Abstract
Deposition of metallic powders from aqueous solutions without an external current source is presented. Metallic powders can successfully be produced via galvanic displacement reaction or by electroless deposition from homogenous aqueous solutions or slurries. Formation of powders such as Cu, Ni, Co, Ag, Pd, and Au from homogenous solutions using an appropriate reducing agent or Ag via galvanic displacement reaction was demonstrated. The hydrolysis of metallic ions is a crucial step in the deposition metallic powders via electroless deposition from homogenous solutions. To confirm that the hydrolysis phenomenon is essential in the electroless deposition of metallic powders, oxides, such as Ag2O, Cu2O, and CuO, were suspended in water. It was clearly demonstrated that these oxides can be successfully reduced with an appropriate reducing agent, leading to the precipitation of metallic powders.
Export citation and abstract BibTeX RIS
Without an application of the external current, metallic powders can be formed from aqueous solutions either via galvanic displacement (cementation or immersion plating) or using an appropriate reducing agent.1, 2
In the galvanic displacement reaction, the ions of more noble metal are reduced with the less noble metal according to the reaction
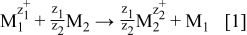
where M1 represents the more noble metal and M2 is the less noble metal. In this way, the less noble metal in terms of electroless deposition acts as a reducing agent of ions of a more positive metal. Typical examples for this type of deposition include Fe/Cu2+, Al/Ag+, Al/Cu2+, etc. In an ideal thermodynamic case, as soon as the surface of the less noble metal is covered with a film of the more noble metal, the deposition should stop. However, as experimentally seen, this is not the case. In this kind of deposition, not only thicker than a monolayer coatings but also powders can be achieved.2, 3
At the beginning, i.e., immediately upon the immersion, the rate of the deposition is the fastest, because the whole surface or more active sites are available for the reaction. As the surface of the less noble metal is covered with the more noble metal, the rate of deposition progressively decreases, because smaller number of sites of the less noble metal is in the contact with solution. Due to porosity or uneven coating, the deposition of the more noble metal will continue as long as the surface of the less noble metal is in contact with the solution. On the other hand, when the less noble metal is completely consumed, then electrons cannot be produced as a consequence of its dissolution (Eq. 1). In this case, the deposition stops.
Various types of the surface morphology and different shapes or sizes of powders can be obtained using this technique of deposition.1–5 The growth of particles of the more noble metal depends on the conditions such as the type of the electrolyte, metal being deposited, temperature, pH, etc.
During the electroless deposition from homogenous aqueous systems, formation of metallic powders into the bulk solution is usually seen when the concentration of the reducing agent is too high or at elevated temperatures. The deposition of thin films proceeds only onto catalytic or previously activated metallic or nonmetallic surfaces. It is important to note that the deposition temperature and concentration of the reducing agent, complexing agents, buffering agents, stabilizers, etc., must be very carefully controlled in order to produce the thin smooth films on properly activated metallic or nonmetallic surfaces.1, 2, 6 The formation of powders is observed both at the activated surface or into the bulk solution. The growth of particles is obviously catalyzed by the metal being deposited, which is the same as the autocatalytic deposition.
Although electrodeposition of metallic powders has been extensively studied over the last century, studying of electroless deposition of powders was in a way neglected. Formation of metallic powders occurs during electroless deposition when the so-called bath instability takes place.1, 2, 6 As such, the electroless deposition of metallic powders was far less investigated than its electrolytic counterpart.
Mechanisms of electroless deposition have not yet been fully explained. Studying of the formation of metallic powders without an external current source may help understanding the mechanistic aspects of electroless deposition.
In the present work, the electroless deposition of metallic powders is presented.
Experimental
For the galvanic deposition, an Al foil was used to deposit silver powders from the acidic or alkaline solutions containing Ag(I) ions. The details for deposition of Ag powders via galvanic deposition are given in the Results and Discussion section.
