Abstract
The aim of this work was using ZnO nanoparticles and a synthesized ion liquid (1-Methyl-3-butylimidazolium bromide) for modification of carbon pate electrode. This modified electrode was applied to determination of isoproterenol (IP). Under optimum pH of 7.0, the oxidation of IP in the presence of ZnO nanoparticles and ion liquid occurred at a potential about 520 mV and oxidation current was more than unmodified electrode. Square wave voltammetry was used for determination of linear dynamic range of the method. The plot of oxidation current vs. concentration was linear in the range of 0.08 to 800.0 μmol L−1 IP and detection limit was calculated to be 0.04 μmol L−1 IP. The diffusion coefficient (D = 2.9 × 10−6 cm2 s−1), and electron transfer coefficient (α = 0.78) for IP were also determined using electrochemical approaches. This method was also examined for determination of IP in pharmaceutical and biological samples.
Export citation and abstract BibTeX RIS
In pharmaceutical industries, determination of drugs is very important in order to analysis of pharmaceutical products and also pharmacokinetics studies. There are several methods for this purpose. However, some of these methods are expensive, complex and time consuming methods. Electrochemical methods are simple, inexpensive, fast and selective methods with minimum sample preparation.1–4 These methods have been applied for determination of drugs in pharmaceutical products and biological samples such as blood serum and urine and in most cases they have shown good accuracy and precision.5–10
Carbon paste electrodes are one of the most common electrodes in electrochemistry due to simple preparation and reproducible surface. However, in order to improve electrical and chemical properties, modification of carbon paste electrodes is necessary. Nano compounds have been used as modifiers for increasing electrical conductivity and active surface area of carbon paste electrodes.10–15 In recent years, metal oxide nanoparticles (such as magnetic nanoparticles),16 metallic nanoparticles (such as gold and silver nanoparticles)17–19 and carbon nano structures (such as carbon nanotubes and graphene) have been used singly or in composite form for modification of electrodes.20–22
In addition, another idea is adding ion liquid compounds in association with paraffin as pasting liquid. It improves conductivity of electrode and measurement of redox current.23–26
Isoproterenol (IP) is a synthetic catecholamine drug which stimulates the heart, increases heart rate and cardiac output like epinephrine. It works thru stimulation of β adrenergic receptors. Also, isoproterenol is a potent bronchodilator.27
In this work, carbon paste electrode was modified by adding zinc oxide nanoparticles and ion liquid as a binder. Electrochemical behavior of IP was investigated at modified electrode (ZnO/ILCPE) and was compared with its behavior at carbon paste electrode modified with ionic liquid (ILCPE), at carbon paste electrode modified with ZnO nanoparticles (ZnO/CPE) and at carbon paste electrode (CPE). The results showed superiority of ZnO/ILCPE to the other electrodes. In comparison to some of previously reported works, this method has long linear dynamic range and low detection limit.28–30 Also, preparation of the modified electrode is very simple and it has good reproducibility and repeatability.
Experimental
Chemicals and apparatus
Isoproterenol was purchased from Merck. Stock solutions were prepared daily by dissolving appropriate amount distilled water to prepare 1 × 10−3 molL−1 and were stored in 4°C until use. Other standard solutions were prepared by dilution of the stock solution with buffer solution. Graphite fine powder (particle size <50 μm) and high viscosity paraffin oil (density = 0.88 g cm−3) were purchased from Merck. All other chemicals were purchased in the analytical grade from Merck and Fluka.
Phosphate buffer (sodium dihydrogen phosphate and disodium monohydrogen phosphate plus sodium hydroxide, 0.1 mol L−1) solutions (PBS) with different pH values were used.
1-Methyl-3-butylimidazolium bromide (ion liquid) was synthesized according to the previous paper.26
All Voltammetric measurements were carried out using μ-Autolab modular electrochemical system (Eco Chem. Utrecht, The Netherlands) equipped with PSTA 20 model and driven by NOVA software (Eco Chem.). All the electrochemical studies were performed at 25 ± 1°C. A conventional three electrode cell assembly was employed for the experiments in a 50 mL glass cell containing an Ag/AgCl electrode as reference electrode, a platinum wire as counter electrode and ZnO/ILCPE as working electrode. All of the potentials were measured and reported vs. Ag/AgCl reference electrode. The pH of the solutions was controlled with a Metrohm pH meter (model 827). X-ray powder diffraction studies were carried out using a STOE diffractometer with CuKα radiation (λ = 1.54 Å).
Synthesis of ZnO nanoparticles
The 100 ml of 0.25 molL−1 zinc nitrate solution was added drop wise to 100 ml of 0.5 molL−1 sodium hydroxide solution. The mixture was stirred by magnetic stirring apparatus (1,500 rpm) at room temperature. Then, the light-green precipitate was separated from solution using buchner funnel. The precipitate was washed with deionized water and ethanol several times. It was dried at 110°C for 15 h, and then calcined at 300°C for 2 h.
