Abstract
Proton conducting intermediate temperature (400–700°C) solid oxide fuel cells (IT-SOFC) have attracted great interest recently. However, for SOFC operation at intermediate temperature, cathode is often considered to be the main factor limiting the overall cell performance. In this study, Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF), which is one of the most active cathode materials and shows significant hydration effect suggesting possible proton conductivity, was investigated as the cathode for proton conducting IT-SOFC at temperature down to ∼450°C. The stability and compatibility of BSCF cathode with BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb), one of the leading proton conducting electrolyte materials, were studied at intermediate temperature in various atmospheres. In addition, experiments were carried out to understand the electrochemical behaviors of BSCF as a mixed proton, oxide ion and electron conducting cathode using BSCF/BZCYYb/BSCF symmetrical cells in simulated air containing different concentrations of water vapor (H2O) and carbon dioxide (CO2), especially at lower temperature of ∼450°C. The results are analyzed from the underlying oxygen electrode reaction mechanism point of view, and the direction for future research to further improve the cathode for proton conducting IT-SOFC is pointed out.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
In recent years, intermediate temperature (400–700°C) solid oxide fuel cells (IT-SOFC) have drawn increasing interest due to the possibility of achieving slower degradation during long-term operation, cheaper sealing and interconnect material choices, as well as higher overall thermodynamic efficiency compared to conventional high temperature (≥ ∼750°C) solid oxide fuel cells (HT-SOFC).1,2 For IT-SOFC, conventional oxide ion electrolyte of yttria-stabilized zirconia (YSZ) suffers from low ionic conductivity,3,4 which dictates unreasonably thin electrolyte and high cost,5,6 while alternative oxide ion electrolytes, such as gadolinium doped ceria (GDC) or samarium doped ceria (SDC), suffer from high electronic transport number, which lowers open circuit voltage (OCV) and system efficiency despites their significantly improved ionic conductivity at intermediate temperature.7–9 In comparison, proton conducting oxides, many of which are perovskite-structured, offer higher ionic conductivity with low electronic transport number as well as lower activation energy at intermediate temperature.10–16 As a result, they are regarded as the preferred electrolyte for IT-SOFC.17
For IT-SOFC, the cathode process is generally regarded as the rate-limiting step due to its high activation energy compared to the electrolyte. In particular, for proton conducting IT-SOFC, there have been a number of perovskite oxides employed as potential cathodes such as La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF), Sm0.5Sr0.5CoO3-δ (SSC), and BaCo0.4Fe0.4Zr0.1Y0.1O3-δ (BCFZY).18–22 Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF), which is considered to be one of the most active cathode materials for oxide ion conducting IT-SOFC,23 could also be a promising cathode material for proton conducting IT-SOFC because of its capability of undertaking significant amount of water and becoming proton conductive at intermediate temperature of ∼600°C and below.24–27 In fact, various researchers have already explored BSCF as the cathode for proton conducting IT-SOFC and obtained varying degrees of success.18,20,28–30 However, there are still many unknown aspects left. For example, the exact influence of water vapor on the cathodic process for such proton conducting SOFC, especially at even lower temperatures of ∼400–500°C is still not clear. On the other hand, the influence of carbon dioxide (CO2), which is always present in air, on the cathode electrochemical behavior for such proton conducting IT-SOFC is also uncertain despite evidence showing questionable stability of BSCF against CO2 in SOFC operation at intermediate temperature.7 In addition, how would the co-presence of H2O and CO2, which is a more realistic situation for practical SOFC operation, influence the electrochemical behavior for proton conducting SOFC with BSCF cathode has never been studied to the best of the authors' knowledge.31,32
In this work, the chemical stability and compatibility of the BSCF cathode with one of the leading proton conducting electrolytes BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb)10–12,14,15 under various conditions were studied. More importantly, BSCF/BZCYYb/BSCF cathode symmetrical cells were used to investigate the influence of water vapor and CO2 on the electrochemical behaviors for the proton conducting IT-SOFC at various temperatures ranging from 650°C to 400°C. The implication of the experimental observations on the underlying oxygen electrode reaction mechanism for proton conducting IT-SOFC versus conventional oxide ion conducting SOFC is discussed, and the direction for future research on designing better cathode for proton conducting IT-SOFC is pointed out.
Experimental
Powder synthesis
Both BZCYYb powder with nominal composition of BaZr0.1Ce0.7Y0.1Yb0.1O3-δ and BSCF powder with nominal composition of Ba0.5Sr0.5Co0.8Fe0.2O3-δ were synthesized by glycine nitrate process (GNP).33 For BZCYYb, stoichiometry amounts of Ba(NO3)2 (#A11305, Alfa Aesar, 99%), ZrO(NO3)2•xH2O (#43224, Alfa Aesar, 99.9%), Ce(NO3)3•6H2O (#11329, Alfa Aesar, 99.5%), Y(NO3)3•6H2O (#12898, Alfa Aesar, 99.9%), and Yb(NO3)3•xH2O (#12901, Alfa Aesar, 99.9%) powders were dissolved in DI water. Then glycine (#G8898, Sigma Aldrich, 99+ %) was added to the solution with molar ratio between glycine and total metal ions of 1:1. The solution was stirred for about 30 minutes in a 2-liter beaker on a hot plate at ∼100°C in order to dissolve the various salts completely. The obtained transparent solution was then heated up on the hot plate set at ∼540°C. Rapid self-combustion process occurred after water was evaporated, and the very fine white powder generated after combustion was collected and calcined in ambient air at 1100°C for 5 hours to form pure BZCYYb perovskite phase. (It is noted that right after combustion, the powder obtained was not BZCYYb, but doped CeO2 and amorphous phases, as reported elsewhere.14) In the case of BSCF powder, the major steps for powder synthesis are similar to that for BZCYYb, except the starting materials now also include Fe(NO3)3·9H2O (#216828, Alfa Aesar, 99%) and Co(NO3)2·6H2O (#239267, Alfa Aesar, 99%), while the molar ratio between glycine and total metal ions was changed to 7:6 for complete combustion. After self-combustion, the powder was calcined at 1000°C for 2 hours in ambient air to form the pure phase.29
Chemical compatibility and stability test
For chemical compatibility test between BSCF and BZCYYb in typical cell fabrication process, intimately mixed powders of the two materials with weight ratio of 1:1 were exposed to ambient air at typical BSCF cathode firing temperature of 1000°C for 5 hours. To test the stability and compatibility of the BSCF and BZCYYb mixture under typical IT-SOFC operation conditions, the powder mixtures were exposed at 450°C in ambient air, humidified nitrogen with nominal composition of 3% H2O/97% N2 (gas compositions are all by volume in this study), and a gas mixture of ∼1% CO2/99% N2 for various time from 24 to 72 hours. Furthermore, the chemical stability of the BZCYYb electrolyte against 1%CO2/99% N2 was also investigated at 750°C by exposing BZCYYb powder to that gas mixture for 24 hours. The samples after various chemical stability/compatibility tests were analyzed by X-Ray diffraction (SIEMENS diffractometer D5000) for phase identification.
BSCF/BZCYYb/BSCF symmetrical cells fabrication
Cathode symmetrical cells with the configuration of BSCF/BZCYYb/BSCF were fabricated. First, electrolyte pellets with diameter of 10 mm were dry-pressed at 200 MPa using 0.2 g BZCYYb powder followed by protected sintering at 1550°C for 5 hours.14 Then, the BSCF slurry was prepared by mixing BSCF powder with alpha-terpineol (#16285, Alfa Aesar, 96%) solvent and organic binder at weight ratio of ∼65: 34: 1, and ball-milled for 24 hours. 4 × 4 mm2 BSCF electrodes were brush painted onto both sides of the sintered electrolyte pellets using the prepared slurry, fully dried in an air oven at 100°C, and calcined at 1000°C for 2 hours in ambient air with heating and cooling rate of 5°C/min. (It is noticed that the BSCF cathode fired at 900°C showed weak bonding to the electrolyte, while the cathode fired at 1000°C showed better bonding to the electrolyte. Thus only symmetrical cells with cathode firing temperature of 1000°C were used for electrochemical testing in this study.) The microstructure of the cross-section in the fabricated symmetrical cell was observed using a field emission scanning electron microscope (FE-SEM, JEOL JSM 6330F).
