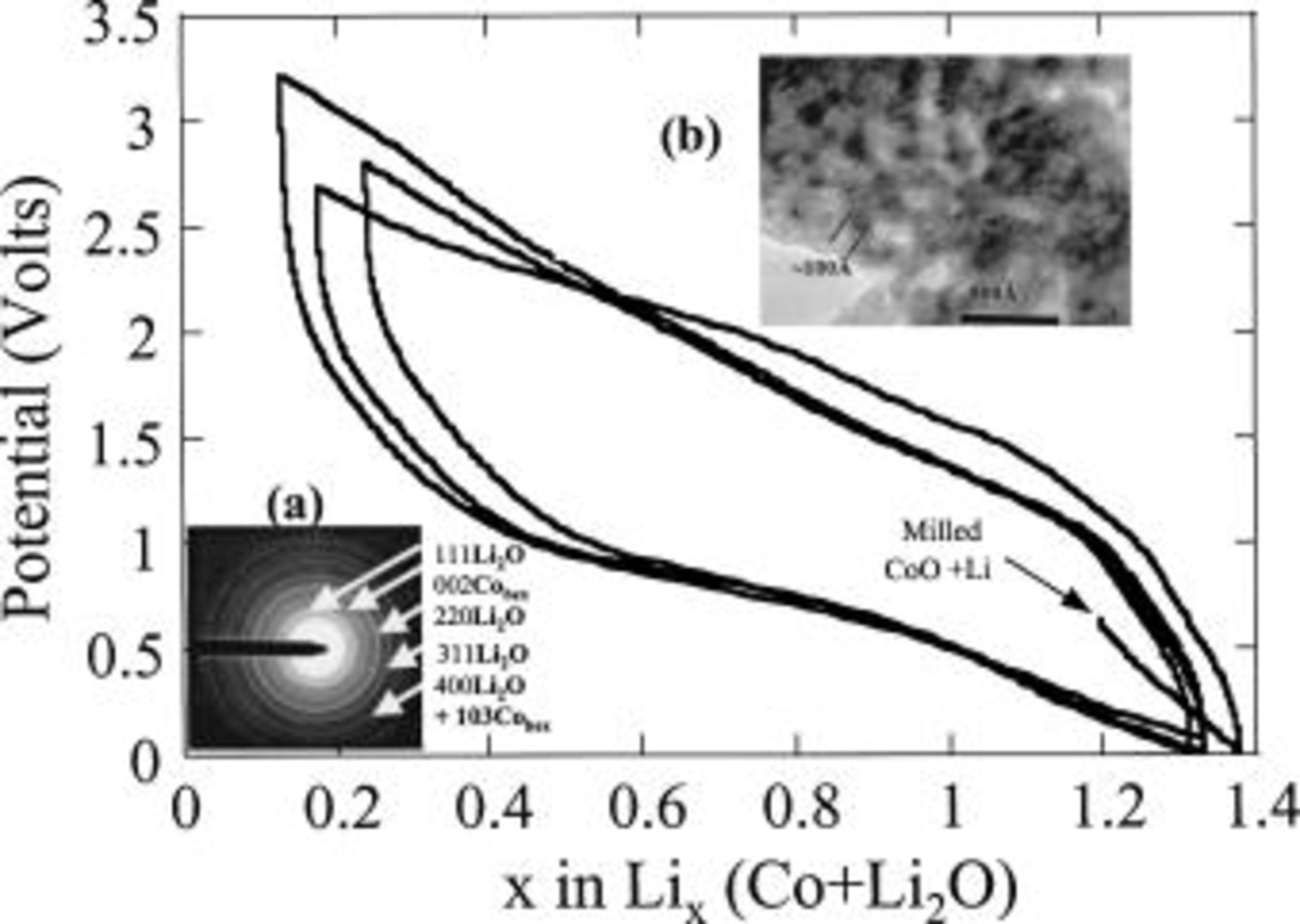
Figure 1. The voltage/composition curve is shown for a composite electrode obtained by ballmilling a mixture of CoO and Li powders, together with its TEM micrograph (a) and SAED pattern (b). Note that the ballmilled composite consists of a mixture of  and
and  fully consistent with the 0.7 V starting cell potential.
fully consistent with the 0.7 V starting cell potential.
