Abstract
A high areal capacity lithium-sulfur battery making use of mass produced aluminum metal foam as a current collector was investigated. A sulfur/Ketjenblack (KB) composite was filled and deposited into the aluminum foam current collector via a predetermined filling procedure, resulting in high sulfur loading. The value for this loading was found to be 17.7 mg sulfur/cm2 by using carboxymethyl cellulose and styrene butadiene rubber (CMC + SBR) as a binder. An operating single-layer pouch-type cell with an S/KB-CMC+SBR on Al foam cathode was created as a result of this synthesis and found to possess an unprecedentedly high areal capacity of 21.9 mAh/cm2. On the basis of the achieved areal capacity, the energy density of a theoretical lithium-sulfur battery was estimated with the assumption of an electrolyte/sulfur ratio of 2.7 μL/mg. This was calculated upon 100% of the pore volume in the S/KB-CMC + SBR on Al foam cathodes and polyolefin separator, along with the inclusion of the weights of the tabs for the current lead and pouch film packaging in the case of a seven-layer pouch-type battery. With this calculation, it was determined that the creation of a lithium-sulfur battery with an energy density of greater than 200 Wh/kg is plausible.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Since the commercial launch of the lithium ion battery (LIB) by Sony in 1991,1 the range of application of this type of battery has been broadened for use in everything from small portable electronic devices to large electric vehicles and stationary batteries capable of storing the power generated by means of solar and wind sources. Such an expansion in the range of applications for LIBs requires them to possess a high energy density, remain safe to use, and to manufactured at a low cost. Considering the requirement for high energy density, there are two general methods by which this can be achieved: increasing the operating voltage or increasing the capacity.
Considering the first method, the use of high operating potential cathodes such as spinel (LiNi0.5Mn1.5O4),2 olivine-type (LiCoPO43 and LiNiPO44), and fluoride phosphate (Li2CoPO4F)5 have been investigated. Such cathode materials are promising for use in transportation applications because of both their high operating potential and high volumetric energy density.
Considering high capacity cathodes, both sulfur (1675 mAh/g)6,7 and air cathodes8–11 have been investigated. These cathodes possess extremely high capacities with the ability to overcome the shortcoming of their low operating potential, thereby increasing their energy density. Issues arise, however, with the use of the air cathode with a lithium metal anode, which include poor reversibility of the discharged product, the occurrence of a high over-potential during charging and discharging, and the penetration of ambient air into the system resulting in the degradation of lithium metal by moisture.
In comparison with the air cathode, the sulfur cathode seems to be a more promising candidate for its use as a high capacity cathode. The sulfur cathode possesses many advantages for practical use: lithium-sulfur batteries have a theoretical energy density (reported to be around 2500 Wh kg−1 and around 2800 Wh L−1)7,12 much higher than that of conventional graphite-LiCoO2 batteries (387 Wh kg−1), and sulfur is both abundant in nature and of low toxicity.12 However, sulfur cathodes are not without their own intrinsic problems such as low ionic and electronic conductivity, large volumetric expansion upon lithiation from S8 to Li2S (around 180%), the dissolution of so-called polysulfide intermediates (Li2Sx where x represents a value between 4 and 8),13 and low sulfur loading in the cathode.14 Nazar et al. proposed the combination of sulfur with an ordered mesoporous carbon material as a host supporting the sulfur, in order to improve the ionic and electronic conductivity of the cathode.7 The mesoporous carbon structure provides an electron pathway and maintains proper spacing within the sulfur electrode through which lithium ions can migrate. These properties thereby accommodate the effect of the volumetric expansion of the sulfur due to lithiation.15–19
The issue of polysulfide dissolution has been improved by taking advantage of the physical and/or chemical adsorbing properties of carbonaceous materials7,17,20,21 and electronically conductive polymers.22–34 Semi-conductive or metallic oxides have been reported to be suitable host materials that can adsorb the polysulfides due to their high polarity.35–37