Electrochemical impedance spectroscopy (EIS) measure-ments
Electrochemical Impedance Spectroscopy (EIS) measurements were carried out using a potentiostat (Gamry Interface 1000) under open circuit condition for the symmetrical cells. The symmetrical cell with silver paste and silver mesh current collector was placed in the hot zone of a sealed quartz tube furnace. Dry simulated air (with the composition of 20% O2/80% N2 with <∼5 parts per million or ∼5 ppm of CO2 or H2O) at a flow rate of 200 ml min−1 was used as the baseline. To test the effect of water vapor on the oxygen electrode behavior, 3%, 10%, and 20% water vapor was introduced into the test chamber by passing the dry simulated air through a water bubbler set at different temperatures, and the connection tubes were heated to ∼100°C to prevent condensation. Before the tests, the symmetrical cell was heated to 750°C in dry simulated air and held for 24 hours to stabilize it. The sequence of the electrochemical experiments for evaluating the moisture effect is described as following: i) The cell was cooled from 750°C to the testing temperature (e.g., 650°C) in dry simulated air, held for 12 hours and then the impedance spectrum was collected. ii) After that, moisture was introduced into the simulated air first at 3% then increased to 10% and finally to 20%, and the impedance spectrum for each level of humidity was collected 2 hours after the adjustment of the moisture content. iii) The cell was then heated back up to 750°C in dry simulated air and held for 12 hours or longer to fully dehydrate the system (including both the cell and testing chamber). This sequence of step i) to ii) and then iii) was repeated for each testing temperature such as 650°C, 550°C, and 450°C to obtain electrochemical responses of the cell to different atmospheres. Similarly, to test the influence of CO2 on the oxygen electrode behavior, 1% CO2 was introduced into the simulated air through a mass flow controller and the impedance spectra for the cell were collected at a given temperature before and after 2 hours of the introduction of 1% CO2, as well as after the removal of CO2 for 24 hours. Before changing to a different testing temperature for CO2 response, the sample was always recovered in the dry simulated air (with <5 ppm CO2 as mentioned before) at 750°C for 24 hours to ensure a complete recovery and a clean surface of both the electrode and the electrolyte. To test the behaviors when the electrode is exposed to both H2O and CO2, which is a more realistic situation for fuel cell operation, the simulated air was passed through the water bubbler at room temperature and then mixed with CO2 to give a gas mixture with nominal composition of ∼1% CO2/3% H2O/20% O2/76% N2, and the sequence of these tests was similar to that for tests in 1% CO2 alone. All impedance data were collected with zero DC bias and AC amplitude of 0.1 mA.
Results
Compatibility and stability of BSCF and BZCYYb in various atmospheres
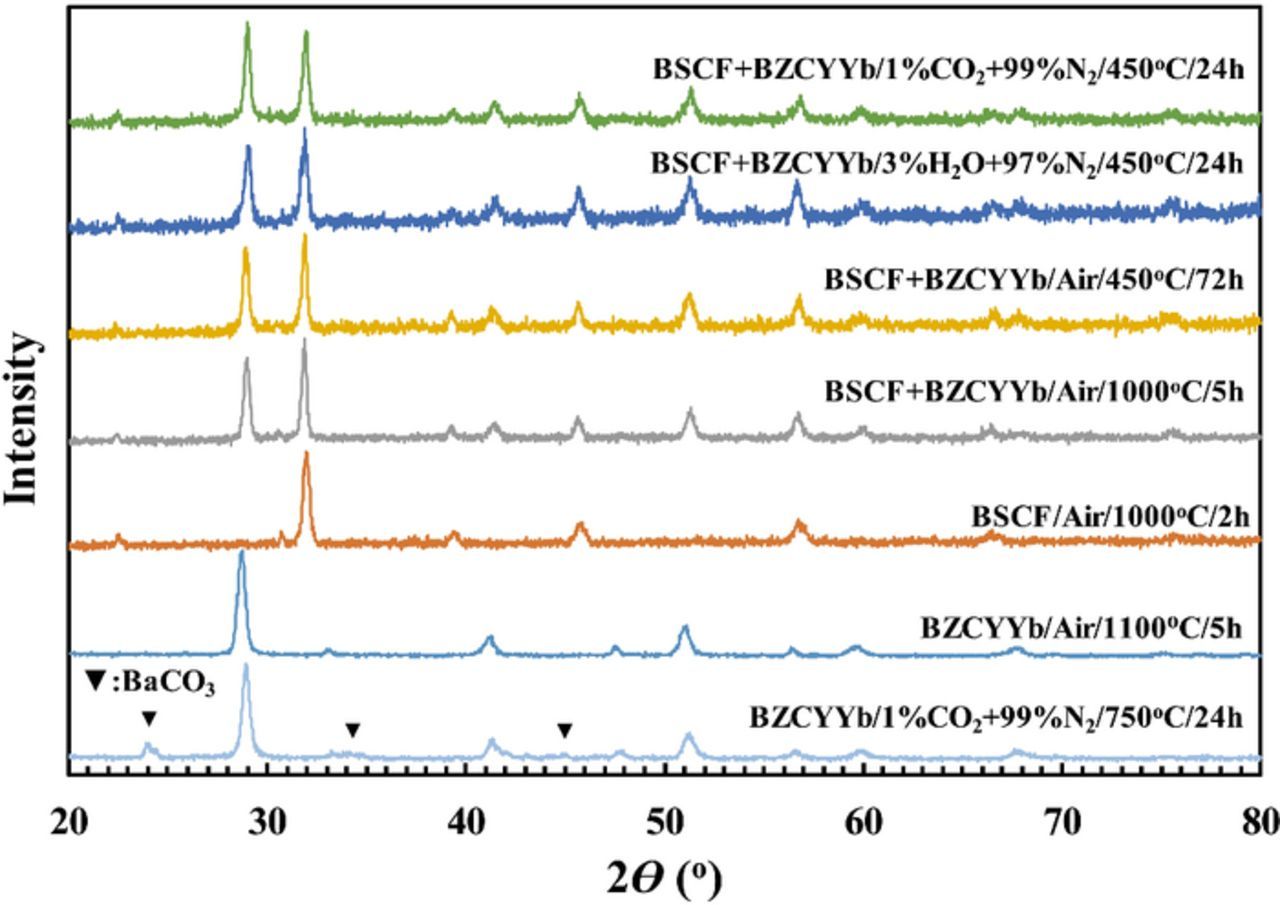
The XRD patterns for the as-synthesized BSCF and BZCYYb powders and their mixtures after various compatibility/stability tests are summarized in Fig. 1. (It's noted that the synthesized BSCF contains some minor unidentified secondary phase as evidenced by the extra peak at ∼31°.) The chemical compatibility between BSCF and BZCYYb was verified with XRD, showing no change for both BSCF and BZCYYb materials after heat-treatment at 1000°C for 5 hours. (Similar result was also obtained for compatibility test at 900°C.) In addition, as shown in Fig. 1, the chemical stability of the BSCF-BZCYYb powder mixture in both ambient air (i.e., containing ∼1–3% H2O and ∼400 ppm CO2) and in N2 containing up to 3% H2O and 1% CO2 at targeted IT-SOFC operating temperature of 450°C was also demonstrated, which is supported by the absence of impurity peaks in the XRD patterns after long time exposure to the various gas mixtures for 24–72 hours. It is worth mentioning that when the BZCYYb electrolyte with Zr doping at 0.1 level was exposed to the gas mixture of 1% CO2/99% N2 at 750°C for 24 hours, some BaCO3 did form, as shown in Fig. 1.
Figure 1. XRD patterns of as-synthesized BSCF and BZCYYb powders and their mixtures after different compatibility/stability tests.
