Abstract
Previously, we studied the anodization of aluminum by adding alcohol to common acidic electrolytes with a focus on film formation efficiency and the hardness of the anodic oxide film. In this study, we focus on the difference in carbon number of monohydric alcohols and select propanol as an additive to confirm whether the effects of adding alcohol on anodization behavior and the growth rate of anodic film are universal regardless of the alcohol type. The tendency of propanol concentration dependence of conductivity and viscosity was generally consistent with results obtained using methanol and ethanol additives; however, unlike other alcohols, the steady state voltage during constant current anodization decreased with an increased amount of propanol. Unlike sulfuric acid only, the addition of propanol clearly improved film growth rate and current efficiency under mild conditions below 100 A‧m−2 and high current density conditions above 500 A‧m−2.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Porous-type anodic oxide films are formed on various metals (e.g., aluminum, titanium, and stainless steel) by anodization in appropriate electrolyte, e.g., an acidic solution and a mixed solution containing fluoride ion. 1–3 Among these films, anodic porous alumina formed on aluminum has attracted attention since the 1990s due to the potential application in nanotechnology research. In addition, anodic porous alumina has been utilized as a template and mask for fabrication of various nanostructured materials 4–8 and as an adherend for coating and adhesion. 9 The device properties and their performance depend on the porous structure of anodic films; thus, it is important to precisely control the dimensions of porous films, e.g., film thickness, pore diameter, interpore distance, pore density, and the regularity of the pore arrangement.
Generally, it is necessary to set the applied voltage according to the dimensions of the desired porous structure and select the optimum electrolyte for the voltage range. Recently, we have investigated the anodization of aluminum in popular acidic electrolytes (e.g., sulfuric and oxalic acids) containing an alcohol as an additive with a focus on film formation efficiency and the hardness of the anodic films. 10–12 Those studies found that the alcohol added to the based-electrolyte suppressed chemical dissolution of the alumina film during anodization, and that the maximum attainable film thicknesses under a constant current anodization increased with an increasing amount of alcohol, which indicates high film formation efficiency. In addition, these effects were significantly higher with polyhydric alcohols (i.e., ethylene glycol and glycerol) than with monohydric alcohols (methanol and ethanol). 11 In 2010, Zaraska et al. reported the effect of normal alcohols (methanol and 1-propanol) on the structural features and regularity of the pore arrangement of self-organized anodic porous alumina formed in phosphoric acid. 13 According to their report, pore arrangement regularity and the uniformity of pore shape increased with increasing normal alcohol content independently of the alcohol type. 13 Several groups have reported interesting results regarding the formation of anodic porous alumina using electrolytes containing various alcohols. 14–27 However, to the best of our knowledge, a detailed investigation of the anodization behavior of aluminum using propanol as an additive has not been conducted to date.
Monohydric alcohols (e.g., methanol, ethanol, 1-propanol, 2-propanol, butanol, and phenol) have a general formula, e.g., Cn H2n+1OH, and contain one hydroxyl group (–OH). In our previous studies, 10–12 methanol (n = 1 for CH3OH) and ethanol (n = 2 for C2H5OH) were used as additives. In this study, to confirm whether the effects of alcohol on anodization behavior and anodic film growth are universal regardless of the type of alcohol, we focused on the difference in carbon number of the monohydric alcohols and selected propanol (n = 3 for C3H7OH) with a three carbon chain (C3-alcohol) as an additive. Here, sulfuric acid was used as the electrolyte with concentrations of 0.3 and 1.5 mol‧dm−3. Propanol has two isomers, i.e., 1-propanol (n-propanol) with the OH group on an end carbon and 2-propanol (isopropanol) with the OH group on the middle carbon; thus, the difference of isomer on film formation was also investigated with a focus on the physical properties (e.g., viscosity and electric conductivity) of the mixed solution, the steady state voltage, and the anodic film's growth rate.