Apart from cathodic improvements, electrolytes, such as glyme-based ionic liquids, have also been investigated to suppress the dissolution of polysulfides.38–40 Glyme-based electrolytes that possess a proper lithium concentration have no electron lone pairs belonging to oxygen atoms in the glyme. This is a results of donations by the lithium cations due to the electron vacancies, and results in the loss of solubility of lithium polysulfides. The use of one such electrolyte, [Li (tetraethylene glycol diethyl ether (G4))] [bis(trifluoromethanesulfonyl) amide (TFSA)]/1,1,2,2–tetrafluoroethyl 2,2,3,3–tetrafluoropropyl ether (HFE), demonstrated an especially low solubility of lithium polysulfides.40 This electrolyte system has received attention owing to its action as a sparingly solvating electrolyte for polysulfides.41 In other approaches, the use of electrolytic additives such as LiNO3 serve to form a protective layer on the lithium metal anode, thereby suppressing the reduction of polysulfides on the anode and impeding the "shuttle effect", which limits their capacity because the sulfur reduction products, LixSy species, cannot be fully re-oxidized.42,43
As described above, sulfur cathodes have been progressing toward their viable use in practical applications. One issue that must be overcome before their practical use can be fully realized is that of low sulfur loading in the cathode and a subsequently low sulfur-loading ratio in the entire cathode.14 Hagen et al. determined that the areal sulfur loading of the majority of the investigated sulfur cathodes was less than 2.0 mg cm−2.45 Gao et al. demonstrated that a low sulfur-loading ratio in the cathode of a lithium sulfur battery (less than around 70%) resulted in the battery possessing a lower energy density than a conventional lithium-ion battery.46 In an effort to remediate this effect, Zhou et al. have investigated a promising method allowing for the achievement of high sulfur loading using a graphene foam as a current collector, and have summarized the previously reported relationship between the sulfur loading and areal capacity.14 The group was able to achieve a high sulfur loading of 10.1 mg cm−2 with a high areal capacity of 13.4 mAh cm−2. However, the mass production of graphene foam is an industry that has not yet been established for practical use.
In the present paper, an attempt is made to verify the possibility of achieving a lithium-sulfur battery with an energy density of 200 Wh kg−1. This will be accomplished using a filling technique that has been we have developed involving the deposit of a sulfur-carbon composite slurry into a three dimensionally (3D) structured aluminum foam current collector, the production of which has been industrially established. Subsequently, a single-layer pouch-type cell possessing high sulfur loading in the cathode was demonstrated using ethylene glycol dimethyl ether (G1) and triethylene glycol dimethyl ether (G3)/HFE instead of G4/HFE as an electrolyte. The energy density of a seven-layer pouch-type lithium-sulfur battery is subsequently estimated, verifying the potential to achieve a lithium-sulfur battery possessing an energy density of more than 200 Wh kg−1.
Experimental
The sulfur/Ketjenblack (KB) composite was prepared by mixing sulfur powder (Sigma-Aldrich, Co. LLC.) with KB (Lion Specialty Chemicals Co., Ltd.) in a 6 to 4 weight ratio using a granulator (Balance Gran, AKIRAKIKO). The mixture was then heated in a N2 atmosphere at a temperature of 155°C for 12 h to form a composite of sulfur and KB. Two different types of the S/KB composite slurry were prepared in the granulator. This involved the use of two binders (namely polyvinylidene difluoride (PVdF, KUREHA Corp.) and carboxymethyl cellulose (CMC, Sigma-Aldrich, Co. LLC.)) and styrene butadiene rubber (SBR, JSR Corporation) (CMC+SBR, at 2:1 weight ratio) with either N-methylpyrrolidone (NMP, Kanto chemical Co., Inc.) or distilled water acting as the solvent, as summarized in Table I. The slurry was filled into the 3D structured aluminum foam (Celmet, Sumitomo Electric Industries, Ltd.). The 3D structured aluminum foam was 1 mm thick with 550 μm pores, as shown in Fig. 1. The filling of the slurry into the aluminum foam was carried out from both sides of the aluminum foam. A single-layer pouch-type full cell was assembled with the configuration of an S/KB on Al foam cathode (70 mm by 70 mm), a Li metal on Cu foil anode (74 mm by 74 mm), and a glyme-based electrolyte. The glyme-based electrolyte was composed of ethylene glycol dimethyl ether (G1, Kishida Chemical Co., Ltd.), triethylene glycol diethyl ether (G3, Kishida Chemical Co., Ltd.), lithium bis (trifluoromethanesulfonyl) imide (LiTFSI, Kanto chemical Co., Inc.), and 1,1,2,2–tetrafluoroethyl 2,2,3,3–tetrafluoropropyl ether (HFE, DAIKIN Industries, Ltd.), which were soaked in a polyolefin separator. The glyme-based electrolyte was a solvate ionic liquid mixture of [Li(G1)2][TFSI], [Li(G3)][TFSI], and HFE in a volume ratio of 1:1:4. The amount of electrolyte used, the pore volume of the S/KB-binder cathode, the separator, and the electrolyte/sulfur (E/S) ratio (μL mg−1) calculated from the electrolyte amount (μL) and Sulfur amount (mg),47–49 are shown in Table II.