Preparation of ZnO/ILCPE
To obtain the best conditions in preparation of the modified electrode, the ratios of ZnO/IL/graphite was optimized. Under optimum conditions, the constituents of the paste electrode contain 0.9 g graphite powder, 0.1 g ZnO nanoparticles, 0.2 g ion liquid and 0.8 g paraffin. The mixture was hand mixed in mortar and pestle for 10 min until uniformly wetted paste obtained. The prepared paste was inserted in to a glass tube (internal radius 2.2 mm) with a copper wire placed in to the glass tube for electrical connection. New surface by pushing an excess the paste and polishing it on a weighing paper was obtained. The unmodified carbon paste electrode was prepared in the same way without adding nanoparticles and ion liquid to the mixture.
Results and Discussion
Nanostructures characterization
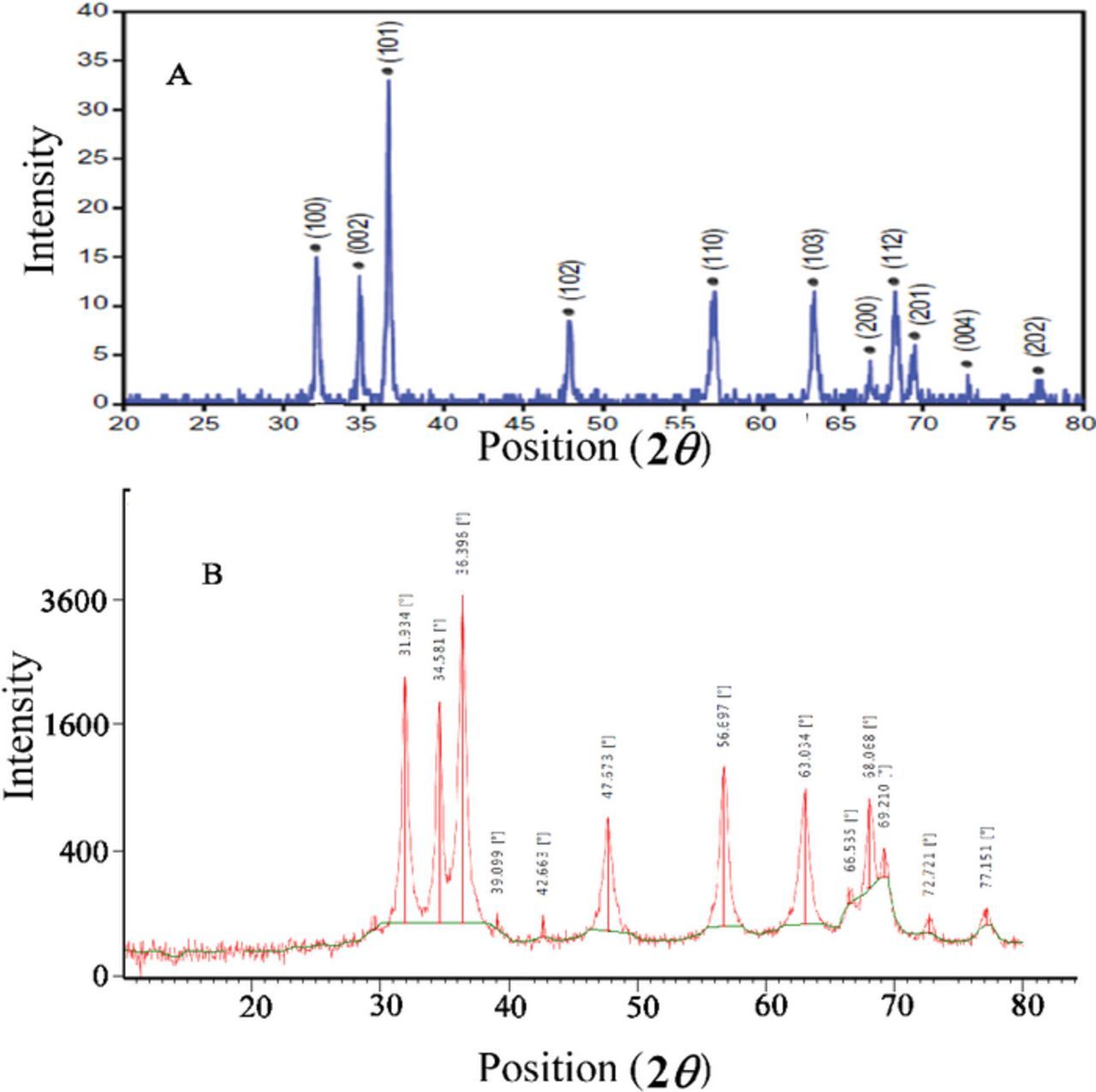
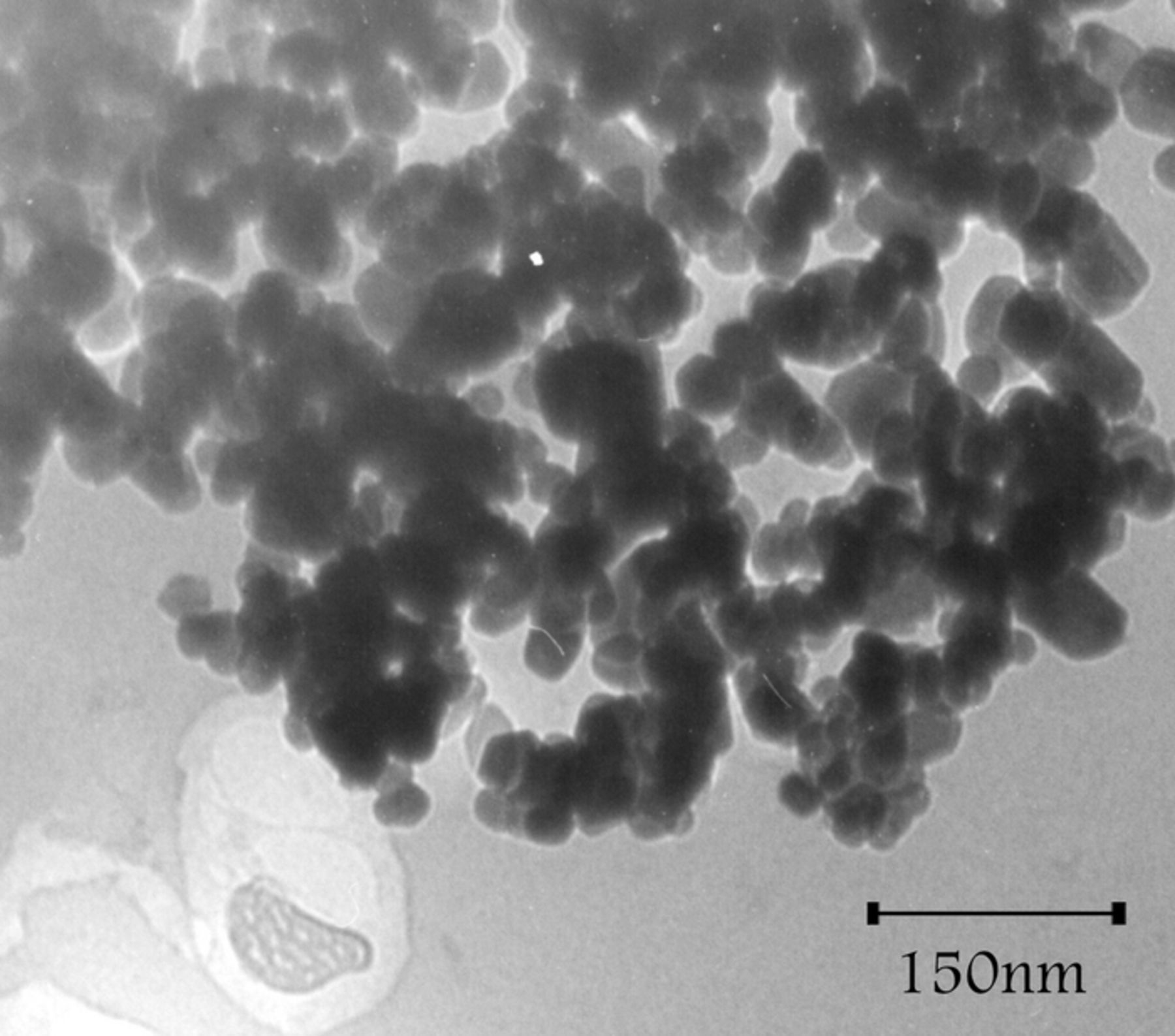
Figure 1 shows X-ray diffraction pattern of ZnO nanoparticles. Average particles size were obtained 49.5 nm using Scherrer equation D = Kλ/(β cos θ) where K is a constant (0.9), λ is the source wavelength (λ = 1.5418 Å), β is the full width at the half-maximum of the ZnO line (101) and θ is the diffraction angle. In XRD pattern the main peaks were observed at the (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202) planes which very close to ZnO reference patterns. The TEM image of synthesized ZnO, shows dark spots that confirm formation of nanoparticles in our synthesis condition (Figure 2).
Figure 1. XRD patterns of ZnO nanoparticles (A) standard XRD pattern, (B) synthesized ZnO nanoparticles pattern.