Materials and Methods
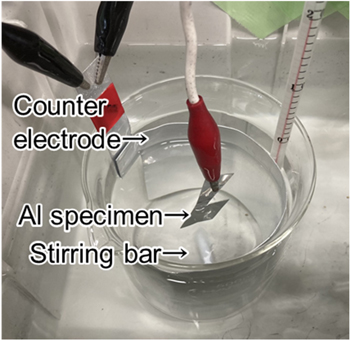
Prior to anodization, aluminum sheets (high purity: 99.99%) with working areas of 10 cm2 were cleaned by immersion in 5 wt% NaOH at 60 °C for 20 s, rinsed in ion-exchanged water, and then immersed in 30 vol% HNO3 at room temperature for 1 min. A 500 ml glass beaker was as the electrolytic cell used for anodization (Fig. 1). An aluminum specimen was set in the electrolyte at the center of the beaker, and a large-area aluminum sheet (99.99%) was used for the cathode and set along the inner wall of the beaker. The glass cell was placed inside a container and the bath temperature was controlled externally with cooling water.
Figure 1. Experimental setup of aluminum anodization.
Download figure:
Standard image High-resolution imageThe anodization was primarily performed in 0.3 and 1.5 mol‧dm−3 H2SO4 containing propanol under a constant current density of 100 A‧m−2. Note that the current density of 100 A‧m−2 is the same as that used in our previous studies. 10–12 In this study, the current density was varied from 50 to 700 A‧m−2 in some experiments. Commercially available sulfuric acid (98 wt%), 1-propanol (99.5 wt%), and 2-propanol (99.5 wt%) were used without further purification. The electrical conductivity and viscosity of a mixed solution of sulfuric acid and propanol were measured at 20 °C using a multifunction water-quality meter (DKK-TOA, MM-60R) and a digital viscometer (ATAGO Co., Ltd, VISCO-895), respectively.
Anodic film growth was examined by measuring the voltage transient under a constant current density using a digital multimeter with a data acquisition system (Keithley, DMM2700). The electrolyte was maintained at a constant temperature of 20 °C using a thermostat and stirred using a magnetic stirrer to prevent local film thickening (i.e., burning 28 ). The stirring speed was set as 300 or 500 rpm depending on conditions. After anodization under different conditions, the thickness of the formed film was measured using an eddy-current coating-thickness tester (Kett Electric Laboratory, LH-373). The surface structure of the anodized aluminum was observed by field-emission scanning electron microscopy (FE-SEM, JEOL, JSM-6701F).
Results and Discussion
Anodization in 0.3 mol‧dm−3 H2SO4 containing propanol
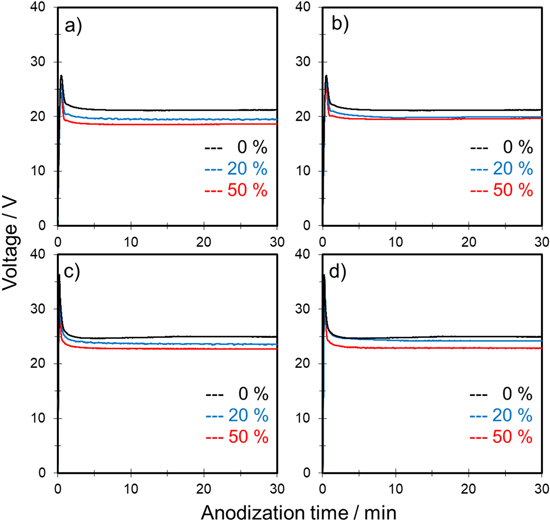
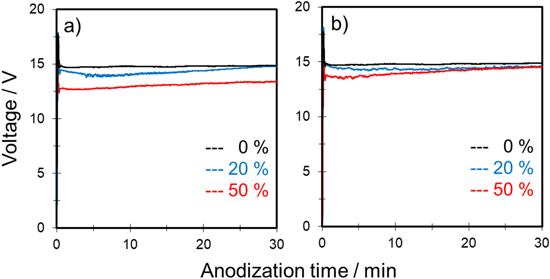
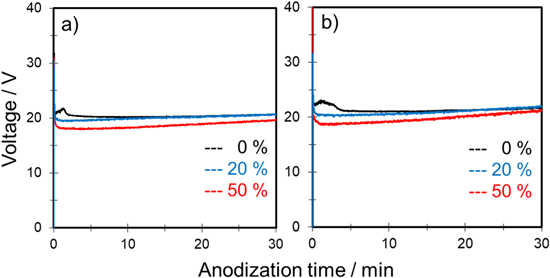
Figure 2 shows typical voltage–time (V–t) curves for the anodization of aluminum in a mixture of 0.3 mol‧dm−3 sulfuric acid and propanol at constant current densities of 50 and 100 A‧m−2. Note that 0.3 mol‧dm−3 sulfuric acid is a suitable electrolyte for self-ordering of anodic porous alumina. 29 The effects of 1-propanol and 2-propanol as an additive were compared at the same concentration (i.e., 20 and 50 vol%). Here, alcohol concentration was indicated in volume percent.