Table I. The composition ratio of the S/KB composite, KB, and binder in the slurry for cathode preparation.
| S/KB composite | KB | Binder | Solvent | Solids ratio/wt% | |
|---|---|---|---|---|---|
| S/KB-PVdF | 60 | 30 | 10 | NMP | 15 |
| S/KB-CMC+SBR | 60 | 30 | 3 | Distilled water | 26–27 |
Figure 1. Appearance and schematic image of 3D structured aluminum foam.
Table II. The specification of the single-layer pouch-type cells with S/KB on Al foam cathodes.
| Pore volume calculated in S/KB-binder cathode/cm3 | Pore volume in separator/cm3 | Amount of electrolyte/ml | E/S ratio/- | |
|---|---|---|---|---|
| S/KB-PVdF on Al | 3.89 | 0.058 | 13.4 | 24.5 |
| S/KB-CMC+SBR on Al | 3.46 | 0.058 | 16.9 | 19.5 |
The charge-discharge property of the sulfur cathodes was evaluated with a cutoff voltage between 1.0 and 3.3 V, using a charge-discharge system (HJ1010SD8, Hokuto Denko Corp.).
Results and Discussion
In order to achieve a high sulfur-loading cathode, 3D structured aluminum foam was selected to act as a support structure and current collector for this system. When filling the aluminum foam with an S/KB slurry, the solids ratio of the slurry is one of the key parameters. For instance, if the solids ratio of the slurry is low, too much space is present following the drying of the S/KB in the Al foam cathode. This results in a low sulfur-loading cathode. The filling of the S/KB slurry into the Al foam must therefore be carried out several times in order to ensure that an excess of space is not present. This process, however, leads to further complications as the filling of the slurry becomes gradually more difficult due to the accumulation of S/KB in the Al foam. A high solids ratio of the slurry is therefore important in order to reduce the number of required fillings to achieve a high sulfur-loading cathode on the 3D structured support and current collector.
To increase the solids ratio in the slurry, the binder concentration in the binder solution must be increased. Compared with a conventional PVdF binder, a CMC+SBR binder demonstrated better dispersion in a solvent. Although the solvents of the two binder solutions that were used are different (NMP and distilled water), the solids ratio of the S/KB-CMC+SBR slurry was found to be 26–27 wt%, which is higher than that of S/KB-PVdF (15 wt%), as shown in Table I. Due to this increase in the solids ratio, the sulfur loading achieved through each filling process is subsequently increased.
A high sulfur loading on the aluminum support can subsequently be attained by carrying out the filling process only once per one side of the aluminum foam. The sulfur loading onto the aluminum foam support by the CMC+SBR binder slurry was successfully achieved at a value of 17.7 mg cm−2, greater than that obtained by the PVdF binder slurry (11.2 mg cm−2).
Single-layer pouch-type full cells were assembled using the S/KB-PVdF on Al foam and S/KB-CMC+SBR on Al foam cathodes with an electrolyte composed of [Li(G1)2] [Li(G3)] [TFSI]2 /HFE. As Dokko et al. reported, [Li (G4)] [TFSI]/HFE demonstrates low solubility of lithium polysulfides.40 As preliminary results indicated, the single-layer pouch-type full cells assembled using the S/KB-PVdF on Al foam and S/KB-CMC+SBR on Al foam cathodes with [Li (G4)] [TFSI]/HFE, delivered less discharge capacity when compared with cells created using [Li(G1)2] [Li(G3)] [TFSI]2 /HFE (not shown).
The difference in this electrochemical property between these cells is considered to be caused by the difference in the solubility of the polysulfide in the electrolyte, as [Li(G1)2] [Li(G3)] [TFSI]2 /HFE is slightly soluble when compared with [Li (G4)] [TFSI]/HFE.44,45 Polysulfides can therefore slightly dissolve into the former electrolyte. In the case of a 2D structured sulfur cathode, the dissolved polysulfide can migrate toward the anode easily. Considering the 3D structure of the sulfur cathode on Al foam, however, this structure possesses the ability to frequently trap the dissolved polysulfides, thereby acting as a shuttle-inhibiting interlayer.46 This characteristic unfortunately does not negate the migration of polysulfides toward the anode, but rather kinetically retards their motion. Therefore, the use of LiNO343 and/or polypyrrole coating33,34 will be necessary to solve the issue posed by polysulfide migration for practical applications.