Figure 2. TEM image of synthesized ZnO nanoparticles.
pH effect
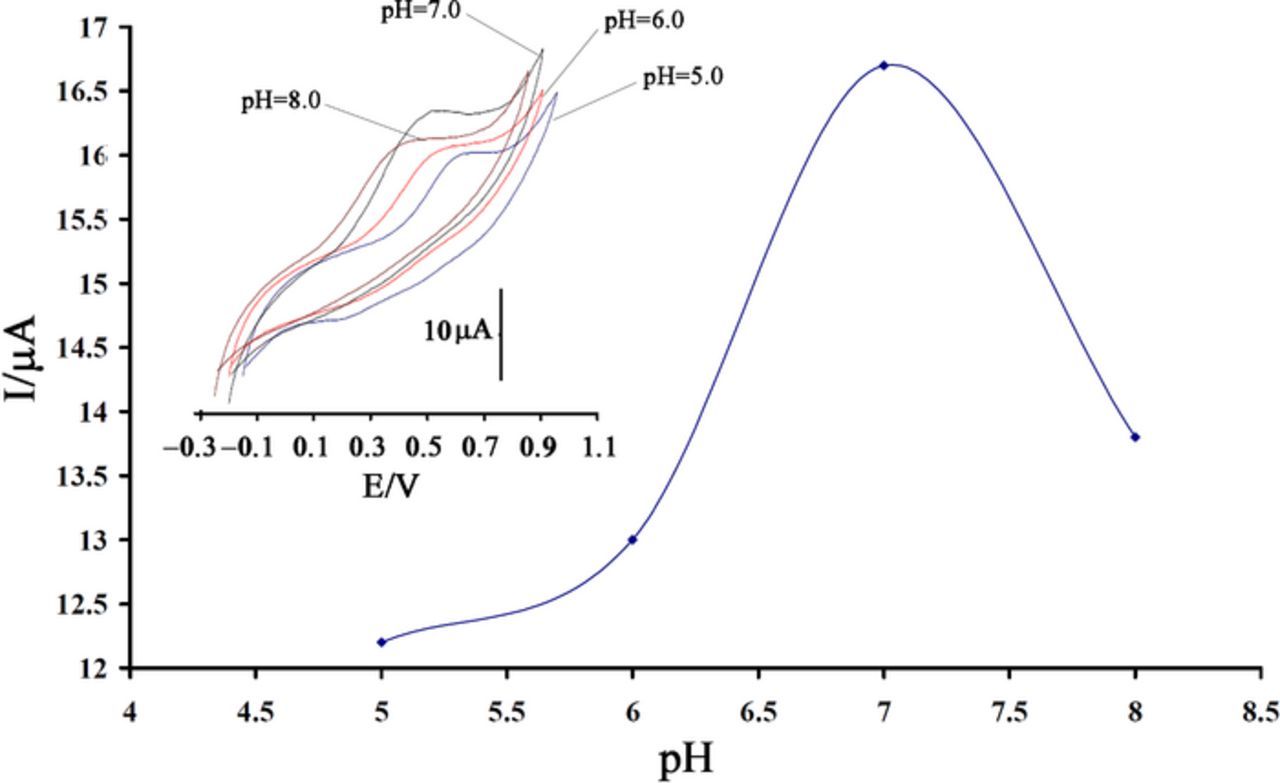
Oxidation of isoproterenol is associated with production of H+, so the pH of solution has effect on the oxidation process. The effect of pH solution on the oxidation of IP at ZnO/ILCPE was investigated using cyclic voltammetry. The maximum peak current was appeared in pH 7.0 (PBS), so this value was selected as optimum pH for further experiments (Figure 3). Also, the results show that the peak potential of the redox couple was pH dependent, and the peak potential was shifted to lower potential with increasing the pH.
Figure 3. Current - pH curve for oxidation of 300.0 μmol L−1 IP at ZnO/ILCPE (scan rate = 100 mV s−1). Inset, Effect of pH on cyclic voltammograms of IP.