Other powders produced from homogenous aqueous solutions without an external current source included Ag, Cu, Ni, Co, Au, and Pd. These powders were synthesized using the formulations for the electroless deposition of corresponding metals described in the available literature.1, 2, 6 The conditions for the formation of powders were sought in the so-called bath instability (either elevated temperatures or increased concentrations of the reducing agents). In all experiments, analytical grade chemicals and distilled water were used. The reducing agents used in the present work included formaldehyde, hydrazine, sodium hypophosphite, and ascorbic acid.
After deposition, powders were separated from the slurry and very carefully washed several times with the distilled water and then with ethanol. Powders were then stored under the ethanol for further examinations.
The electroless deposited powders were analyzed by the scanning electron microscopy (SEM) or, where required, by the x-ray diffraction (XRD).
Results and Discussion
Galvanic deposition of metallic powders
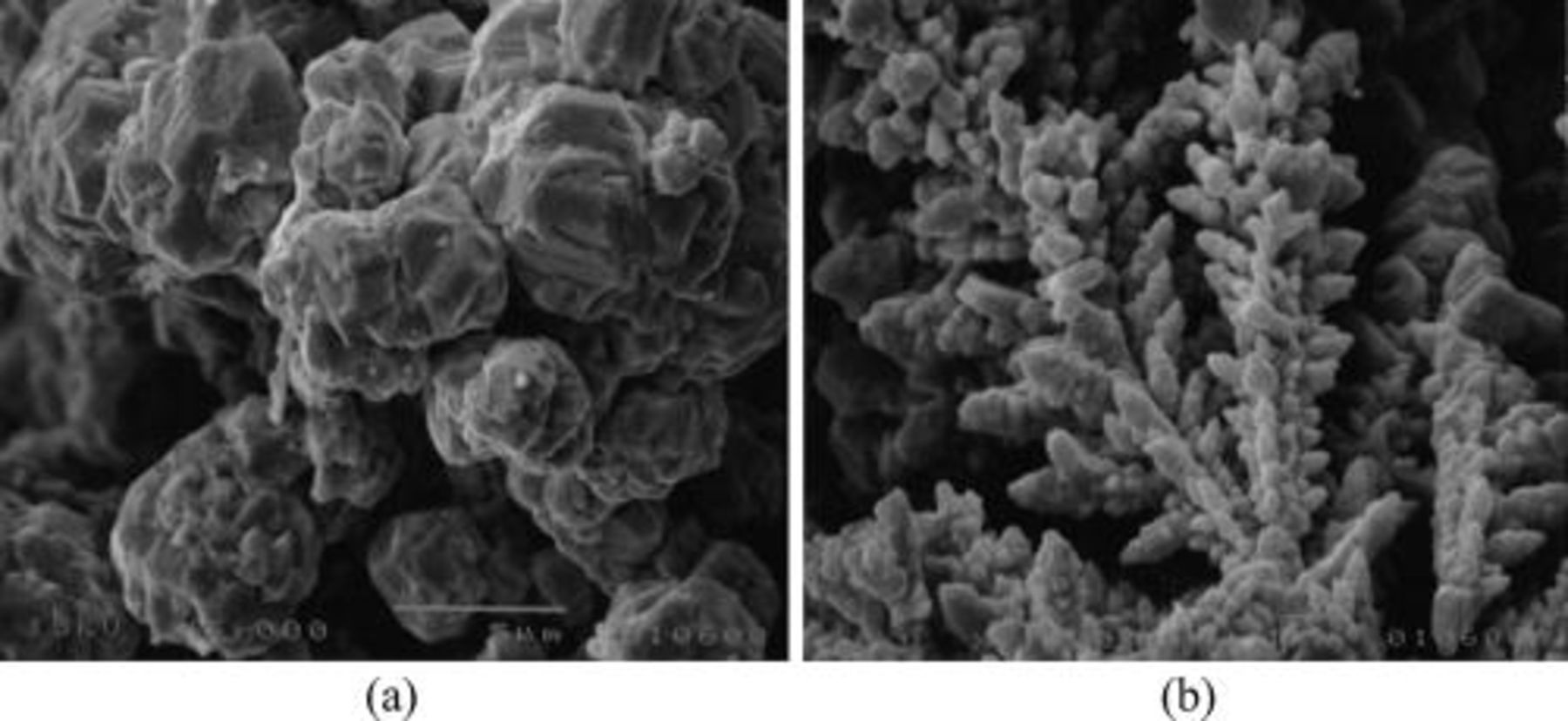
The SEM images of Ag powder produced from a solution containing 0.01 M AgNO3 dissolved in a diluted NH4OH solution at pH of about 10 (a) or from a 0.01 M AgNO3 and 0.5 M citric acid solution at pH of about 2 (b) by the galvanic deposition on an Al foil are presented in Fig. 1.