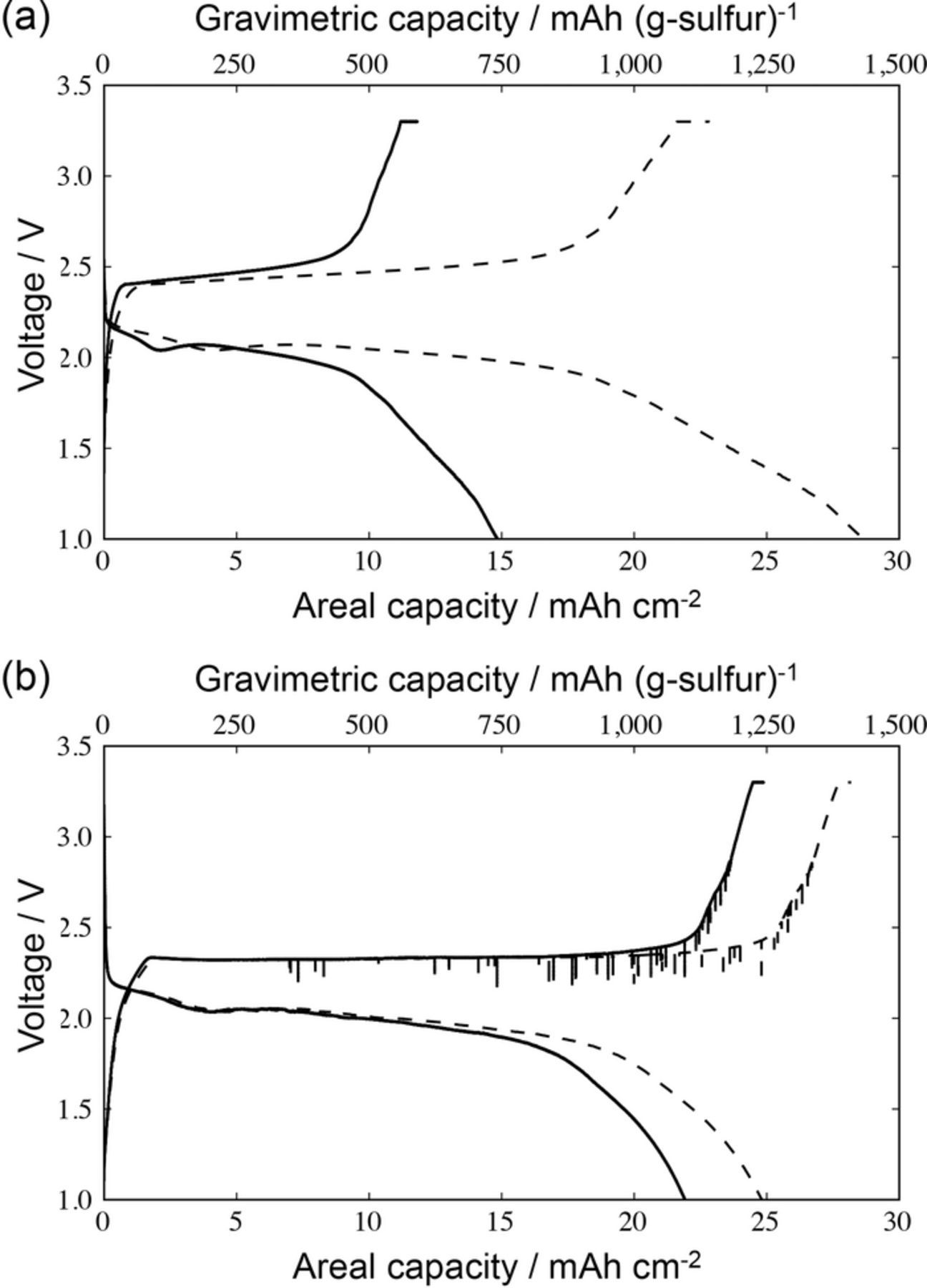
The charge-discharge curves of the pouch-type full cells created using the S/KB-PVdF on Al foam and S/KB-CMC+SBR on Al foam cathodes are shown in Figures 2a and 2b, respectively. The discharge curves of the S/KB-PVdF and S/KB-CMC+SBR cells demonstrate the typical behavior of a sulfur cathode: S8 is converted to Li2Sx (where x is a value between 3 to 8) at around 2.2 V and Li2Sx is converted to Li2S at around 2.0 V.7,47 In the case of a charge-discharge rate of 0.01 C, a difference in the overvoltage was not confirmed. Conversely, the gravimetric discharge capacities of the S/KB-PVdF and S/KB-CMC+SBR cells, obtained when the cells were discharged to 1.0 V, were found to be 1433 and 1241 mAh (g-sulfur)−1, respectively. The difference in the gravimetric capacities was attributed mainly to the behavior shown at the end of the charge or discharge curves. The rate of change of the voltage observed toward the end of the charge or discharge curves of S/KB-CMC+SBR was found to be larger than that of S/KB-PVdF.