Apart from testing for the compatibility and stability of the materials, the microstructure of a fabricated BSCF/BZCYYb/BSCF cathode symmetrical cell is shown in Fig. 2. The thickness of the BSCF electrode layer was around 30 μm. Relatively large particle size of ∼1–3 μm for the BSCF electrode was observed, which may be attributed to the high surface energy of BSCF and the strong tendency to coarsen.23,34,35 The porosity of the BSCF electrode was estimated to be ∼22% based on the analysis of SEM image using the software of ImageJ (version 1.50i).
Figure 2. SEM image of the cross-section of a fabricated BSCF/BZCYYb/BSCF symmetrical cell.
Effect of moisture on BSCF cathode electrochemical behavior
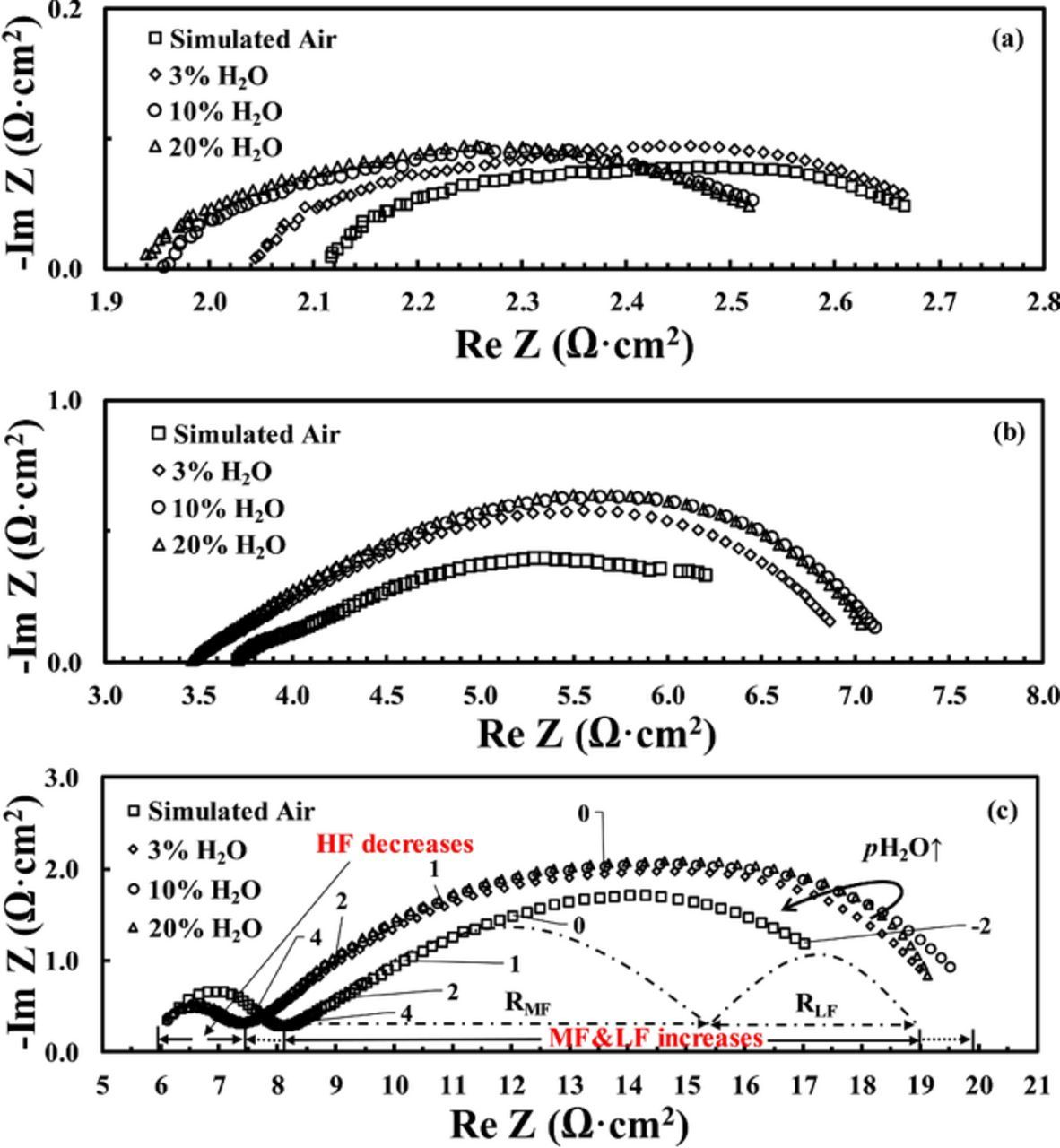
Figs. 3a–3c show the impedance spectra for the BSCF/BZCYYb/BSCF cathode symmetrical cell in the dry simulated air (as explained, it is a gas mixture of 20% O2 and 80% N2 with <∼5 ppm H2O and CO2 as supplied from Airgas) and simulated air humidified with ∼3%, ∼10%, and ∼20% of moisture at 650°C, 550°C, and 450°C, respectively. At 650°C, as shown in Fig. 3a, with the introduction of 3% H2O, the ohmic resistance RO decreases from 2.11 Ohm•cm2 to 2.04 Ohm•cm2. As the moisture content increases further to 10% and 20%, a continued decreasing of RO was observed. On the other hand, for the electrode interfacical polarization resistance Rp, due to the nature of mixed ionic and electronic conduction for the BZCYYb electrolyte and the absence of precise ionic transference number,36 accurate Rp number cannot be obtained readily. As a result, only the apparent interfacial resistance Rai, which is the direct difference between the low frequency intercept and the high frequency intercept on the real axis in an impedance spectrum, is used for the analysis and discussions in this study as in many previous reports.25,37 It can be seen from Fig. 3a that comparing with dry simulated air, Rai increases from ∼0.6 Ohm•cm2 to ∼0.7 Ohm•cm2 with the introduction of 3% moisture. However, with further increase of H2O concentration to 10% and beyond, Rai starts to decrease back to ∼0.64 Ohm•cm2 and stabilizes. It is noted that the observation of the decrease of Rai with increasing moisture content beyond 3% H2O for BSCF cathode over BaCe0.9Y0.1O3-δ (BCY10) proton conducting electrolyte had been reported before.25 Nevertheless, to the best of the authors' knowledge, the initial increase in Rai from dry simulated air to 3% humidified air for proton conducting IT-SOFC with BSCF cathode has not been reported before, and such an observation implies that the introduction of moisture seems to slow down at least certain part(s) of the oxygen electrode reaction for the BSCF/BZCYYb/BSCF cathode symmetrical cell.
Figure 3. Impedance spectra for a BSCF/BZCYYb/BSCF symmetrical cell in dry simulated air (20%O2/80%N2 with <∼5ppm H2O or CO2) versus simulated air humidified with various concentrations of moisture at (a) 650°C, (b) 550°C, and (c) 450°C, respectively.
At reduced temperature of 550°C, which is shown in Fig. 3b, when 3% moisture was introduced, RO still decreases; however, no continued decreasing of RO was observed with the further increase in moisture content beyond 3%, suggesting saturation of the hydration effect by 3% of moisture at that temperature. On the other hand, an increase in Rai was observed with the introduction of 3% moisture compared with dry simulated air, and, in contrast to the observations at 650°C, total Rai does not show a decrease when moisture concentration was further increased to 10% and beyond.
When the temperature was further reduced to 450°C, the overall impedance spectra (shown in Fig. 3c) clearly separate into one semicircle at the high frequency (HF, 106∼104 Hz) range and one large depressed semicircle, which most likely represents two overlapped arcs-one at the middle frequency (MF, ∼104 to ∼100 Hz) range and the other at the low frequency (LF, ∼100 to 10−2 Hz) range. At this temperature, when 3% moisture was introduced, the change in RO becomes negligible. On the other hand, for Rai, the HF part decreases significantly, while the MF and LF parts show an obvious increase with the introduction of moisture. In addition, the effect of moisture seems to reach saturation at 3%, as further increase in moisture content does not produce significant differences at 450°C.