Electrochemical behavior of IP at ZnO/ILCPE
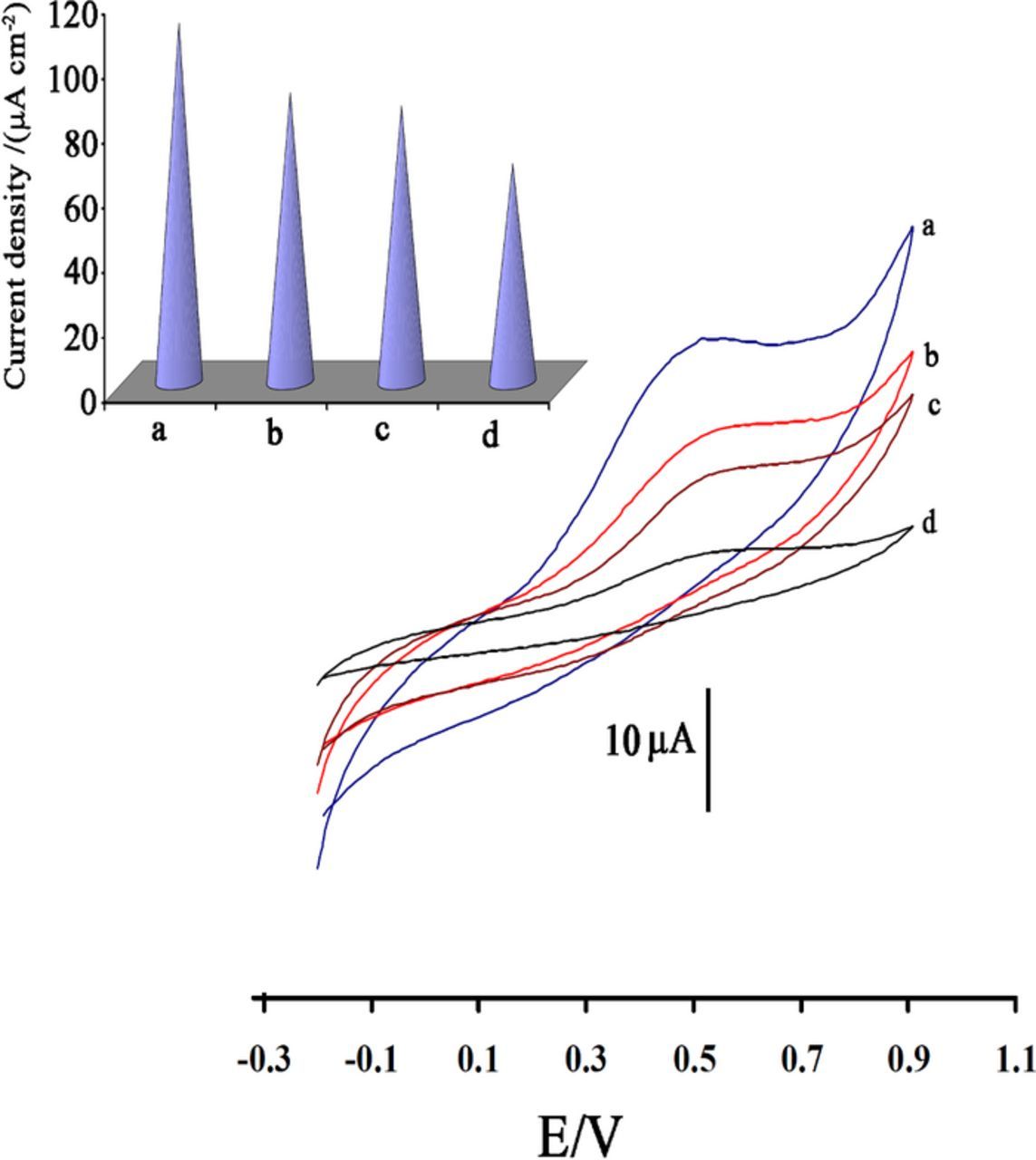
Cyclic voltammograms of 400 μmol L−1 of IP at different electrodes were shown in Figure 4. In the presence of ZnO nanoparticles and ion liquid, maximum oxidation current was obtained around 520 mV vs. the Ag/AgCl/KClsat (Figure 4a). At ZnO/CPE, ILCPE and unmodified CPE lower oxidation currents were observed (Figures 4b,4c,4d). Figure 4 (inset) shows the current density derived from the cyclic voltammograms of 400 μmol L−1 IP (pH 7.0) at the surface of different electrodes. In addition, at ZnO/ILCPE oxidation of IP was occurred at lower potential. In contrast, modification of CPE with ZnO nanoparticles and ion liquid causes increasing oxidation current and decreasing the oxidation potential (overpotential) that indicated catalytic ability of ZnO/ILCPE.
Figure 4. Cyclic voltammograms of (a) ZnO/ILCPE, (b) ILCPE, (c) ZnO/CPE and (d) CPE in the presence of 400 μmol L−1 IP at pH 7.0.