Figure 2. Voltage–time curves for anodization of aluminum in 0.3 mol·dm−3 sulfuric acid with various concentrations of (a), (c) 1-propanol and (b), (d) 2-propanol at a constant current density of (a), (b) 50 and (c), (d) 100 A·m−2 and temperature of 20 °C at 500 rpm.
Download figure:
Standard image High-resolution imageIn Fig. 2, the black curve shows the V–t curves for anodization of aluminum under standard conditions, i.e., 0.3 mol‧dm−3 H2SO4 only. Regardless of the current density, the voltage increased sharply at the beginning of anodization, which suggests formation of a barrier-type oxide film on the aluminum. Then, the voltage decreased and reached steady state voltage in all cases. The initial voltage transient was commonly observed during formation of porous-type alumina film at constant current anodization. 2
When propanol was added to a sulfuric acid-based solution, the steady state voltage decreased by increasing the amount of propanol regardless of the type. Although the shapes of all V–t curves were almost similar, burning was observed frequently under the following three conditions: (1) 0.3 mol‧dm−3 H2SO4 only, (2) 0.3 mol‧dm−3 H2SO4 with 20 vol% 1-propanol, and (3) 0.3 mol‧dm−3 H2SO4 with 20 vol% 2-propanol under a constant current density of 100 A‧m−2.
Figure 3a shows the influence of propanol concentration on the initial peak voltage. As shown, the peak voltages during anodization of the samples with burning were all greater than approximately 35 V. When the peak voltage was less than 35 V, anodization proceeded stably without burning, which indicates that addition of propanol to a sulfuric acid-based solution is effective in preventing the onset of burning.
Figure 3. Relationship between (a) peak voltage at the beginning of anodization and (b) the voltage after anodization for 30 min and propanol concentration in the 0.3 mol·dm−3 sulfuric acid–water–propanol system. The anodization conditions were the same as those in Fig. 2.
Download figure:
Standard image High-resolution imageWhen the sulfuric acid-based solution was used without propanol, the voltage after anodization for 30 min (nearly steady state voltage) was approximately 21 V for 50 A‧m−2 and 25 V for 100 A‧m−2 (Fig. 3b). In contrast, when the mixed solution of sulfuric acid and propanol was used, the steady voltage decreased compared to that of sulfuric acid only. In particular, the voltage was lower with 50 vol% propanol with both current densities. When a monohydric alcohol (i.e., methanol and ethanol) or polyhydric alcohol (i.e., ethylene glycol and glycerol) was added to a sulfuric acid-based solution, anodization voltage increased compared to using only sulfuric acid in our previous study. 11 However, when propanol was added, the voltage decreased with the propanol concentration in all cases. In other words, the propanol addition effect observed in this study works inversely to the addition effects of other alcohols observed in a previous study. 11
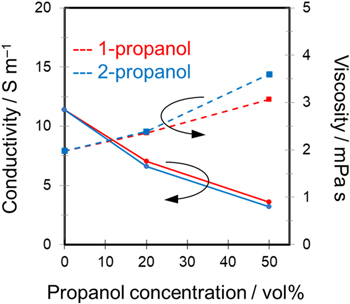
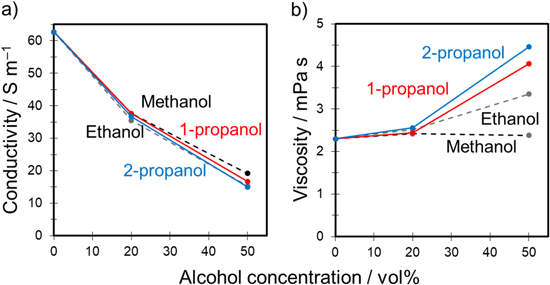
In our previous studies using sulfuric acid–water–alcohol systems, the influences of the type and concentration of alcohol on the physical properties, e.g., viscosity and electric conductivity, of the mixed solution were investigated. 10,11 In the current study, the physical properties of the mixed solutions containing propanol were measured in the same manner (Fig. 4). We found that conductivity decreased with increasing propanol concentration regardless of the isomer type. Adding up to 50 vol% propanol reduced conductivity from approximately 12 (0.3 mol‧dm−3 H2SO4 only) to 4 S m−1. In contrast, adding propanol increased the solution viscosity. The viscosity of the sulfuric acid-based solution without propanol was approximately 2 mPa·s. The viscosity of the mixed solutions increased gradually with the addition of propanol. The mixed solution containing 50 vol% 2-propanol exhibited somewhat higher viscosity of 3.6 mPa·s than that of the mixed solution containing 50 vol% 1-propanol (3.1 mPa·s). The experimental viscosities of 1-propanol and 2-propanol have been reported as 2.197 and 2.414 mPa·s at 20 °C, 30 respectively; thus, the viscosity of the mixed solution containing 2-propanol is considered to be higher than that of the mixed solution containing 1-propanol. In addition, the concentration-dependent trends of decreasing conductivity and increasing viscosity with increasing alcohol concentration were generally consistent with that observed in our previous studies. 10,11
Figure 4. Conductivity and viscosity of a 0.3 mol·dm−3 sulfuric acid–water–propanol system as functions of propanol concentration at 20 °C.