Figure 2. Charge-discharge curves of lithium sulfur batteries with the cathode comprised of (a) S/KB-PVdF on Al foam and (b) S/KB-CMC+SBR on Al foam (solid and sparse lines represent areal capacity and gravimetric capacity, respectively). The charge-discharge test was carried out with the cutoff voltage between 1.0 and 3.3 V, the C-rate for charge and discharge was 0.03 C and 0.01 C, respectively, for S/KB-PVdF on Al foam, respectively, and the C-rate for charge and discharge was 0.01 C and 0.01 C, respectively, for S/KB-CMC+SBR on Al foam, respectively.
Although the difference at the end of the discharge cycles may be caused by the diffusion of polysulfides through the [Li(G1)2] [Li(G3)] [TFSI]2 /HFE electrolyte owing to their slight solubility in said electrolyte, this difference may also be accounted for by the difference in both uniformity and utilization efficiency of the sulfur active material owing to the use of different binders. While the CMC binder is known to uniformly cover active materials,51 the PVdF binder only partially makes contact with the active materials,48 directly exposing said materials to the electrolyte solution. These binder characteristics would therefore contribute to the observed behavior at the end of the charge or discharge curves of S/KB-PVdF and S/KB-CMC+SBR.
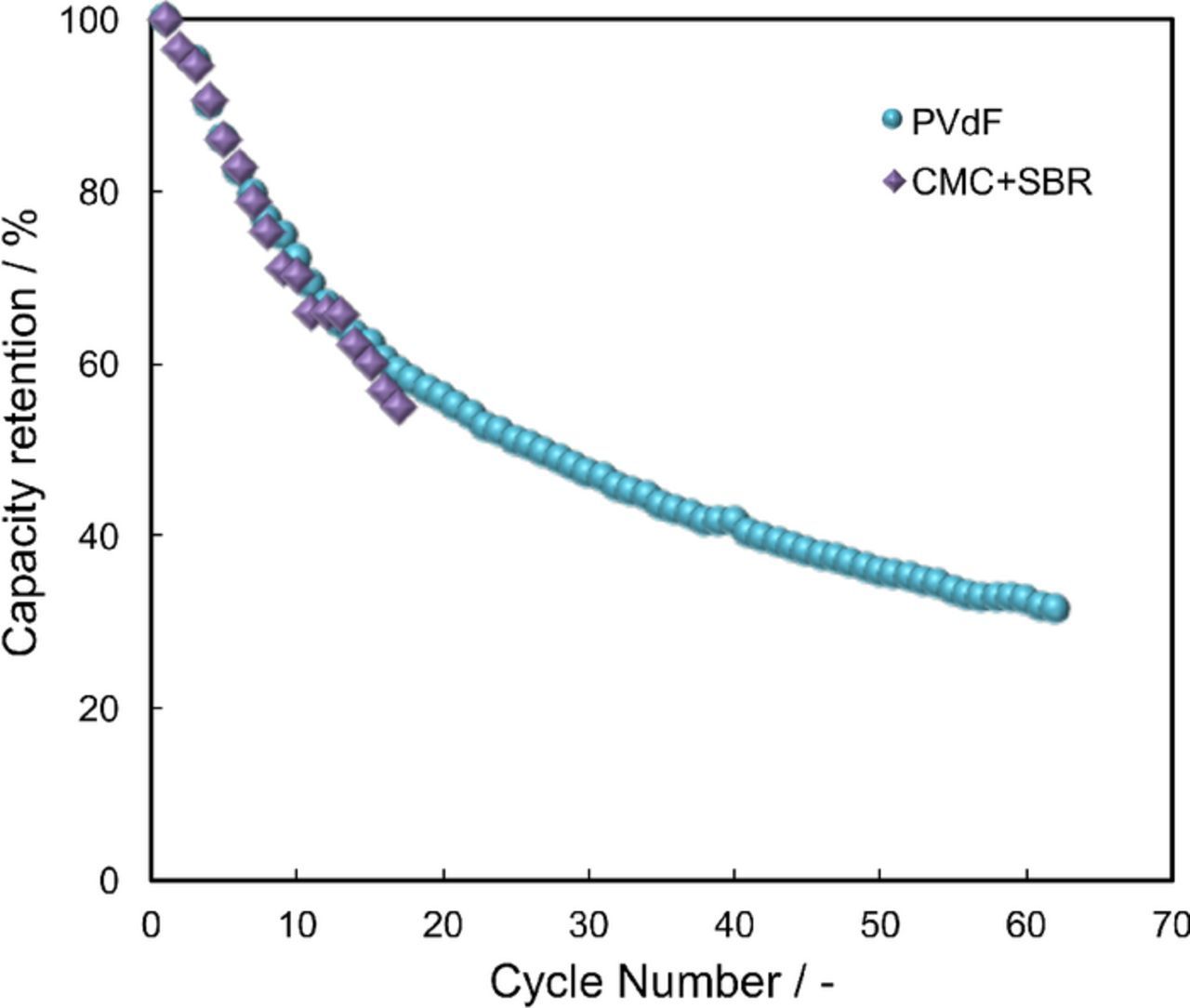
The charge-discharge cycle property of the pouch-type full cells created using the S/KB-PVdF on Al foam and S/KB-CMC+SBR on Al foam cathodes are shown in Figure 3. Both the S/KB-PVdF and S/KB-CMC+SBR cells showed a steep reduction in discharge capacity with charge-discharge cycles. Although the charge-discharge cycle property of the S/KB-PVdF and S/KB-CMC+SBR cells is far from what is required for practical applications of the sulfur cathodes, there