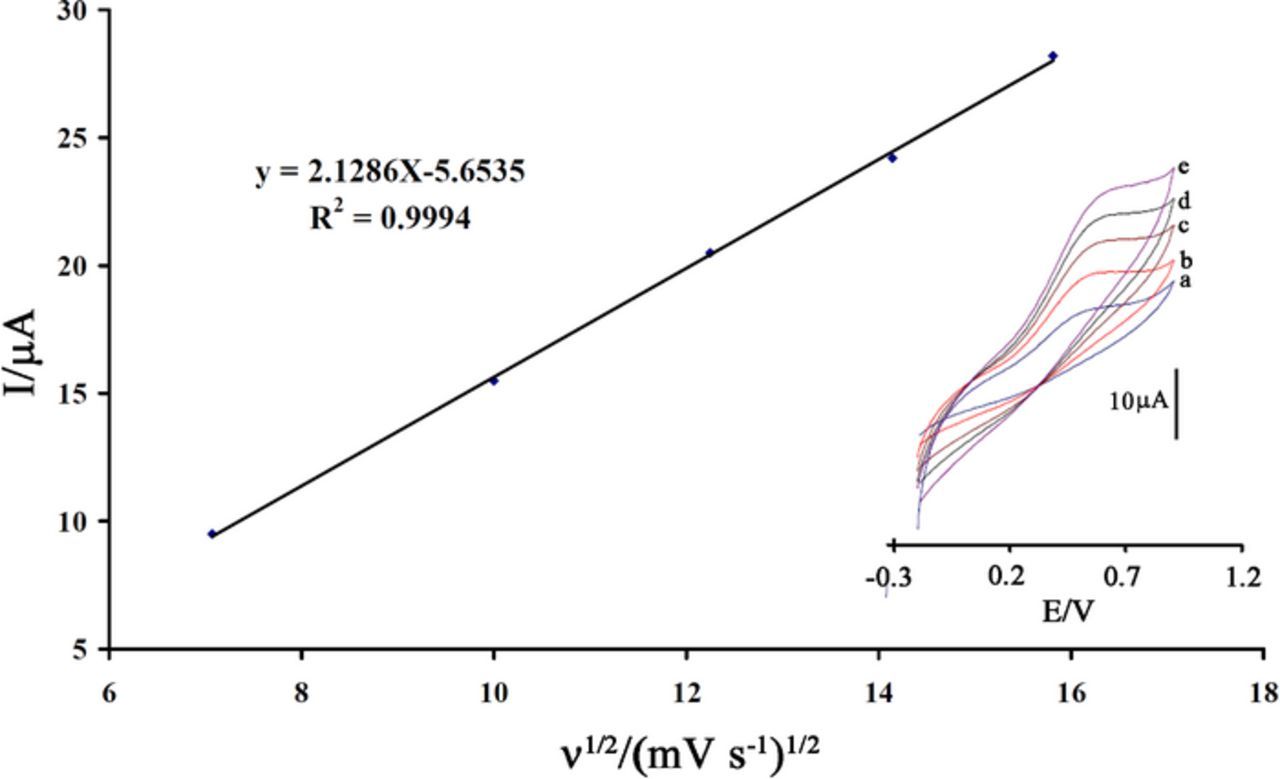
In order to study the effect of potential scan rate (ν) on the oxidation current, cyclic voltammograms of IP at various scan rates were recorded at pH 7.0 (Figure 5). Linear relation between peak current and square root of scan rate was obtained where indicates a diffusion controlled oxidation reaction occurs at the surface of ZnO/ILCPE.
Figure 5. Plot of Ipa vs. ν1/2 for the oxidation of 300.0 μmol L−1 IP at ZnO/ILCPE. Inset: cyclic voltammograms of IP at ZnO/ILCPE at different scan rates of (a) 50, (b) 100, (c) 150, (d) 200, and (e) 250 mV s−1 in pH 7.0.
To obtain transfer coefficient (α), linear relationship between peak potential (Epa) and ln(ν) was used according to equation 1.31
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/3/B38/revision1/d0001.gif)
Where m = RT/ [(1 – α) nαF]. The value of α = 0.78 was obtained from this equation.