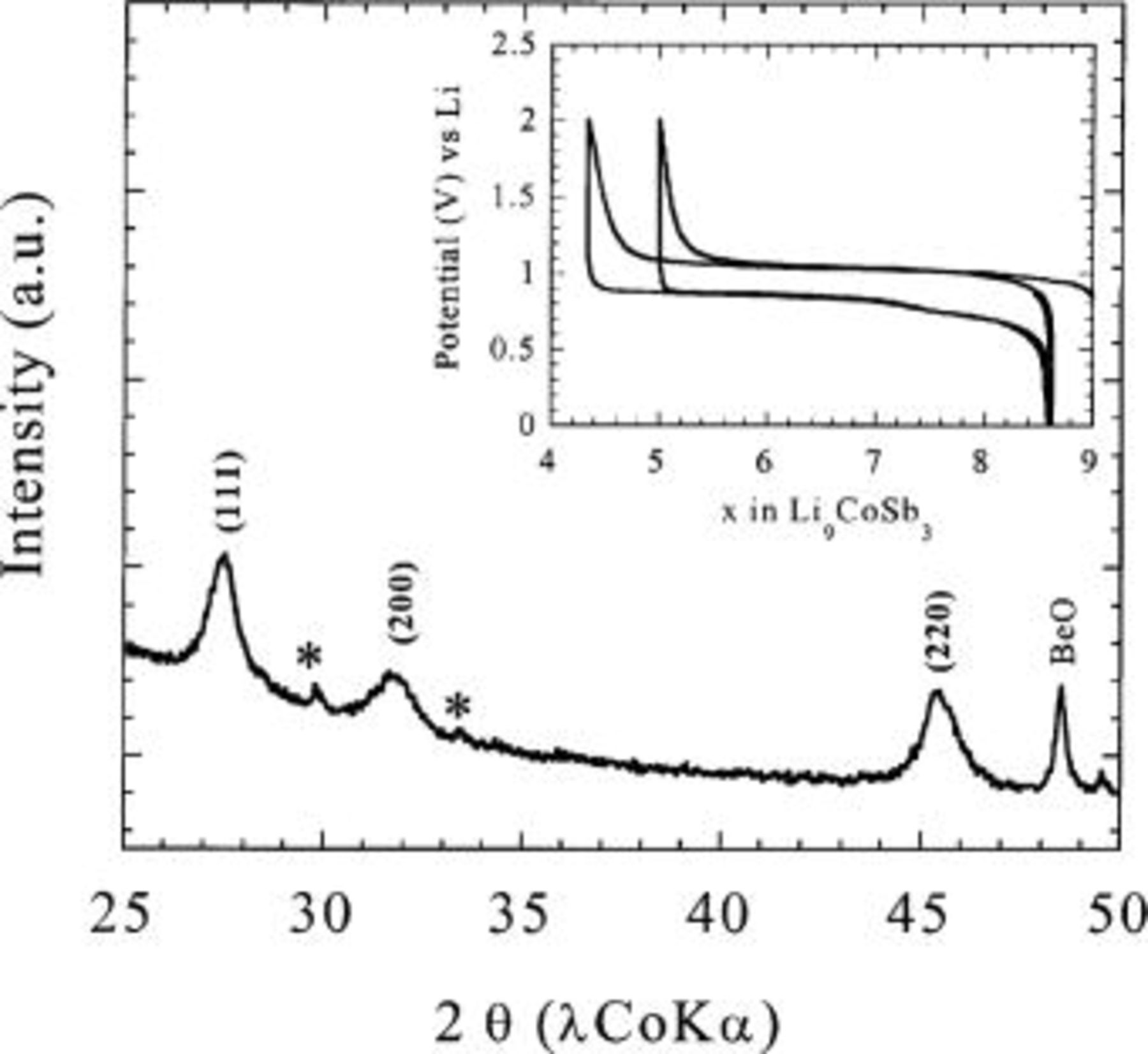
Figure 2. X-ray powder pattern of a powder obtained after ballmilling of a  composite, together with its electrochemical behavior in Li half cells. The
composite, together with its electrochemical behavior in Li half cells. The  -ray diagram is analogous to that of
-ray diagram is analogous to that of  The two extra peaks around 30° and 33.5° in 2θ, denoted by asterisks, are due to the cell hardware.