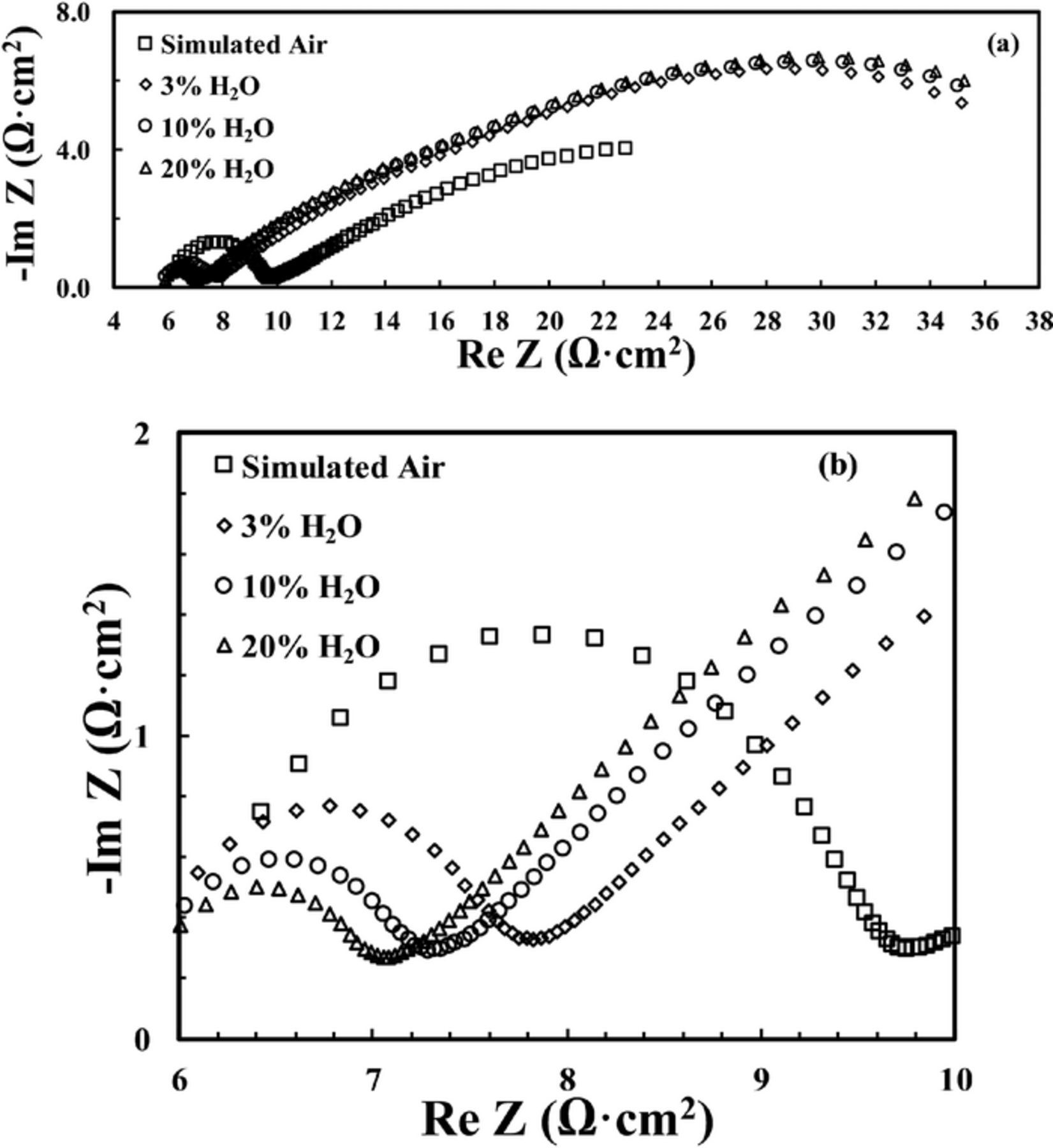
Additional EIS measurements were conducted at 500–400°C because of the clear separation of impedance spectra into two semicircles (HF and MF-LF) in that temperature range. Gradual decrease in the HF semicircle was observed with increasing concentration of moisture as shown, for example, in Fig. 4 for 475°C. For comparison, impedance spectra for another symmetrical cell in both pure O2 and dry simulated air at 450°C are given in Fig. 5a, which shows almost no difference in the size of the HF semicircle, while a significant increase was observed in the MF-LF semicircle in dry simulated air versus in pure O2. On the other hand, as in Fig. 5b, when moisture content was increased in O2, the HF semicircle shows the similar gradual decrease.
Figure 4. Impedance spectra for a BSCF/BZCYYb/BSCF symmetrical cell at 475°C in dry and wet (with various moisture content) simulated air: (a) the full impedance spectra, (b) zoom in to show the changes at the high frequency (HF) portion.
Figure 5. Impedance spectra for a BSCF/BZCYYb/BSCF symmetrical cell at 450°C: (a) comparison of entire impedance spectra between dry simulated air and dry O2 including zoom-in of the high frequency (HF) part, (b) zoom-in of the impedance spectra for O2 with various concentrations of moisture showing the high frequency (HF) part, (c) comparison of impedance spectra between 3% humidified simulated air and 3% humidified O2.
Effect of CO2 on BSCF cathode electrochemical behavior
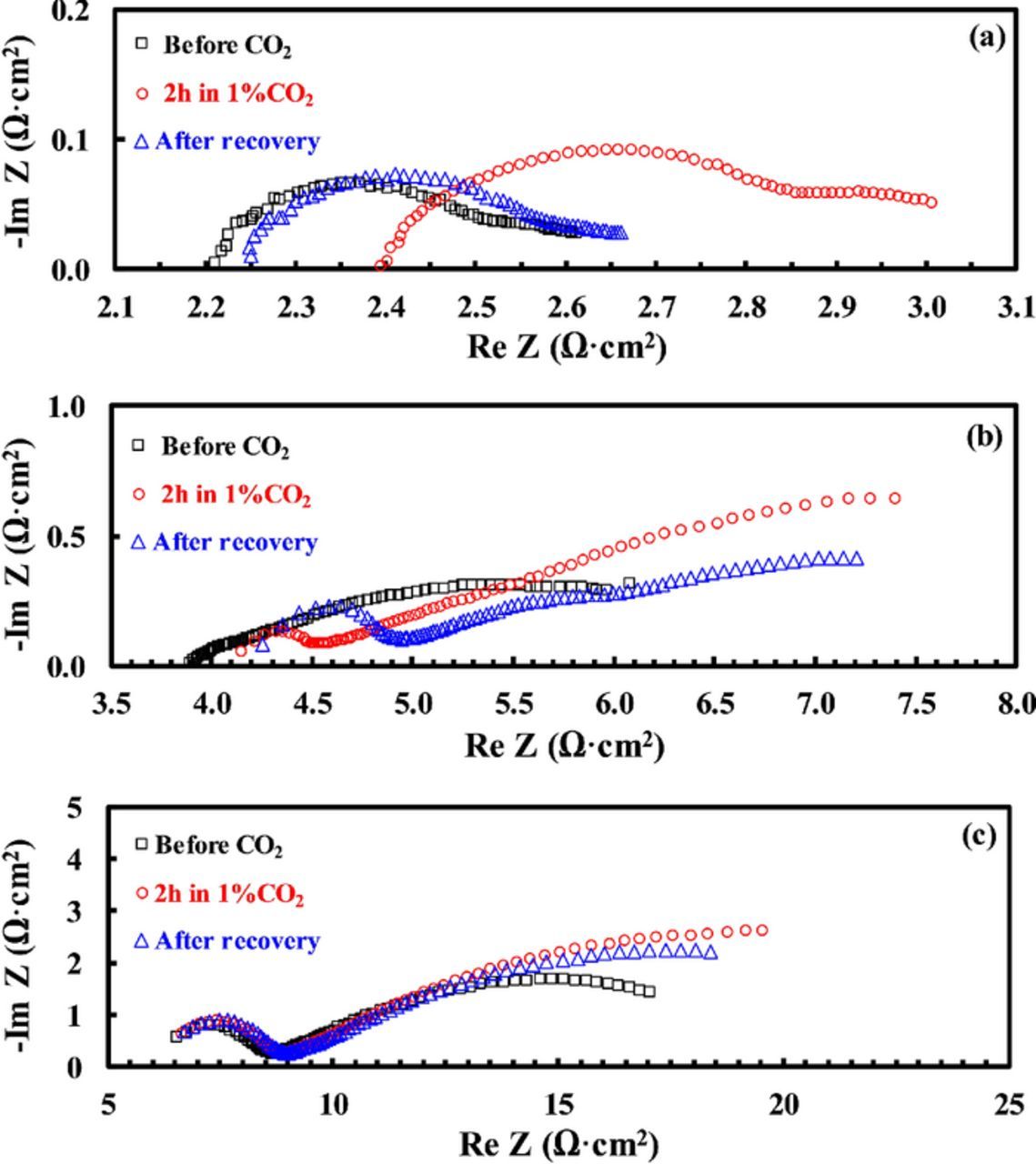
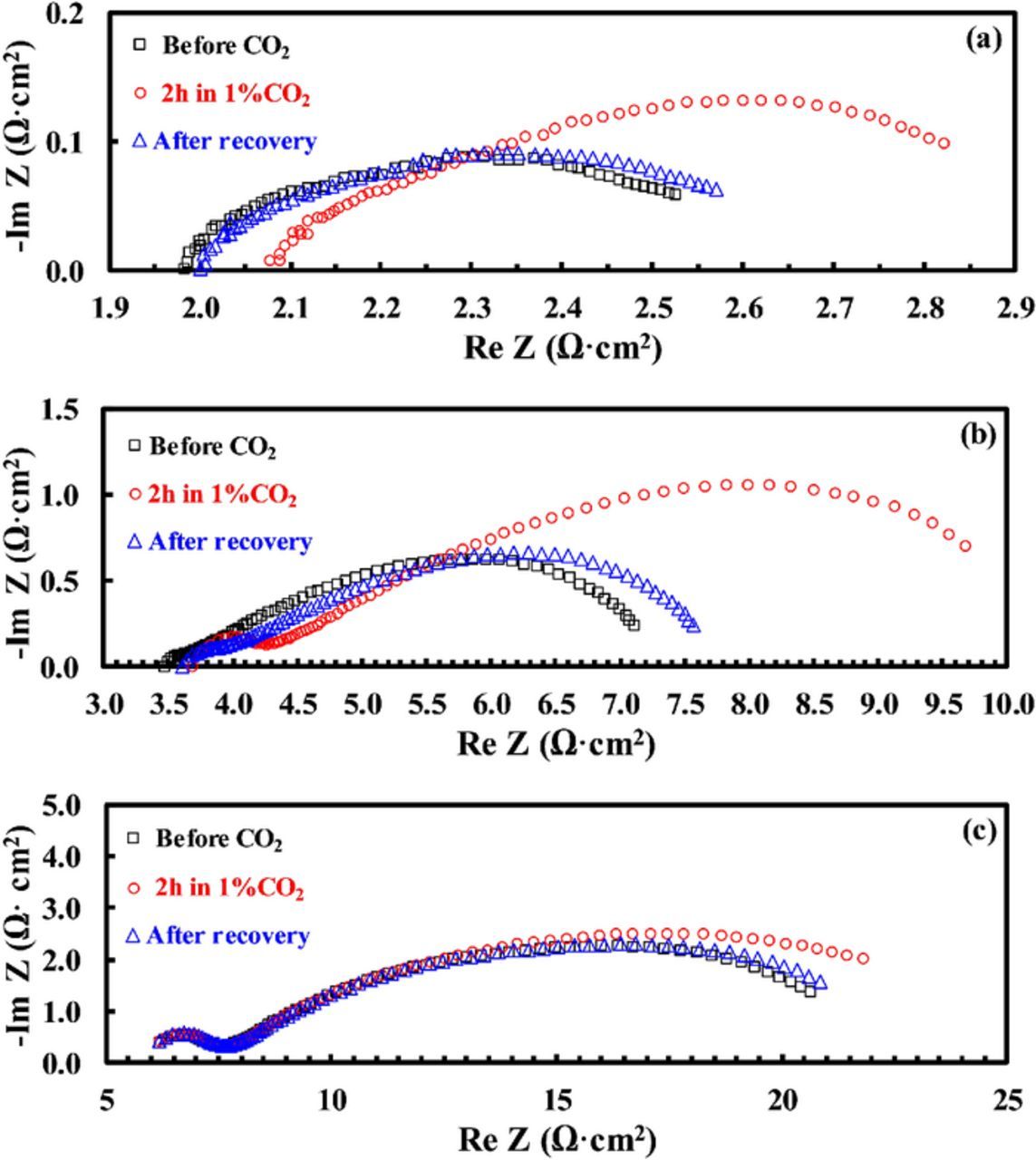
The effect of CO2 alone (i.e., without the presence of moisture) on the BSCF/BZCYYb/BSCF symmetrical cell at 650°C, 550°C, and 450°C is shown in Fig. 6. At 650°C, as shown in Fig. 6a, increase in RO from 2.21 ohm·cm2 to 2.39 ohm·cm2 and in Rai from 0.49 ohm·cm2 to 0.72 ohm·cm2, respectively, were observed after the introduction of 1% CO2 into the dry simulated air for 2 hours. After the removal of CO2 for 24 hours, almost complete recovery in both RO and Rai was observed. At 550°C, as shown in Fig. 6b, both RO and Rai increased after the introduction of 