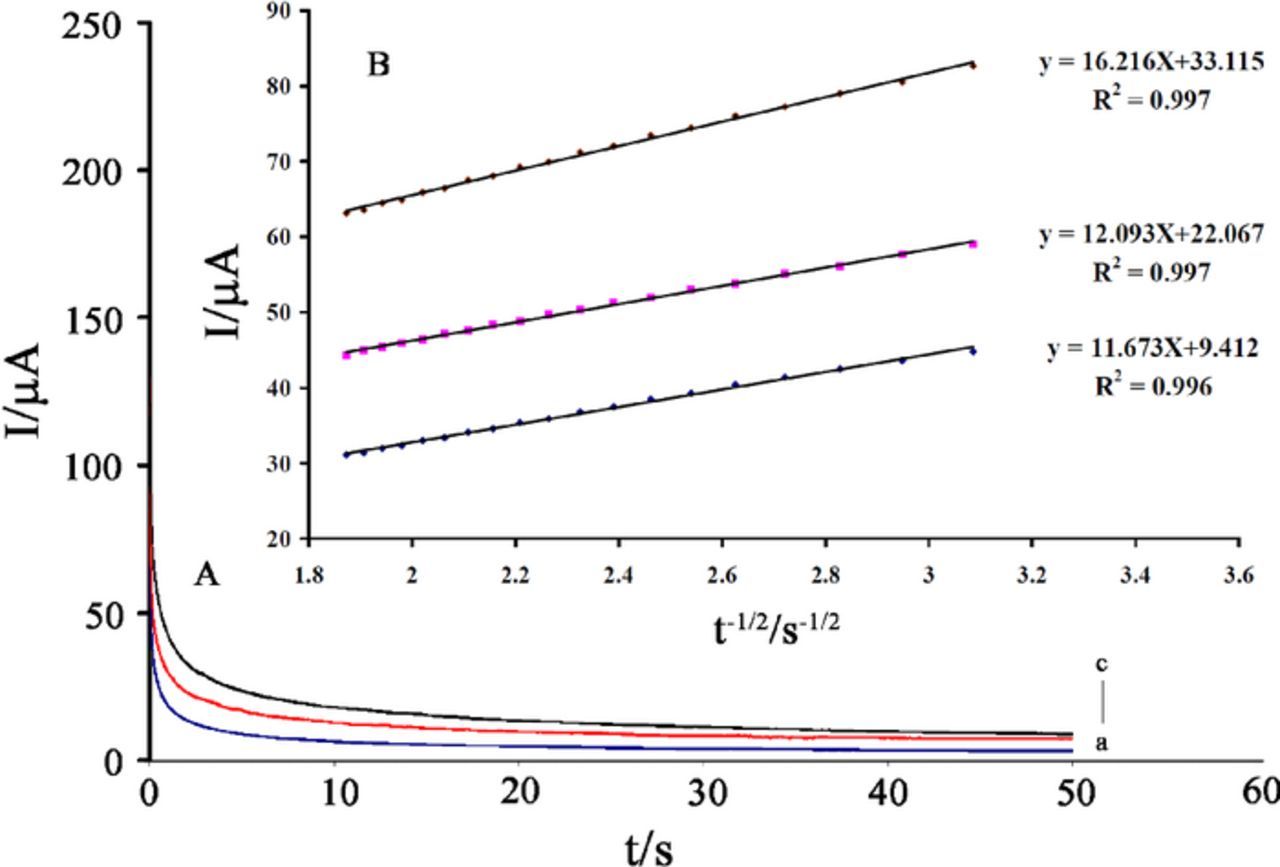
Chronoamperometric measurements of IP at ZnO/ILCPE were carried out by setting the working electrode potential at 700 mV vs. Ag/AgCl/KClsat for the various concentrations of IP in buffer solutions (pH 7.0) (Figure 6). Under diffusion control, a plot of I vs. t−1/2 will be linear, and the value of D can be obtained from the slope. The slopes of the straight lines for different concentrations of IP were plotted vs. IP concentration and the value of the D was found to be 2.9 × 10−6 cm2 s−1 using the Cottrell equation.32
Figure 6. (A) Chronoamperograms obtained at the ZnO/ILCPE in the presence of a) 200, b) 400 and c) 600 μmol L−1 IP (pH 7.0). (B) Cottrell's plot for the data from the chronoamperograms.
Analytical features of the method
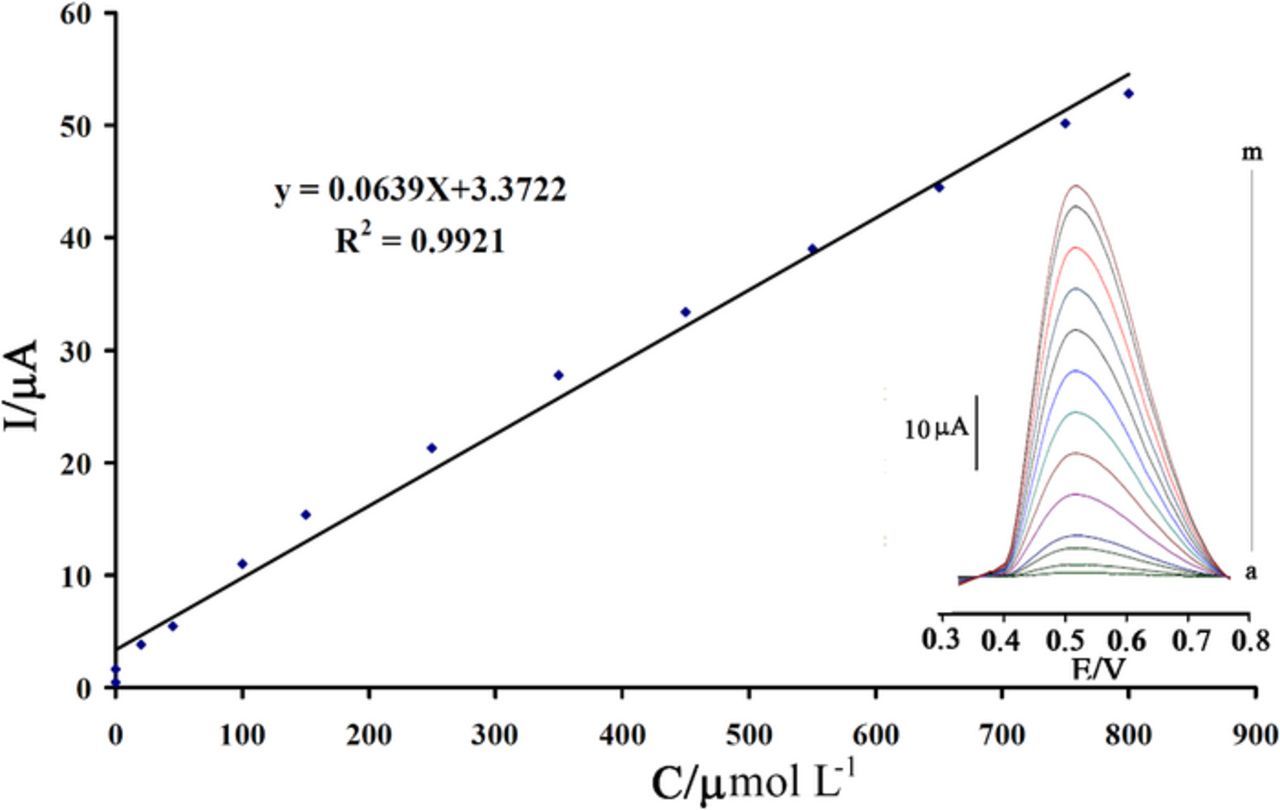
Square wave voltammetry was used for determination of linear dynamic range of the method. The plot of oxidation current vs. concentration was linear in the range of 0.08 to 800.0 μmol L−1 IP (I (μA) = 0.0639 CIP + 3.3722, R2 = 0.992) (Figure 7). The lower detection limit of the method was determined using the equation Cm = 3Sb/m, where Sb is the standard deviation of the blank response (μA) and m is the slope of the calibration curve. Using the data from calibration curve and measurements of blank, the lower detection limit was obtained to be 0.04 μmol L−1 IP. In order to examine the reproducibility of preparation of modified electrode, three electrodes were prepared according to proposed method. Then, they were used for determination of IP in the solution containing 50 μmol L−1 IP. The RSD values were found to be 1.1% and 1.5% for Epa and I, respectively.
Figure 7. Calibration curve for determination of IP. Inset shows Square wave voltammograms in the buffer solution (pH = 7.0) containing different concentrations of IP in the range of 0.08 to 800.0 μmol L−1 IP.
Interferences