The two extra peaks around 30° and 33.5° in 2θ, denoted by asterisks, are due to the cell hardware.
The electrochemical properties of these samples were reported in Fig. 1 and 2. Note that 1.2 Li from the  composite can be removed, while 2Li can be removed from the electrochemically formed sample.10 This difference is rooted in the nanoparticle size, which is about 100 Å (Fig. 1b) for the BM sample, compared to 20 Å for the electrochemically made sample,10 thus leading to the expectation of a very difficult
composite can be removed, while 2Li can be removed from the electrochemically formed sample.10 This difference is rooted in the nanoparticle size, which is about 100 Å (Fig. 1b) for the BM sample, compared to 20 Å for the electrochemically made sample,10 thus leading to the expectation of a very difficult  conversion process. For the BM
conversion process. For the BM  sample, the oxidation curve is similar to that observed with an electrochemically made
sample, the oxidation curve is similar to that observed with an electrochemically made  composite. These support the use of mechanical milling in the presence of Li metal powders as a new way of obtaining Li-based reduced composites.
composite. These support the use of mechanical milling in the presence of Li metal powders as a new way of obtaining Li-based reduced composites.
We further exploited the possibility of using room temperature milling of Li powders to synthesize phases, such as  that usually require high temperature and a controlled environment. Highly divided
that usually require high temperature and a controlled environment. Highly divided  powders were synthesized by mixing stoichiometric amounts of lithium and Sb powders together with
powders were synthesized by mixing stoichiometric amounts of lithium and Sb powders together with  of dodecane. The best synthesis results were obtained for grinding times ranging from 1 to 2 h. The XRD pattern of a sample ballmilled for 1 h exhibited Bragg peaks (Fig. 3b) that were indexed with
of dodecane. The best synthesis results were obtained for grinding times ranging from 1 to 2 h. The XRD pattern of a sample ballmilled for 1 h exhibited Bragg peaks (Fig. 3b) that were indexed with  corresponding to the
corresponding to the  fcc phase with
fcc phase with  This contrasted with the high temperature
This contrasted with the high temperature  phase (Fig. 3a) made from Li foil and Sb powders in sealed SS tubes. Although the high temperature and ballmilled samples exhibited similar voltage profiles, as shown in the insets of Fig. 3a and b, respectively, the BM sample exhibited lower capacity. No simple explanation was identified for the latter feature.
phase (Fig. 3a) made from Li foil and Sb powders in sealed SS tubes. Although the high temperature and ballmilled samples exhibited similar voltage profiles, as shown in the insets of Fig. 3a and b, respectively, the BM sample exhibited lower capacity. No simple explanation was identified for the latter feature.