1% CO2, and the relative increase in Rai due to CO2 poisoning becomes larger comparing with 650°C. Also, only incomplete recovery was observed with the removal of CO2 even after 24 hours. At further reduced temperature of 450°C, little change in RO was observed with the introduction of 1% CO2, while large increase in Rai was still observed. In addition, because of the clear separation of the impedance spectra into one semicircle at the HF range and another at the MF-LF range, the increase in Rai due to CO2 at 450°C occurs almost exclusively to the MF-LF part. At this temperature, very little recovery was observed after the removal of CO2 for 24 hours.
Figure 6. Plots showing the change in impedance spectra for a BSCF/BZCYYb/BSCF symmetrical cell in dry simulated air (20% O2/80% N2) before exposure to CO2, after exposure to 1% CO2 for 2 h, and after recovery (i.e., removal of 1% CO2) for 24 hours at (a) 650°C, (b) 550°C and (c) 450°C, respectively.
In comparison, the effect of 1% CO2 for 3% humidified simulated air on the BSCF/BZCYYb/BSCF symmetrical cell at 650, 550, and 450°C is shown in Fig. 7. At 650°C, almost the same behavior was observed as both RO and Rai increase with the introduction of 1% CO2, and they are largely recoverable with the removal of CO2. However, at lower temperatures of 550 and 450°C, it is seen that the presence of moisture significantly improves the reversibility for CO2 poisoning. In fact, at 450°C, the presence of 3% moisture makes the cathode much less sensitive to 1% CO2. To illustrate the results better, based on the collected impedance spectra (as shown in Figs. 6 and 7), the estimated value of RO and Rai are summarized in Table I, as well as their relative changes after being poisoned for 2 hours and after the recovery by removing 1% CO2 for 24 hours in both dry and 3% humidified simulated air.
Figure 7. Plots showing the change in impedance spectra for a BSCF/BZCYYb/BSCF symmetrical cell in 3% humidified simulated air before exposure to CO2, after exposure to 1% CO2 for 2 hours, and after recovery (i.e., removal of 1% CO2) for 24 hours at (a) 650°C, (b) 550°C and (c) 450°C, respectively.