Figure 1. Ag powders produced via galvanic deposition from (a) 0.01 M AgNO3 dissolved in diluted NH4OH (pH 10, 22°C) and (b) 0.01 M AgNO3 dissolved in 0.5 M citric acid solution (pH 2, 22°C) on an aluminium foil.
In the alkaline solutions (pH 10) on a flat aluminium substrate, globular powders of silver were produced (a), while in acidic solution (pH 2), dendritic (b) forms were produced.
The production of silver powder from an acidic solution using the aluminium substrate can be explained with the following reaction
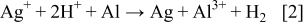
Similarly, in alkaline solutions containing [Ag(NH3)2]+ ions, silver powder is produced onto aluminium foil via galvanic deposition according to the reaction
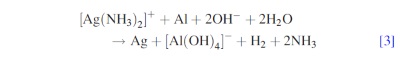
It is worth to note that in both acidic or alkaline conditions, the simultaneous hydrogen evolution reaction takes place due to aluminium dissolution, which is experimentally seen as bubble formation.
In general terms, the experimental observations here suggest that the surface of the less noble metal is not uniformly covered with the more noble metal. Rather, the film of more noble metal is porous. The existence of pores allows the electrolyte to penetrate through and get in contact with the less noble metal. Under these conditions, when in the contact with an appropriate electrolyte, the less noble metal, M2, will dissolve according to the reaction
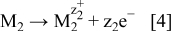
The produced electrons are further transferred and used for the reduction of ions of the more noble metal, M1
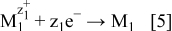
The sum of the Eqs. 4, 5 gives Reaction 1, which describes the galvanic deposition. In this way, the growth of the film or powders of the more noble metal occurs further. Due to porosity of the deposited metal, particles can be obtained via galvanic deposition on powdery or flat substrates. Depending on the conditions, by this type of deposition, different shapes of powders can be produced, as illustrated by Fig. 1.
It seems that the galvanic deposition produces powders when the simultaneous hydrogen evolution reaction takes place
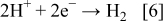
This is demonstrated for the examples of Al/Ag in the present work or Al/Cu in alkaline solutions.3 The simultaneous hydrogen evolution (as bubbles) may significantly change the hydrodynamic conditions at the surface at which the deposition of a more noble metal takes place. Changes in the hydrodynamic conditions may lead to the growth of the particles of various shapes.
For other systems, where the simultaneous hydrogen evolution is not evident, it seems that in order to produce powders by this type of deposition, it is necessary that the surface of the less noble metal is not completely covered by the more noble metal. An incomplete surface coverage of the less noble metal may form porous (spongy) deposits with a poor adhesion, thus leading to the formation of various shapes of powders.
Electroless deposition of metallic powders from homogenous aqueous solutions
Figure 2 presents the SEM micrographs of Cu powders obtained by electroless deposition with formaldehyde as the reducing agent.
Figure 2. SEM micrographs of Cu powders produced from an alkaline CuSO4 solution containing sodium potassium tartrate as a complexing agent of Cu(II) ions and formaldehyde as a reducing agent.