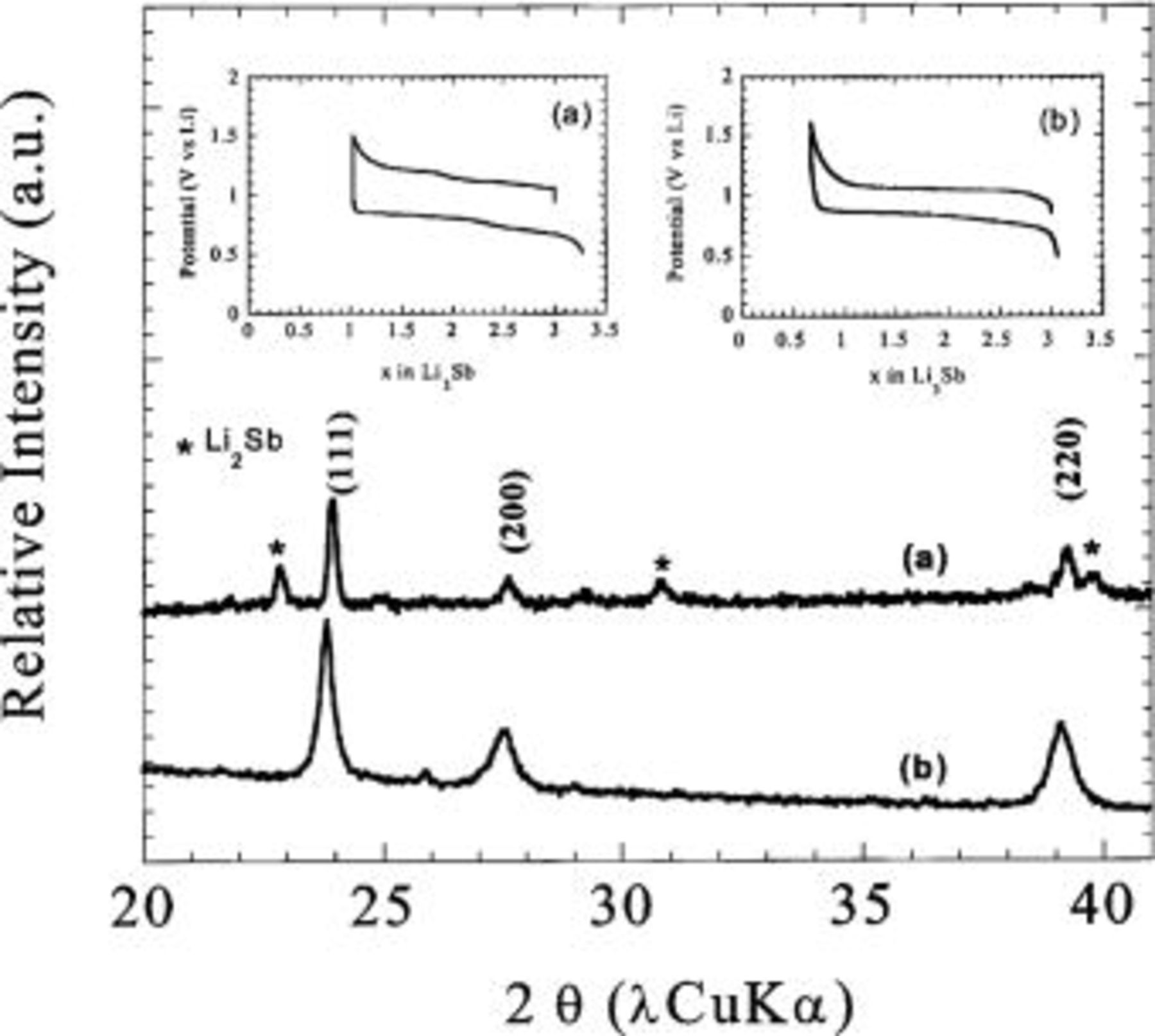
Figure 3. X-ray powder pattern of  made at (a) high temperature as compared to
made at (a) high temperature as compared to  prepared by (b) ballmilling. Their corresponding electrochemical signature, referred to as a and b, are shown as inset. While the
prepared by (b) ballmilling. Their corresponding electrochemical signature, referred to as a and b, are shown as inset. While the  curves are similar, note that the ballmilled sample is devoid of non indexed extra peaks.
curves are similar, note that the ballmilled sample is devoid of non indexed extra peaks.