remains room for improvement in bettering the capacity retention. Several approaches addressing such drawbacks of sulfur-based cathodes have been previously are reported.33,34,42,43,49 The addition of LiNO342,43 to the electrolyte (which has already been widely adopted for many sulfur cathode systems), along with the use of a polypyrrole coating33,34 or amino-functionalized reduced graphene oxide coating49 are all examples of such attempts to remediate the drawbacks of these systems. Therefore, there exist numerous solutions for addressing the problem of a steep reduction of the discharge capacity of these cells with charge-discharge cycles. It is worth noting that the decrease rate of the capacity of the S/KB-PVdF and S/KB-CMC+SBR cells was almost same, indicating that the capacity retention did not depend on the binders in the case of PVdF and CMC+SBR.
Figure 3. The charge-discharge cycle property of the pouch-type full cells created using the S/KB-PVdF on Al foam and S/KB-CMC+SBR on Al foam cathodes. The charge-discharge test was carried out with the cutoff voltage between 1.0 and 3.3 V, the C-rate for charge and discharge was 0.01 C and 0.01 C for S/KB-PVdF and S/KB-CMC+SBR on Al foam, respectively.
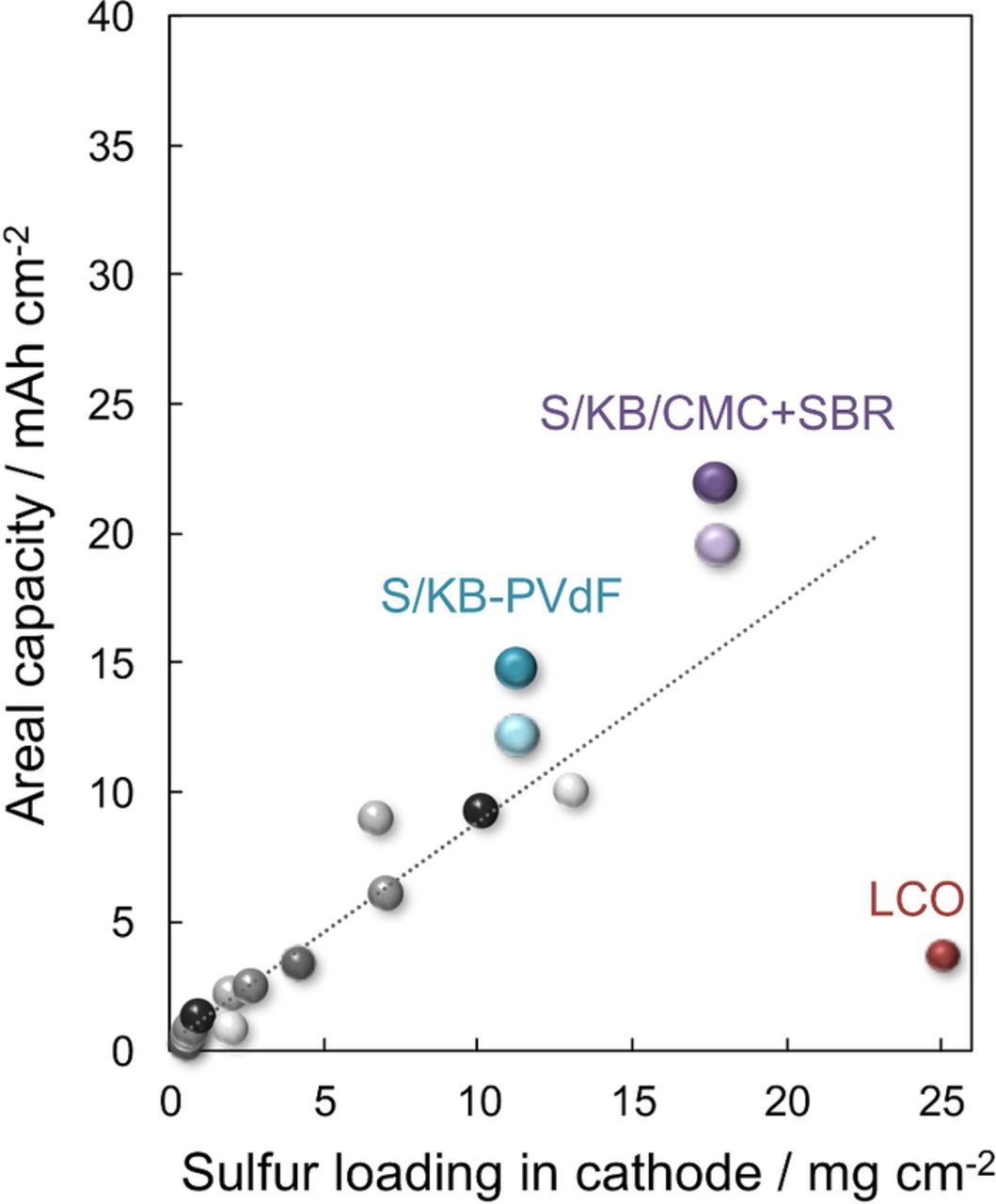
Considering now the areal capacity of the S/KB-PVdF and S/KB-CMC+SBR systems, the discharge capacity of S/KB-CMC+SBR was found to be 21.9 mAh cm−2, which is higher than that observed for S/KB-PVdF (14.8 mAh cm−2). In the case of a lower cutoff voltage of 1.5 V, the discharge capacity was found to be 19.6 and 12.2 mAh cm−2, respectively. This difference is attributed to the difference in sulfur loading in the aluminum foam supported cathodes. Figure 4 shows the sulfur loading in cathode and discharge areal capacity of the