This Cu powder was produced from an alkaline CuSO4 solution containing sodium potassium tartrate as a complexing agent of Cu(II) ions. The reduction of Cu(II) complexed ions with formaldehyde is described with the following simple reaction
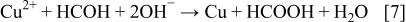
The electroless deposition of copper accompanies the simultaneous hydrogen evolution as is presented with the reaction below
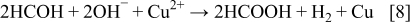
A combination of the Eqs. 7, 8 leads to
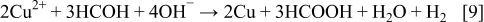
It is obvious from Fig. 2 that Cu powders are agglomerated. The individual particles are predominantly larger than 1 μm, and they have a very rough surface morphology.
Similarly, other metallic powders can be produced by the electroless deposition when the bath instability takes place.1, 2, 6, 7 A few examples, such as Ni powder produced with hydrazine, Co powder produced with hydrazine or hypophosphite, and Ag powder produced with formaldehyde, are presented in Fig. 3. Hypophosphite, hydrazine, and formaldehyde were used as reducing agents of the respective metal ions.
Figure 3. Powders of (a) nickel, produced by electroless deposition with hydrazine, (b) silver, produced with formaldehyde, (c) cobalt, produced with hypophosphite, and (d) cobalt, produced with hydrazine.
Based on the results presented in Fig. 3a, the produced nickel particles are agglomerated globules with a rough surface morphology. The size of these particles, according to the SEM image, is estimated to be between 100 and 300 nm. Silver powders obtained from an alkaline solution containing [Ag(NH3)2]+ and formaldehyde as a reducing agent, based on the SEM image (Fig. 3b), represent agglomerates composed of globular silver particles with the size less than 1 μm. The shapes of Co particles produced by the reduction with hypophosphite (Fig. 3c) and hydrazine (Fig. 3d) are quite different. While the Co powders deposited via the reaction with hydrazine represent the three-dimensional (3D) dendrites, particles produced with hypophosphite are platelets. In both cases, the size of the majority of particles is estimated to be more than 1 μm. The difference in the shape of Co particles can be attributed to their various chemical compositions. It is known that a reduction of Co(II) ions with hypophosphite leads to the incorporation of phosphorous and formation of amorphous deposits,1 while the reduction with hydrazine produces pure Co.7
The results presented in Figs. 2 and 3 show that the shape, size, and morphology of the particles are significantly influenced by the reducing agent and by the nature of the metal deposited.
Deposition of metallic powders with ascorbic acid as a reducing agent
Many metallic powders can be successfully deposited from aqueous solutions using the reducing agents such as formaldehyde, hypophosphite, borohydride, dimethylamine borane (DMAB), or similar. All mentioned reducing agents have been very successfully used in the electroless deposition of thin uniform and continuous films of various metals. Ascorbic acid, as an environmentally friendly reducing agent of various metallic ions (e.g., silver, gold, and copper), has particularly attracted researchers in the field of electroless deposition.1 In the present work, Ag, Cu, and Au powders were produced by the electroless deposition from alkaline or acidic solutions using ascorbic acid as the reducing agent.