Recently, it was shown2
13
14 that  -type phases hold great potential with respect to applications in Li-ion cells. However, the challenge consists in synthesizing such compounds as single phases. Their structure15 can be viewed as an fcc framework of Pnictogens (P, N, As, Sb,..) having 12 available cationic sites per formula unit, with Li and Ti statistically partially occupying the 8 tetrahedral and four octahedral sites. They are usually synthesized by
-type phases hold great potential with respect to applications in Li-ion cells. However, the challenge consists in synthesizing such compounds as single phases. Their structure15 can be viewed as an fcc framework of Pnictogens (P, N, As, Sb,..) having 12 available cationic sites per formula unit, with Li and Ti statistically partially occupying the 8 tetrahedral and four octahedral sites. They are usually synthesized by  introducing, within an argon dry box, a mixture of stoichiometric amounts of
introducing, within an argon dry box, a mixture of stoichiometric amounts of  Ti, and P powders in an arc welded SS container,
Ti, and P powders in an arc welded SS container,  placing the container into an oven, whose temperature is raised to 950°C at 15°C per hour, and held at this temperature for 48 h, and
placing the container into an oven, whose temperature is raised to 950°C at 15°C per hour, and held at this temperature for 48 h, and  quenching the tube in air. The resulting crystallized powders were always found (Fig. 4a) to be contaminated by second phases, primarily
quenching the tube in air. The resulting crystallized powders were always found (Fig. 4a) to be contaminated by second phases, primarily  TiP, and
TiP, and  whose cumulative amounts always exceeded 25%, despite efforts to modify the annealing protocol or lithium stoichiometry. Hence, the need to search for a different synthesis path to fully exploit the Li reactivity within these phases was clear. In continuing our demonstration of the positive attributes of Li powder ballmilling to synthesize Li-based electrodes, we decided to extend this method to the preparation of
whose cumulative amounts always exceeded 25%, despite efforts to modify the annealing protocol or lithium stoichiometry. Hence, the need to search for a different synthesis path to fully exploit the Li reactivity within these phases was clear. In continuing our demonstration of the positive attributes of Li powder ballmilling to synthesize Li-based electrodes, we decided to extend this method to the preparation of 
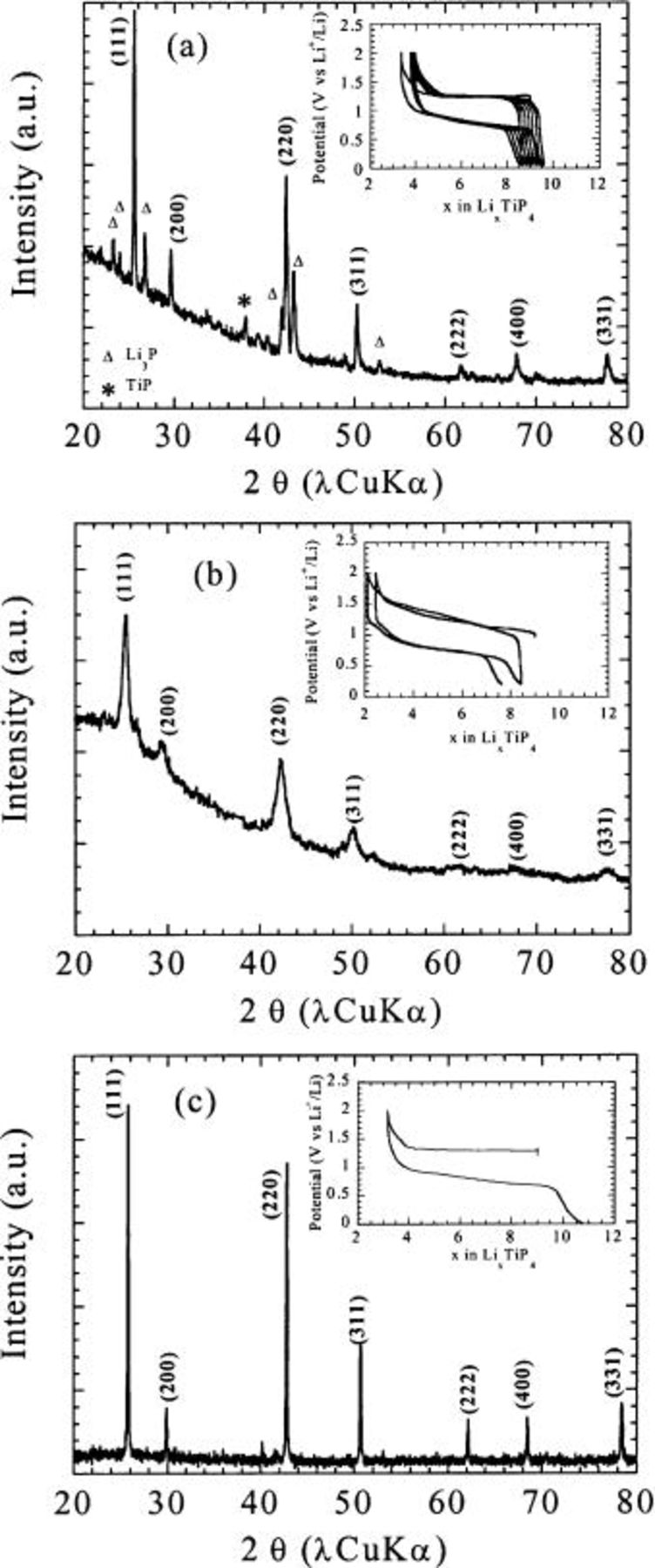
Figure 4. X-ray powder patterns of  made by (a) high temperature synthesis, by (b) ballmilling, and by (c) ballmilling followed by 500°C post-treatment, together with their corresponding voltage composition curves. Note the single phase character of the ballmilled samples and the post treatment benefit on sample crystallinity.