Download figure:
Standard image High-resolution imageThe dielectric constant of the medium affects the reaction and properties of the mixed solution, e.g., conductivity and degree of dissociation. 31,32 The dielectric constant of pure 1-propanol and 2-propanol at 20 °C is reported to be 20.81 and 18.62, respectively. 31 The dielectric constant of a water–1-propanol system varies from 80.37 (1-propanol = 0 wt%) to 66.54 (1-propanol = 20 wt%) and 44.29 (1-propanol = 50 wt%) at 20 °C. 31 The dielectric constant of the mixed solution decreases with increasing amounts of 1-propanol. The dielectric constant of a water–2-propanol system varies from 80.37 (2-propanol = 0 wt%) to 65.72 (2-propanol = 20 wt%) and 43.68 (2-propanol = 50 wt%) at 20 °C. 31
Generally, this decrease in dielectric constant makes it more difficult for the dissociation reaction to proceed in a medium. If the concentration of dissociated ions decreases by increasing the amount of propanol, the conductivity of the solution would also decrease. In our previous studies, it was considered that adding alcohols decreased the conductivity and increased the viscosity of the mixed solution, which in turn increased the voltage during anodization. However, when propanol was added, the opposite effect on anodization voltage was observed despite observing the same decrease in conductivity as with the addition of other alcohols, e.g., methanol and ethanol.
Relationship Between Film Thickness and Charge
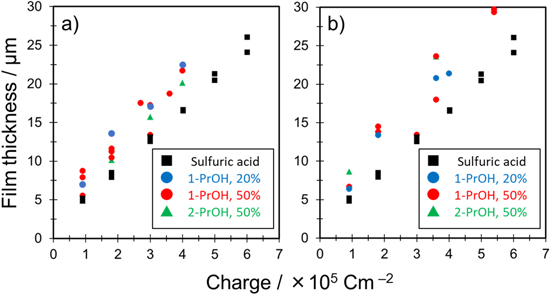
To clarify the effect of adding propanol to the sulfuric acid-based solution on film growth efficiency, the thickness of the films, which were formed under various anodization conditions, was summarized by the electric charge (Fig. 5). Here, average film thickness was calculated from measurements taken from five observation areas on one side of each specimen.
Figure 5. Relationship between film thickness and charge under different anodization conditions in the 0.3 mol·dm−3 sulfuric acid–water–propanol system. Current density was (a) 50 and (b) 100 A·m−2. The thickness of films formed in 0.3 mol‧dm−3 sulfuric acid only at a constant current density of 50 A‧m−2 is plotted in black square as reference values.
Download figure:
Standard image High-resolution imageFirst, the thickness of films formed in 0.3 mol‧dm−3 H2SO4 only at a constant current density of 50 A‧m−2 was plotted in a black square as a function of the charge. Two experiments were performed under each charge condition. No burning was observed under this condition; thus, these data were used as a baseline for comparison. In the case of 0.3 mol‧dm−3 H2SO4 only, the film thickness increased nearly linearly with increasing anodization time (the film thickness is directly proportional to the charge). Note that variations in the data may be due to the accuracy of the film thickness measurement.