Table I. Ohmic resistance RO and apparent interfacial resistance Rai for the BSCF/BZCYYb/BSCF cathode symmetrical cell before and after being poisoned by 1% CO2 for 2 hours and then recovery by removing 1% CO2 for 24 hours in both dry and 3% humidified simulated air. The relative change values (i.e., ΔRO/RO and ΔRai/Rai), as given in brackets, are calculated against the baseline values obtained from before the 1% CO2 poisoning.
| 650°C | 550°C | 450°C | |||||
|---|---|---|---|---|---|---|---|
| Temperature Condition | Dry | 3%H2O | Dry | 3%H2O | Dry | 3%H2O | |
| RO (Ω·cm2) and ΔRO/RO | Before Poisoning | 2.21 | 1.98 | 3.89 | 3.46 | 6.08 | 5.76 |
| After poisoning | 2.39 (+8.1%) | 2.07 (+4.5%) | 4.15 (+6.6%) | 3.68(+6.4%) | 6.24 (+2.6%) | 5.70 (−1.0%) | |
| After 24 h recovery | 2.25 (+1.8%) | 2.00 (+1.0%) | 4.26 (+9.5%) | 3.61 (+4.3%) | 6.28 (+3.2%) | 5.66 (−1.7%) | |
| Rai (Ω·cm2) and ΔRai/Rai | Before Poisoning | 0.49 | 0.72 | 2.99 | 3.83 | 14.07 | 16.20 |
| After poisoning | 0.72 (+46.9%) | 0.96 (+33.3%) | 6.37 (+113%) | 6.76 (+76.5%) | 24.64 (+75.1%) | 20.53 (+26.7%) | |
| After 24 h recovery | 0.49 (0.0%) | 0.79 (+9.7%) | 4.87 (+62.9%) | 4.17 (+8.9%) | 21.08 (+49.8%) | 17.32 (+6.9%) | |
Discussion
The results from compatibility and chemical exposure tests show that at the targeted proton conducting IT-SOFC operating temperature of ∼450°C, the combination of BSCF cathode and BZCYYb electrolyte has demonstrated the desired compatibility in processing and stability in practical air. However, the observation of reactivity of BZCYYb electrolyte to 1% CO2/99% N2 to form BaCO3 at 750°C seems to contradict the earlier study showing stability of BZCYYb in a gas mixture of 50% CO2/50% H2 at 750°C.19 Whether such a discrepancy is due to the different balance gas (N2 versus H2) used, or the variation in sample stoichiometry (e.g., Ba to (Ce+Zr+Y+Yb) molar ratio), or other factors is not clear at the moment.
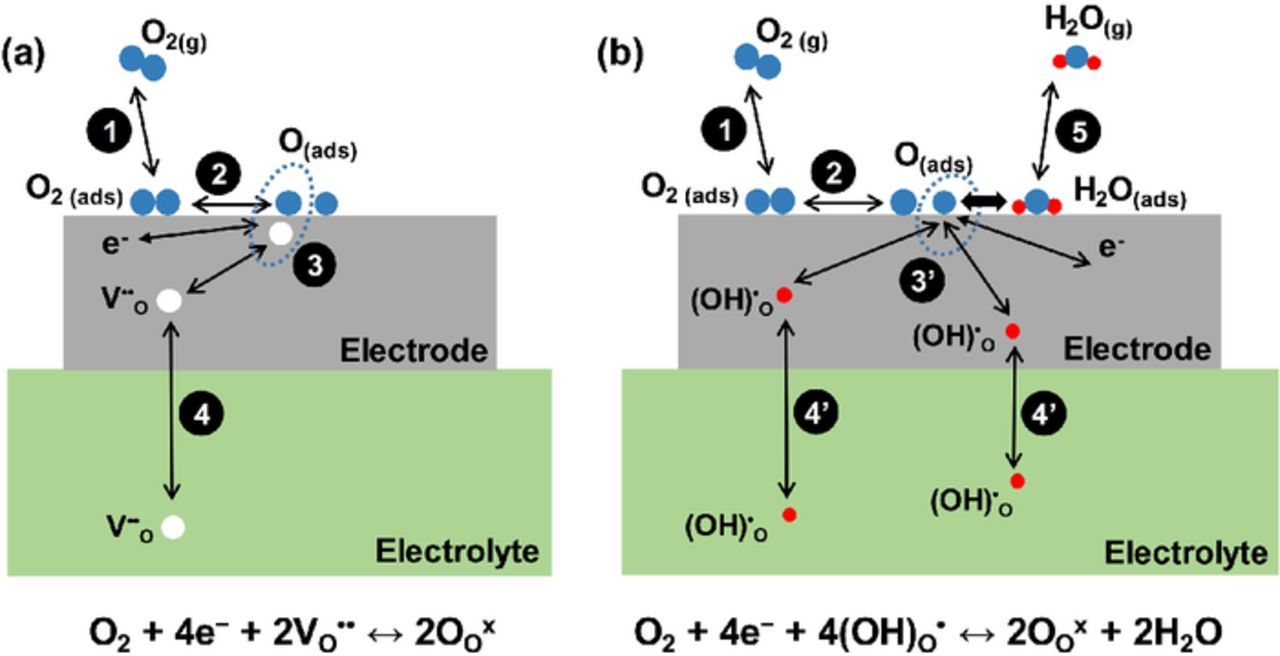
Besides that, in order to understand the various observed phenomena concerning the effects of moisture and CO2 on the electrochemical behaviors for the BSCF/BZCYYb/BSCF symmetrical cell, fundamental oxygen electrode reaction processes are first summarized. Shown in Fig. 8a is the conventional cathode reaction pathway for an ideal oxide ion based SOFC with a mixed ionic and electronic conductor (MIEC) electrode. In comparison, shown in Fig. 8b is the ideal cathode reaction pathway for a "pure" proton conducting SOFC with MIEC electrode, which means the electrolyte is fully hydrated and conducts only proton while the electrode conducts electron (hole) and proton upon hydration. The elementary steps corresponding to the illustrated pathways are summarized in Table II.
Figure 8. Schematics showing the reaction species involved and the elementary steps (also refer to Table II) for the reversible oxygen electrode reactions for (a) ideal oxide-ion based SOFC with MIEC electrode and (b) ideal "pure" proton conducting SOFC with MIEC electrode.
Table II. Elementary steps of the reversible oxygen electrode reactions for the ideal oxide-ion based SOFC (step 1,2,3,4) and the ideal "pure" proton conducting SOFC (step 1,2,3',4',5), both with MIEC electrodes.
| Elementary Steps | Frequency range | |
|---|---|---|
| 1 Mass transfer of O2 molecule in gas phase and adsorption on electrode surface | O2(g)↔O2 (ads) | LF |
| 2 Adsorbed O2 molecule dissociation | O2 (ads)↔2O (ads) | MF |
| 3 Charge transfer for ideal oxide ion conducting electrolyte | O(ads) + V· ·O + 2e−↔OOX | HF |
| 3' Charge transfer for ideal pure proton conducting electrolyte | O (ads) + 2e− + 2OH·O↔H2O(ads) + 2OXO | HF |
| 4 Mass transfer of oxide ion in the bulk of electrode and electrolyte | V· ·O(electrode)↔VO(electrolyte)· · | Very HF (〉〉 106 Hz) |
| 4' Mass transfer of prton in the bulk of electrode and electrolyte | OH·O(electrode)↔OHO(electrolyte)· | Very HF (〉〉 106 Hz) |
| 5 H2O transport and desorption | H2O(ads)↔H2O(g) | LF |
For the conventional ideal oxide ion based SOFC, the overall oxygen electrode reaction follows:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/164/2/F81/revision1/d0001.gif)
When the electrode is MIEC, the overall oxygen electrode reaction consists of elementary steps (and their reverse steps) of 1) mass transport of O2 molecule in the gas phase and adsorption on the electrode surface; 2) dissociation of adsorbed O2 molecule into adsorbed oxygen atoms; 3) charge transfer and combining of lattice oxygen vacancy with surface adsorbed oxygen atom and electrons to form lattice oxygen; and 4) mass transport of oxide ion (oxygen vacancy) in the bulk of electrode and electrolyte.
In comparison, for the ideal pure proton conducting SOFC, in principle, the oxygen electrode reaction would follow a different pathway:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/164/2/F81/revision1/d0002.gif)
When the electrode is MIEC with proton as the sole ionic charge carrier, apart from the common elementary steps of O2 molecules gas phase transport and adsorption (step 1) and adsorbed O2 molecule dissociation (step 2), alternative elementary steps of 3') charge transfer and combining of proton with adsorbed oxygen atom and electrons to form water, and 4') mass transport of proton in the bulk of electrode and electrolyte, as well as 5) water molecule transport in the gas phase and adsorption/desorption on the electrode surface also need to be taken into consideration.