made by (a) high temperature synthesis, by (b) ballmilling, and by (c) ballmilling followed by 500°C post-treatment, together with their corresponding voltage composition curves. Note the single phase character of the ballmilled samples and the post treatment benefit on sample crystallinity.
The experiments were carried out by mixing, within an argon dry-box, raw powders of Ti, P, and Li elements in the right stoichiometry to reach the  reported composition.14 The mixture was placed into an SS vial containing an SS ball whose weight was 10 times greater than the mass of the initial powder mixture. The optimum milling parameters were determined, and the best results were obtained for a grinding time of 12 consecutive hours at room temperature. The XRD powder pattern for the BM powder (Fig. 4b) showed Bragg peaks reminiscent of the
reported composition.14 The mixture was placed into an SS vial containing an SS ball whose weight was 10 times greater than the mass of the initial powder mixture. The optimum milling parameters were determined, and the best results were obtained for a grinding time of 12 consecutive hours at room temperature. The XRD powder pattern for the BM powder (Fig. 4b) showed Bragg peaks reminiscent of the  phase with an
phase with an  axis of 6.02(2) Å only, as compared to 5.96 Å for the reported literature value (JCPDS 21-0529). Although such a lattice parameter value (6.02 Å) is not accurately determined due to the limited number of broad Bragg peaks used to extract it, we believe its difference with the reported one (5.96 Å) to be meaningful and indicative of the high density of lattice/structural defects introduced during the BM process. Surprisingly, no second phase could be detected, either by X-rays or by TEM measurements (not shown here), suggesting once again the profound advantages of the BM synthesis. However, caution has to be exercised in over-interpreting such a result, since the samples could be contaminated by amorphous phases.
axis of 6.02(2) Å only, as compared to 5.96 Å for the reported literature value (JCPDS 21-0529). Although such a lattice parameter value (6.02 Å) is not accurately determined due to the limited number of broad Bragg peaks used to extract it, we believe its difference with the reported one (5.96 Å) to be meaningful and indicative of the high density of lattice/structural defects introduced during the BM process. Surprisingly, no second phase could be detected, either by X-rays or by TEM measurements (not shown here), suggesting once again the profound advantages of the BM synthesis. However, caution has to be exercised in over-interpreting such a result, since the samples could be contaminated by amorphous phases.