S/KB-PVdF and S/KB-CMC+SBR systems, which are plotted in conjunction with the cathodic sulfur loading and areal capacity data summarized and reported by Zhou et al. (Table III),14 and are summarized in Table IV. Many approaches have been used to increase the areal capacity via improving the structure of the carbon supports, as are summarized in Figure 4. However, the areal capacity increase achieved using an aluminum foil current collector was limited because of insufficient ionic and electronic pathway through the cathode. The dotted line was projected by a linear approximation method used in the previously reported plots. As is clearly shown in Figure 4, the areal capacities of the S/KB-PVdF and S/KB-CMC+SBR systems are plotted higher than the dotted line, indicating an effective utilization of the sulfur active material. This can be attributed to the relatively large pore volume in the cathode. The pore volume in the cathode was calculated from the weight and density of sulfur, KB, binder, and aluminum, and is summarized in Table II.
Table III. The cathodic sulfur loading and areal capacity data, and reference lists summarized and reported by Zhou et al.14
| Sulfur loading | Areal capacity | |
|---|---|---|
| in cathode/mg cm−2 | of cathode/mAh cm−2 | References |
| 0.5 | 0.3 | 35 |
| 0.6 | 0.7 | 50 |
| 0.7 | 0.9 | 51 |
| 0.9 | 1.3 | 52 |
| 2 | 0.9 | 53 |
| 2 | 2.2 | 54 |
| 2.6 | 2.5 | 55 |
| 4.2 | 3.4 | 56 |
| 6.7 | 9 | 57 |
| 7 | 6.1 | 58 |
| 10.1 | 9.3 | 14 |
| 13 | 10 | 59 |
| LCO loading in cathode/mg cm-2 | Areal capacity/mAh cm-2 | References |
| 25 | 3.7 | 57 |
Table IV. Sulfur loading in Al foam of the cathode, along with the areal capacity and estimated gravimetric capacity of seven-layer pouch-type lithium-sulfur batteries as a function of lower cutoff voltage.