The actual system considered here would approach the ideal oxide-ion based system (Fig. 8a) in dry condition. On the other hand, when significiant concentration of moisture is present, proton is generated in the BZCYYb electrolyte as following:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/164/2/F81/revision1/d0003.gif)
The system would then approach the ideal "pure" proton-based system (Fig. 8b) in humidified condition especially when the moisture content is high (≥3%) and at lower temperature (e.g., ∼450°C and below) when the BZCYYb electrolyte and the BSCF electrode become fully hydrated with oxygen vacancy V••O replaced by proton OH•O.
As shown in Figs. 3a and 3b, the decrease in RO at 650 and 550°C with the introduction of moisture into the simulated air could be attributed to the hydration of the BZCYYb electrolyte and the change of conducting species from oxide ion to proton yielding higher ionic conductivity.19,36 However, at temperature below ∼500°C, negligible reduction in RO was observed (see Fig. 3c and Fig. 4). Such a phenomenon could be attributed to the enhanced affinity of proton conducting oxide electrolyte (BZCYYb here) for water below the temperature of ∼450°C,38,39 which is supported by the TGA from previous reports suggesting that the dehydration of proton conducting oxides and associated weight loss only occur significantly at temperature above ∼450°C.25 The implication is that despite the dry simulated air used, which is supposed to give moisture content of only ∼5 ppm, the actual moisture content in the system due to various leakage might be sufficient to hydrate the electrolyte and make it proton conductive at temperature of ∼450°C and below. Therefore, strictly speaking, the so-called "dry simulated air" is only a loosely used term to indicate that the moisture content is, qualitatively, much lower than the 3% used for comparison. Though the difference in actual moisture content between the so-called "dry simulated air" and 3% humidified air may not be large enough to influence the ohmic resistance at ∼450°C and below, it is, however, adequate to significantly impact the oxygen electrode processes, as discussed below.
For the apparent interfacial resistance Rai, in the temperature range of 650°C to 450°C, as shown in Fig. 3 and Fig. 4, the overall Rai seems to increase with the introduction of moisture, especially for the middle to low frequency (MF-LF) semicircle. Generally, the MF-LF semicircle is believed to be associated with the mass transport process of oxygen molecules and the oxygen adsorption/dissociation process on the BSCF electrode.40 The observed increase in that part upon moisture introduction could be attributed to the strong adsorption of H2O on the surface of BSCF electrode and the BZCYYb electrolyte, both of which have high affinity for water. This would result in the reduced number of active sites for the adsorption/dissociation of oxygen molecules. In addition, strongly adsorbed water molecules on the BSCF surface could also greatly impede the transport (or diffusion) of oxygen species on the electrode surface. Both effects would slow down the overall cathode reaction process, leading to the increased Rai, especially in the MF-LF range.
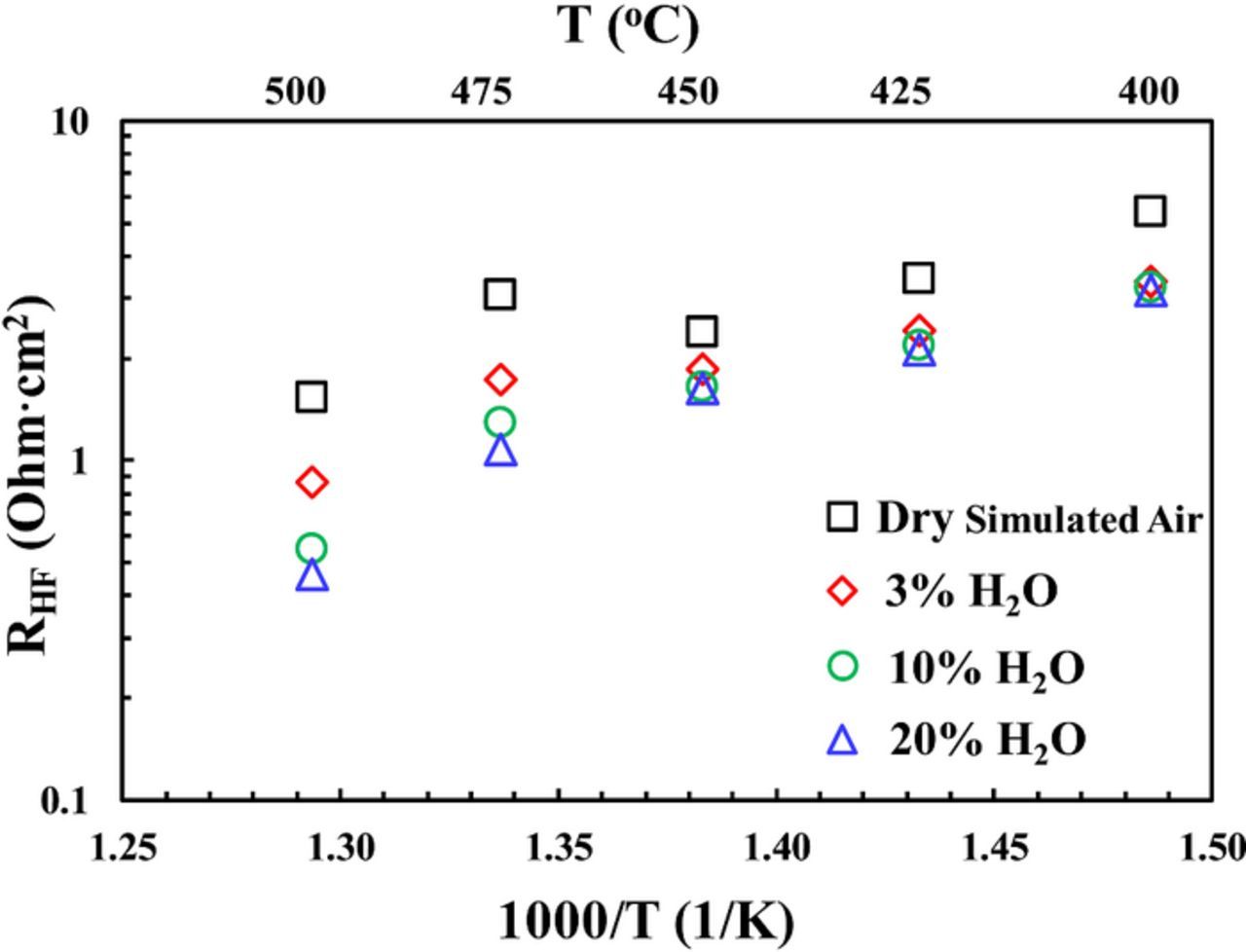
On the other hand, as shown in Figs. 3c and 4, an opposite trend with respect to the moisture effect was observed in the high frequency (HF) part of the impedance spectra. Fig. 9 summarizes the HF resistances obtained from 500°C to 400°C for a symmetrical cell in both dry simulated air and humidified air with different moisture content. It is observed that the decrease in HF resistance due to the introduction of moisture is more significant at higher temperature (e.g., 500 and 475°C) than at lower temperature (e.g., 450°C or below). Such results suggest that the activation energy for the HF resistance is significantly different in dry simulated air from those in humidified air. (It is noted that there appears to be a deviation at 475°C in Fig. 9. Whether it is due to experimental error or other factors is not clear at this moment and will be investigated in the future.) Therefore the HF semicircle is attributed to the resistance from the charge transfer step and not the grain boundary (GB), because previous study suggests that the activation energy for the GB resistance remains almost the same in humidified versus dry atmosphere.41 Such an assignment of the HF semicircle at temperature below ∼500°C to the charge transfer step is also consistent with literature.25,40
Figure 9. High frequency resistance RHF in dry simulated air versus simulated air containing up to 20% moisture at temperatures from 500 to 400°C (CO2 concentration <∼5 ppm).