Thus, to clarify this issue, the ballmilled samples were annealed at various temperatures and success was achieved by obtaining crystallized samples with  devoid of any impurity phases (Fig. 4c) at temperatures as low as 300 and 500°C. The sample Li composition could not be ascertained accurately, since it was reported2
16 that changing the Li content from
devoid of any impurity phases (Fig. 4c) at temperatures as low as 300 and 500°C. The sample Li composition could not be ascertained accurately, since it was reported2
16 that changing the Li content from  to 9 for the phosphide phase did not change the
to 9 for the phosphide phase did not change the  axis by more than 0.05%. Therefore, an
axis by more than 0.05%. Therefore, an  value of 8.5 was determined by atomic absorption analysis. Annealing at temperatures greater than 900°C did result in the presence of impurity phases similar to those observed for the high temperature synthesis method, suggesting the metastability of
value of 8.5 was determined by atomic absorption analysis. Annealing at temperatures greater than 900°C did result in the presence of impurity phases similar to those observed for the high temperature synthesis method, suggesting the metastability of  and once again stressing the advantage of BM.
and once again stressing the advantage of BM.
We performed electrochemical tests using  half cells to further validate the mechanical milling-induced formation of pure
half cells to further validate the mechanical milling-induced formation of pure  phase. The voltage/composition curves for
phase. The voltage/composition curves for  cells made of either the high temperature, room temperature ball-milled, or 500°C reannealed ballmilled sample, as the positive electrode, are shown in Fig. 4a, b, and c, respectively. Note that whatever the sample elaboration process, more than 6 lithium atoms can react reversibly with
cells made of either the high temperature, room temperature ball-milled, or 500°C reannealed ballmilled sample, as the positive electrode, are shown in Fig. 4a, b, and c, respectively. Note that whatever the sample elaboration process, more than 6 lithium atoms can react reversibly with  yielding capacities of at least 700 mAh/g, twice as much as the capacities obtained from carbonaceous materials. However, these samples exhibit differences in the voltage profile during oxidation. While the high temperature and the 500°C BM sample showed a well-defined plateau on oxidation, the room temperature ballmilled sample was devoid of this feature. This is mainly due to the nanosized character of the room temperature ballmilled produced particles, which generates many new indefinite interfaces with various interfacial energies, resulting in some potential distribution. In contrast, the origin of an oxidation plateau on charge as compared to a sloppy voltage discharge for the 500°C ballmilled and heat-treated sample is nested in amorphization/recrystallization matters, as discussed in a separate paper.14 Finally, though not plotted herein, the capacity retention of BM samples did not exhibit any improvement compared to the HT ones. We could only achieve 20 cycles at C/5 while maintaining 80% of the initial capacity. Therefore, efforts still have to be made toward the optimization of such recently identified electrode materials.
yielding capacities of at least 700 mAh/g, twice as much as the capacities obtained from carbonaceous materials. However, these samples exhibit differences in the voltage profile during oxidation. While the high temperature and the 500°C BM sample showed a well-defined plateau on oxidation, the room temperature ballmilled sample was devoid of this feature. This is mainly due to the nanosized character of the room temperature ballmilled produced particles, which generates many new indefinite interfaces with various interfacial energies, resulting in some potential distribution. In contrast, the origin of an oxidation plateau on charge as compared to a sloppy voltage discharge for the 500°C ballmilled and heat-treated sample is nested in amorphization/recrystallization matters, as discussed in a separate paper.14 Finally, though not plotted herein, the capacity retention of BM samples did not exhibit any improvement compared to the HT ones. We could only achieve 20 cycles at C/5 while maintaining 80% of the initial capacity. Therefore, efforts still have to be made toward the optimization of such recently identified electrode materials.
Conclusions
We showed that Li electrochemically driven displacement reactions, which involve extensive bond breakage, atomic reorganization, and formation of new bonds can be mimicked by Li-assisted mechanical milling. Similarly, we illustrate the benefits of ballmilling in the presence of Li powder to synthesize, under mild conditions, new metastable Li-based phases. Therefore, these BM-made phases suffer from poor capacity retention and further work is required on that issue. Overall, these ballmilling-driven Li reduction reactions are of great value in screening new materials, since they are free of the complexities associated with high temperature processes in sealed environments. Needless to say, such an approach is being widely implemented with a wide variety of materials.
Acknowledgments
The authors would like to give special thanks to L. Dupont for enabling TEM studies, and L. Aymard, A. Rougier and R. Janot for technical discussions.
Université de Picardie Jules Verne assisted in meeting the publication costs of this article.