Next, the thickness of the films formed in the mixed solution with different propanol concentrations at a constant current density of 50 A‧m−2 was overlaid and plotted in the same manner (Fig. 5a). Generally, if anodization is performed at a constant current density for the same time (i.e., the same electric charge), the film thickness should be nearly the same at approximately the same voltage. However, the thickness of the film formed in the mixed solution was clearly higher with propanol than without propanol at the same charge. Although there is no clear difference in the effect of different isomers on the film growth rate, adding propanol to the electrolyte appears to have a particular effect on film growth rate unlike sulfuric acid only.
The thickness of the films formed in the mixed solution at a constant current density of 100 A‧m−2 was plotted in the same manner (Fig. 5b). Here, the black squares are the same data as in Fig. 5a. Note that the film thickness was measured at average sites rather than at abnormal sites, e.g., the burned area. When the film thicknesses were compared at the same charge, it was clear that thicker films were formed in the mixed solution containing propanol. A higher film growth rate implies that the current efficiency was effectively improved by adding propanol. No clear effect on film growth rate was observed with other alcohols examined in our previous studies; 10–12 thus, this effect is specific to propanol.
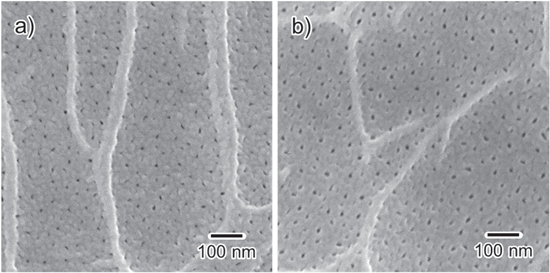
Assuming that the same amount of alumina continues to be produced at the same charge density and constant electrode surface, the film thickness would increase as the porosity increases. Figure 6 shows typical surface SEM images of alumina films formed in 0.3 mol·dm−3 H2SO4 with 50 vol% 1-propanol at 100 A·m−2 for 30 and 60 min. Nanopores were generated in the scallops, which were formed by alkaline etching during pretreatment, and only few were generated on the ridges. Although the pore diameter slightly increased with prolonged anodization, it was <20 nm. The porosity of anodic porous alumina formed in typical anodization conditions is ∼10%. The relationship between the porosity α, interpore distance dint, and pore diameter dpore is given by
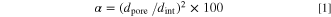
Therefore, if α = 10%, then dpore/dint ≈ 0.32. The SEM image in Fig. 6a indicates that the porosity of the outermost surface should be less than 10%. Alternatively, the porosity of the films formed in 0.3 mol·dm−3 H2SO4 with 50 vol% 1-propanol is not excessively large.
Figure 6. SEM images of the surface of anodized aluminum prepared in 0.3 mol·dm−3 sulfuric acid with 50% 1-propanol at 100 A·m−2 and 20 °C at 500 rpm for (a) 30 and (b) 60 min.
Download figure:
Standard image High-resolution imageGenerally, the current efficiency of porous-type oxide growth is considered much lower than that of the barrier-type growth (approaching 100%) because Al3+ cations are being shed into the electrolyte via direct cation ejection mechanism without contributing to the oxide formation, and depending on the anodization conditions. 2 Therefore, in this study, the improved current efficiency (the proportion of the Al3+ ions produced during anodization that are retained in the anodic film) was attributed to propanol addition.
Anodization in 1.5 mol‧dm−3 H2SO4 containing propanol
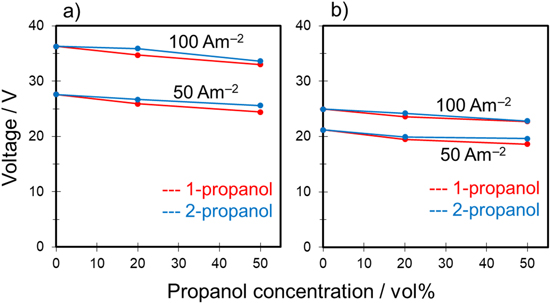
Relatively high concentrations of sulfuric acid (e.g., 200 g‧dm−3) are in industrial applications; thus, the versatility of the additive effect of propanol on film growth was investigated by varying the concentration of sulfuric acid. Figure 7 shows typical V–t curves for anodization of aluminum in a mixture of 1.5 mol‧dm−3 sulfuric acid and propanol at a constant current density of 100 A‧m−2. No burning was observed in all cases. When the sulfuric acid-based solution was used without propanol, the voltage after anodization for 30 min was approximately 15 V. Regardless of the concentration of a sulfuric acid-based solution, the voltage decreased as the amount of propanol increased, as shown in Fig. 2.