It should be noted that similar behavior of decrease in HF semicircle with increase of moisture content from 3% to 10 and 20% in simulated air had been reported before for the sytem of Sm0.5Sr0.5CoO3-δ (SSC)-BaCe0.8Sm0.2O3-δ (BCS) composite cathode over BCS proton conducting electrolyte at 500°C,42 while in another study on the the system of BSCF over BaCe0.9Y0.1O3-δ (BCY10) electrolyte at 600°C,25 the total apparent interfacial resistance decreases with increasing moisture content from 3% to 30%. Nevertheless, to the best of the authors' knowledge, no previous study has systematically compared the BSCF cathode behavior over proton conducting electrolyte between dry and humified conditions, as reported here. The signficiant decrease in the HF semicircle for the oxygen electrode upon the introduction of moisture in the current study is hypothesized to be due to i) the intrinsically faster kinetics for the charge transfer step 3') via proton (Fig. 8b) comparing with the conventional charge transfer step 3) via oxide ion (Fig. 8a) and/or ii) the greater concentration of H2O(ads) reactant for the reverse reaction of step 3). Both explanations are consistent with the observation of a continued decrease of high frequency resistance RHF with increasing concentration of H2O, especially at temperatures of 475°C and 500°C (see Fig. 4b and Fig. 9): As moisture content increases, the BSCF electrode and the BZCYYb electrolyte can become more and more hydrated, leading to the continued increase in proton conduction and decrease in oxide ion conduction. On the other hand, the concentration of surface H2O(ads) would increase as well. Either way, the overall charge transfer process 3') would become faster with greater moisture content. Further study will be needed to clarify the exact origin for such a phenomenon.
Furthermore, such explanations could also be supported by the comparison of the impedance spectra obtained in simulated air versus in pure O2 at 450°C, as shown in Fig. 5a. The HF semicircles in simulated air and in pure O2 are essentially the same, which suggests that they are not sensitive to the amount of O2 available, and should represent the charge transfer step of the electrode reaction. On the other hand, the MF-LF semicircle is significantly smaller in pure O2 comparing with that in dry simulated air, which is consistant with the attribution of the MF-LF semicircle to the oxygen adsorption/mass transport of oxygen molecules in the gas phase. With the introduction of moisture to O2, RHF becomes smaller and smaller with increasing moisture content, as shown in Fig. 5b, while the MF-LF semicircle becomes significantly larger, which is similar to the behavior in simulated air. In addition, as shown in Fig. 5c, the impedance spectra in 3% humidified air and in humidified O2 are very similar including the MF-LF part, and this can be understood as significant surface sites over BSCF electrode are now occupied by adsorbed water, which leads to limited reaction sites for O2 adsorption, making the overall electrode reaction less sensitive to the oxygen gas concentration.
For the effect of CO2 on the cathode electrochemical behavior, adding CO2 to the dry simulated air obviously poisons the BSCF electrode as evidenced by the increase in Rai (shown in Fig. 6 and Table I). In addition, 1% CO2 also seems to cause an increase in RO for the BZCYYb electrolyte at higher temperature such as 650°C and 550°C (as shown in Table I). This is most likely due to the bulk reaction between CO2 and the BZCYYb electrolyte, as evidenced by the XRD pattern in Fig. 1 showing the existence of BaCO3 impurity after the exposure of the BZCYYb powder to 1%CO2/99%N2 at 750°C for 24 hours. In comparison, at lower temperature of 450°C, no obvious change in RO was observed, which is consistent with the XRD pattern for the BSCF+BZCYYb powder mixture after 24 hours of exposure to 1%CO2/99%N2 in Fig. 1, suggesting sufficient chemical stability against 1%CO2 for the BSCF electrode and BZCYYb electrolyte at that temperature.
In addition, the increase of Rai upon the introduction of 1% CO2 into the dry simulated air, especially for the MF-LF semicircle (shown in Figs. 6b and 6c) could be attributed to the adsorption of CO2 on the BSCF and BZCYYb surfaces, which would substantially occupy the active surface sites for oxygen adsorption and slow down the overall reaction. On the other hand, when the oxygen electrode process shows clear separation into HF and MF-LF semicircles at lower temperature such as 450°C, the HF semicircle does not appear to be influenced much by the adsorption of CO2 (Fig. 6c). This is also consistant with the attribution that the HF semicircle represents the charge transfer process. Furthermore, as observed in Fig. 6 and summarized in Table I, the CO2 poisoning is largely recoverable upon the removal of 1% CO2 in the dry simulated air at 650°C, but it gets less reversible at lower temperature of 550°C and 450°C. This is likely due to the relative strong adsorption and high desorption temperature (>600°C) for CO2 on BSCF surface as reported before.7,31,32
With the presence of 3% moisture, the extent of poisoning caused by 1% CO2 reduces and the recovery becomes much more complete, especially at lower temperature of 550°C and 450°C, as shown in Fig. 7 and in Table I. The less extent of CO2 poisoning and faster and more complete recovery in the presence of moisture comparing with dry condition could be attributed to the strong adsorption of moisture on the BSCF and BZCYYb surfaces, especially at lower temperature of 550°C and 450°C, which leads to the formation of adsorbed surface bicarbonate species (i.e., adsorbed -HCO3) apart from typical surface carbonate (adsorbed -CO2) on the electrode and electrolyte. According to Yan et al., the surface bicarbonate species have weaker bonding and much lower desorption temperature of ∼400°C comparing with the desorption temperature of ∼600°C for surface carbonate species.31
Finally, considering that the CO2 concentration in ambient air is much lower than the 1% used in this study and there will always be some moisture in air, the results observed suggest that proton conducting IT-SOFC with BSCF cathode might be insensitive to typical CO2 poisoning for operation at reduced temperature of ∼450°C. On the other hand, the observed apparent interfacial resistance on the order of 10–15 Ω•cm2 at this temperature is still much higher than ideal. Therefore, alternative SOFC cathodes that have relatively lower affinity for water adsorption, high activity for oxygen dissociative adsorption, as well as high proton conductivity would be promising candidates for proton conducting IT-SOFC.
Conclusions
BSCF cathode demonstrates chemical compatibility with BZCYYb proton conducting electrolyte up to 1000°C and stability at 450°C in air containing 3% H2O and up to 1% CO2. For a BSCF/BZCYYb/BSCF symmetrical cell, ohmic resistance RO decreases with the introduction of moisture, which is consistent with typical hydration behavior of the BZCYYb proton conducting electrolyte. For the apparent interfacial resistance Rai, the middle and low frequency (MF-LF) semicircle increases with the introduction of moisture.25,42,43 Such an increase is attributed to the occupation of the BSCF electrode surface active sites by water molecules that inhibit oxygen adsorption/dissociation and surface diffusion. On the other hand, the high frequency (HF) semicircle, which corresponds to the charge transfer process in the oxygen electrode reaction, could be clearly separated at ≤∼500°C and it shows significant reduction with the introduction of moisture. Such a phenomenon is hypothesized to be related to the intrinsically faster charge transfer process involving proton vs the conventional pathway involving only oxide ion and/or the greater availability of reactant, in particular adsorbed water H2O(ads), for the reverse reaction of the charge transfer step in the oxygen electrode reaction over the proton conducting electrolyte. On the other hand, introducing 1% CO2 to simulated air causes obvious poisoning for the BSCF/BZCYYb/BSCF symmetrical cell. While CO2 poisoning becomes less reversible at lower temperature, the presence of moisture helps reduce the extent of CO2 poisoning and improves the reversibility especially at reduced temperatures of 450°C. This is attributed to the co-adsorption of H2O and CO2 on BSCF and BZCYYb surfaces, as well as the formation of bicarbonates on surfaces, which tend to bond weaker and desorb at lower temperature comparing with surface carbonate species. Considering water molecules adsorb strongly on BSCF at ∼450°C and below, which tends to interfere with the oxygen adsoption as shown in this study, designing alternative cathodes with reduced tendency for water adsorption while maintaining fast oxygen adsorption and high proton conductivity appears to be a promising direction in the future development of cathodes for proton conducting IT-SOFC.
Acknowledgment
The authors thank FIU College of Engineering for new faculty start-up fund. The authors would also like to acknowledge the use of materials characterization facilities at FIU Advanced Materials Engineering Research Institute (AMERI).