| Sulfur loading in cathode/mg cm−2 | Areal capacity of the cathode/mAh cm−2 | Gravimetric energy density estimated as the seven-layer pouch-type lithium-sulfur batteries/Wh kg−1 | |
|---|---|---|---|
| S/KB-PVdF on Al 3.3–1.0 V | 11.2 | 14.8 | 141 |
| S/KB-PVdF on Al 3.3–1.5 V | 11.2 | 12.2 | 116 |
| S/KB-CMC+SBR on Al 3.3–1.0 V | 17.7 | 21.9 | 208 |
| S/KB-CMC+SBR on Al 3.3–1.5 V | 17.7 | 19.6 | 186 |
The gravimetric energy density of a pouch-type full battery was estimated based on the areal capacity of the S/KB-PVdF on Al and S/KB-CMC+SBR on Al cathodes, as shown below in Table IV. The theoretical pouch-type full battery is composed of seven sheets of the S/KB-binder on Al cathode, six sheets of a double-sided Li on Cu anode, and two sheets of a single-sided Li on Cu anode, wherein the thickness of the Cu current collector is 10 μm. Fourteen sheets of a polyolefin separator divided each cathode and anode. The amount of Li metal to be used was calculated from the set capacity ratio of cathode and anode of 1:1 and the areal discharge capacity obtained in Figure 2. The amount of electrolyte to be used was calculated based upon 100% of the pore volume in the S/KB-binder cathodes and polyolefin separator. The E/S ratio is 4.8 and 2.7 for the S/KB-PVdF and S/KB-CMC+SBR on Al foam cathodes, respectively. The weights of both the tabs for the current lead and the pouch film for packaging are included. From the estimation of the gravimetric energy density, the seven-layer pouch-type lithium-sulfur batteries were calculated to possess a gravimetric energy density of 208 Wh kg−1 and 141 Wh kg−1 in the case of S/KB-CMC+SBR on Al foam and S/KB-PVdF on Al foam cathodes, respectively, when the battery is operated between 3.3 and 1.0 V. The estimated gravimetric energy densities are summarized in Table IV. Through these results, it can be determined that there is potential for the creation of a lithium-sulfur battery with a gravimetric energy density of more than 200 Wh kg−1. Additionally, there is still room to improve the capacity and energy density of the battery through the addition of a LiNO3 additive.42 Although the lowering of the E/S ratio remains challenging, it may remediated by a process of sparingly solvating electrolytes.53
Conclusions
The potential for the creation of a high areal capacity lithium-sulfur battery using a metal foam current collector was investigated. A cathodic high sulfur loading of 17.7 mg cm−2 was demonstrated by using an aluminum foam current collector and CMC+SBR binder. Although much improvement is required to achieve a better charge-discharge property before these cells can be put to practical use, the operation of a single-layer pouch-type cell with S/KB-CMC+SBR on Al cathode was achieved possessing an unprecedented high areal capacity of 21.9 mAh cm−2. On the basis of these results, the energy density of a lithium-sulfur battery was estimated based upon the assumption of an E/S ratio of 2.7 μL mg−1. This ratio is calculated based upon 100% of the pore volume in the S/KB-CMC+SBR on Al foam cathodes and polyolefin separator, in the case of a seven-layer pouch-type battery, including the weight of the tabs for the current lead and the pouch film for packaging. The results indicated the possibility of achieving a lithium-sulfur battery with an energy density of greater than 200 Wh kg−1.
Although electrodes possessing the necessary high areal capacity are already an established technology, the development of a suitable electrolyte to permit the required current density for high C-rate operation of the electrodes remains a challenge. By increasing the areal capacity by a large amount, lithium-sulfur batteries will subsequently be able to be employed in the field of stationary energy storage rather than solely in the transportation industry as is the current situation, as a result of this type of battery's low power density and low cost. The utilization of aluminum foam is a promising tactic for the reduction of the battery cost because the mass production of metal foam has already been realized for the NiMH battery. Therefore, these electrodes, which possess enormously high areal capacity, are promising for their potential use in low cost stationary energy storage.
Acknowledgment
This work was partly supported Advanced Low Carbon Technology Research and Development Program, Specially Promoted Research for Innovative Next Generation Batteries (ALCA Spring)" from the Japan Science and Technology Agency (JST), Japan.