To study the effect of potential interferences, the influence of various foreign species on the signal of 10 μmol L−1 IP were investigated at ZnO/ILCPE. The tolerance limit was taken as the maximum concentration of foreign substances which caused no more than ±5% relative error in the determination of IP. The results showed that 1000 fold of glucose, fructose, lactose, sucrose, Na+, K+, CO32−, SCN−, F−, Br−, Ca2+, SO42−, 800 fold aniline, phenylalanine, methionine, valine, glycine, histidine and tryptophan, and saturated starch solution did not effect on IP oxidation current.
Real sample analysis
With the aim of evaluation of proposed method for analysis of real samples, the concentration of IP was measurement in a pharmaceutical sample) Injection solution (and two biological samples. The results presented in Table I showed that ZnO/ILCPE has retained its efficiency for the determination of IP in real samples with satisfactory results.
Table I. Determination of IP in real samples.
| No. | Sample | Added (μmol L−1) | Expected (μmol L−1) | IP Founded (μmol L−1)* | Recovery (%) |
|---|---|---|---|---|---|
| 1 | Injection solution | - | 10.0 | 9.94 ± 0.44 | 99.4 |
| 2 | 10.0 | 20.0 | 20.57 ± 0.62 | 102.85 | |
| 3 | Urine | - | - | < detection limit | |
| 4 | 20.0 | 20.0 | 20.75 ± 0.83 | 103.75 | |
| 5 | 20.0 | 40.0 | 40.86 ± 0.93 | 102.15 | |
| 6 | Serum | - | - | < detection limit | |
| 7 | 50.0 | 50.0 | 49.57 ± 1.01 | 99.14 |
*Average of three replicated measurements. ± Standard deviation
Conclusions
The modified CPE was developed for determination of IP. Modification of CPE with ZnO and ion liquid causes increasing oxidation current. But, in the presence of both modifiers, maximum oxidation current was obtained and peak potential was shifted to lower potential. Proposed method has long linear dynamic range with 4 orders of magnitude. The modified electrode was applied for determination of IP in pharmaceutical and biological samples and satisfactory results were obtained.
Acknowledgment
The author gratefully acknowledges Islamic Azad University, Falavarjan branch research council for support of this work (301/28310).