Figure 7. Voltage–time curves for anodization of aluminum in 1.5 mol·dm−3 sulfuric acid with various concentrations of (a) 1-propanol and (b) 2-propanol at a constant current density of 100 A·m−2 and temperature of 20 °C at 300 rpm.
Download figure:
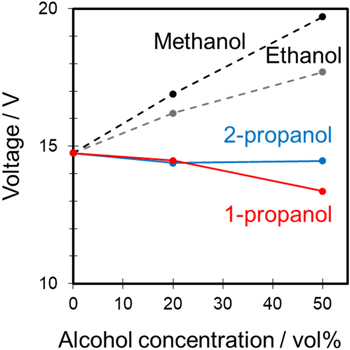
Standard image High-resolution imageFigure 8 shows the relationship between the voltage after anodization for 30 min and alcohol concentration in the mixed solutions. The voltage plots are presented as the average of four experiments. The results for the mixed solutions containing methanol and ethanol, which were used as additives in our previous study, 11 are also plotted in Fig. 8 for comparison. The voltage for sulfuric acid only was approximately 15 V. This value was used as a reference value to examine additive effects. Although all additive alcohols were monohydric alcohols, the anodization voltage clearly increased with methanol and ethanol addition, and the voltage decreased with propanol addition. In particular, the final voltage after 30 min for sulfuric acid containing 50 vol% 1-propanol was the lowest at approximately 13 V.
Figure 8. Relationship between voltage after anodization for 30 min and alcohol concentration in the 1.5 mol·dm−3 sulfuric–water–alcohol system.
Download figure:
Standard image High-resolution imageWe considered that the increased voltage can be attributed to the high solution resistance of the electrolyte used for anodization, which was a consequence of the low ionic conductivity and high viscosity. The conductivity and viscosity of the mixed solutions containing methanol and ethanol 11 are plotted in Fig. 9 for comparison. The tendency of the change in conductivity of the four solutions containing different alcohols was nearly the same as that shown in Fig. 9a. The viscosity of the mixed solution containing propanol was higher than that obtained by adding methanol and ethanol. The higher viscosity of the mixed solution containing 2-propanol was consistent with the trend shown in Fig. 4.
Figure 9. (a) Conductivity and (b) viscosity of a 1.5 mol·dm−3 sulfuric acid–water–alcohol system as functions of alcohol concentration at 20 °C.
Download figure:
Standard image High-resolution imageThese changes in the solution properties led us to expect increased electrical resistance when adding propanol; however, as shown in Figs. 7 and 8, the actual anodization voltage decreased when propanol was added. In other words, it is clear that conductivity and viscosity are not the only factors controlling anodization voltage during film formation. Although the conductivity and viscosity of the sulfuric acid and propanol mixture were measured without an electric field, actual film formation proceeded under an electric field. Therefore, the electrooxidation and dehydrogenation of propanol and/or the intermediates involved in electrooxidation may affect anodization voltage.
Effect of propanol on growth rate of anodic films under high current density conditions
Anodization at high current densities of 500 and 700 A‧m−2 was performed to take advantage of the voltage lowering effect of the addition of propanol. Here, 1-propanol, which has a relatively high voltage lowering effect, was used as an additive. Although no significant difference was observed in the anodization behavior between sulfuric acid only and the mixed electrolyte containing 20% 1-propanol, decreased voltage was observed in the 50% 1-propanol system even for anodization under high current density, as shown in Fig. 10, and for anodization under a mild current density of 100 A‧m−2, as shown in Fig. 7.
Figure 10. Voltage–time curves for anodization of aluminum in 1.5 mol·dm−3 sulfuric acid with various concentrations of 1-propanol at a constant current density of (a) 500 and (b) 700 A·m−2 and temperature of 20 °C at 300 rpm.
Download figure:
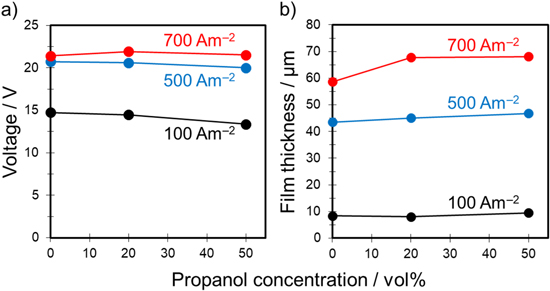
Standard image High-resolution imageFigure 11a shows the relationship between the voltage after anodization for 30 min and 1-propanol concentration in the mixed solutions. Here, the voltage plots are presented as the average of two experiments. Regardless of current density, the voltage for anodization in the mixed solutions was nearly the same as or lower than that of the sulfuric acid only. The thicknesses of the films formed under these conditions shown in Fig. 11a are summarized in Fig. 11b. Measurements were taken three times for each of the front and back surfaces, for a total of six observation areas, and the average film thickness was calculated.
Figure 11. Relationship between (a) voltage after anodization for 30 min and (b) film thickness and 1-propanol concentration in the 1.5 mol·dm−3 sulfuric acid–water–propanol system. The anodization conditions were the same as those in Fig. 10.
Download figure:
Standard image High-resolution imageFor the sulfuric acid only condition, the film thickness was approximately 8.4 μm for 100 A‧m−2, 44.5 μm for 500 A‧m−2, and 58.3 μm for 700 A‧m−2. Generally, the film thickness depends on the charge; thus, if the current density increases by a factor of five or seven, the film thickness is expected to increase accordingly. In fact, the film thickness (44.5 μm) at 500 A‧m−2 and that (58.3 μm) at 700 A‧m−2 were 5.3 and 6.9 times thicker than the film thickness (8.4 μm) at 100 A‧m−2, respectively.
When anodization was conducted in the mixed solution containing 20% 1-propanol, the film thickness was approximately 8.0 μm for 100 A‧m−2, 45.8 μm for 500 A‧m−2, and 68.1 μm for 700 A‧m−2. When adding 50% 1-propanol, the film thickness was approximately 9.5 μm for 100 A‧m−2, 47.6 μm for 500 A‧m−2, and 65.9 μm for 700 A‧m−2. For example, when 20% 1-propanol was added, the film thickness (45.8 μm) at 500 A‧m−2 and that (68.1 μm) at 700 A‧m−2 were 5.7 and 8.5 times thicker than the film thickness (8.0 μm) at 100 A‧m−2, respectively. The rate of increase in the thickness of these films was higher than the rate of increase in current density (i.e., increased by a factor of five or seven), which means that the film formation efficiency is specifically improved by adding propanol. In other words, the current efficiency obtained with propanol was significantly higher than that obtained using only sulfuric acid.
The reactivity of C3-alcohols has been studied in fuel cell research. 33–35 Generally, it is well known that 1-propanol (CH3CH2CH2OH) oxidizes to propanal (CH3CH2CHO) and propionic acid (CH3CH2COOH), and 2-propanol (CH3CH(OH)CH3) oxidizes to acetone (CH3COCH3). Thus, the adsorption and electrooxidation of 1-propanol and 2-propanol at the electrode surface is considered to occur during the anodization process for aluminum in sulfuric acid because the anodization voltage for film formation is sufficiently high to oxidize propanol. To the best of our knowledge, the electrooxidation and dehydrogenation of propanol has not yet been clarified; however, the increase in film formation efficiency obtained via addition of propanol will provide new insights in terms of industrialization.
Conclusions
In this study, the effect of adding propanol to a sulfuric acid-based solution on anodization behavior of aluminum was investigated with a focus on the physical properties (e.g., viscosity and electric conductivity) of the mixed solution, the steady state voltage, and the anodic film's growth rate. Moreover, propanol has two isomers, i.e., 1-propanol and 2-propanol; thus, the difference of isomer in film formation was investigated. The following conclusions were obtained.
- (1)The steady state voltage during constant current anodization decreased with an increasing amount of propanol regardless of the isomer type and sulfuric acid concentration. In addition, adding propanol to a sulfuric acid-based solution was effective in terms of preventing the onset of burning.
- (2)
- (3)Adding propanol improved the film growth rate and current efficiency unlike sulfuric acid only, even under high current density conditions, e.g., greater than 500 A‧m−2. However, there was no clear difference in the effect of different isomers on film growth rate.
Acknowledgments
This work was supported in part by the Light Metal Educational Foundation of Japan.