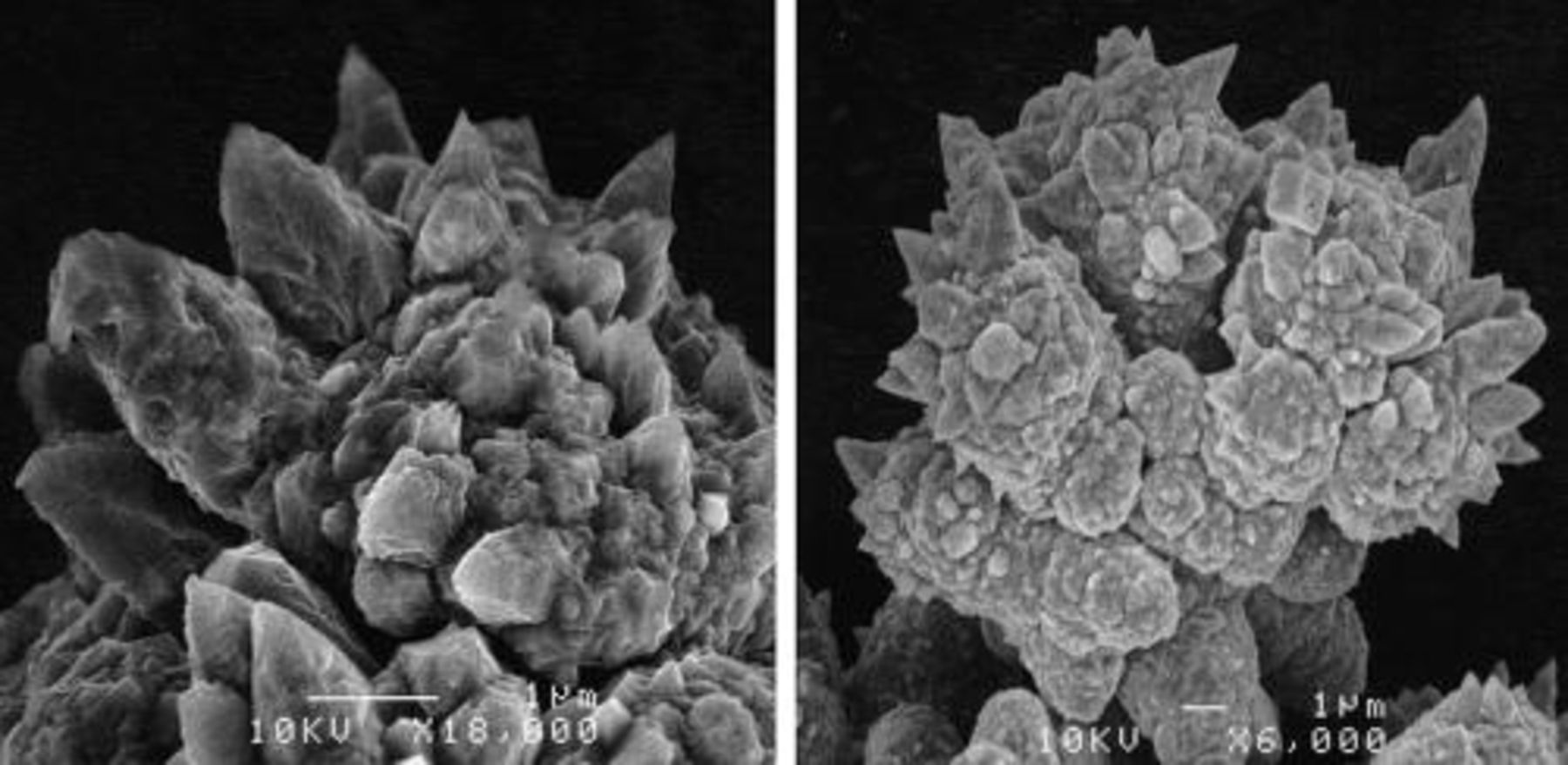
The SEM micrographs of silver particles produced from a solution of AgNO3 in water (a) and from an alkaline [Ag(NH3)2]+ solution (b) with ascorbic acid as the reducing agent are shown in Fig. 4. As these images show, there is a significant difference in the shapes of silver particles produced in acidic or alkaline solutions using the same reducing agent. In both acidic (Fig. 4a) or alkaline (Fig. 4b) solutions, the agglomerated powders were obtained. In the acidic solutions, platelets or geometric crystals of individual particles of silver, with majority being 1 μm in size or more, were found. On the other hand, in the alkaline solutions, spherical-like particles with a rough surface morphology were obtained. The size of these spherical particles is less than 1 μm, with majority being in the range of 300–500 nm. The difference in the shape of produced powders can be attributed to various reaction mechanisms in the alkaline and acidic conditions.
Figure 4. Particles of silver, produced from (a) a solution of AgNO3 in water (a) and (b) an alkaline [Ag(NH3)2]+ solution with ascorbic acid as a reducing agent.
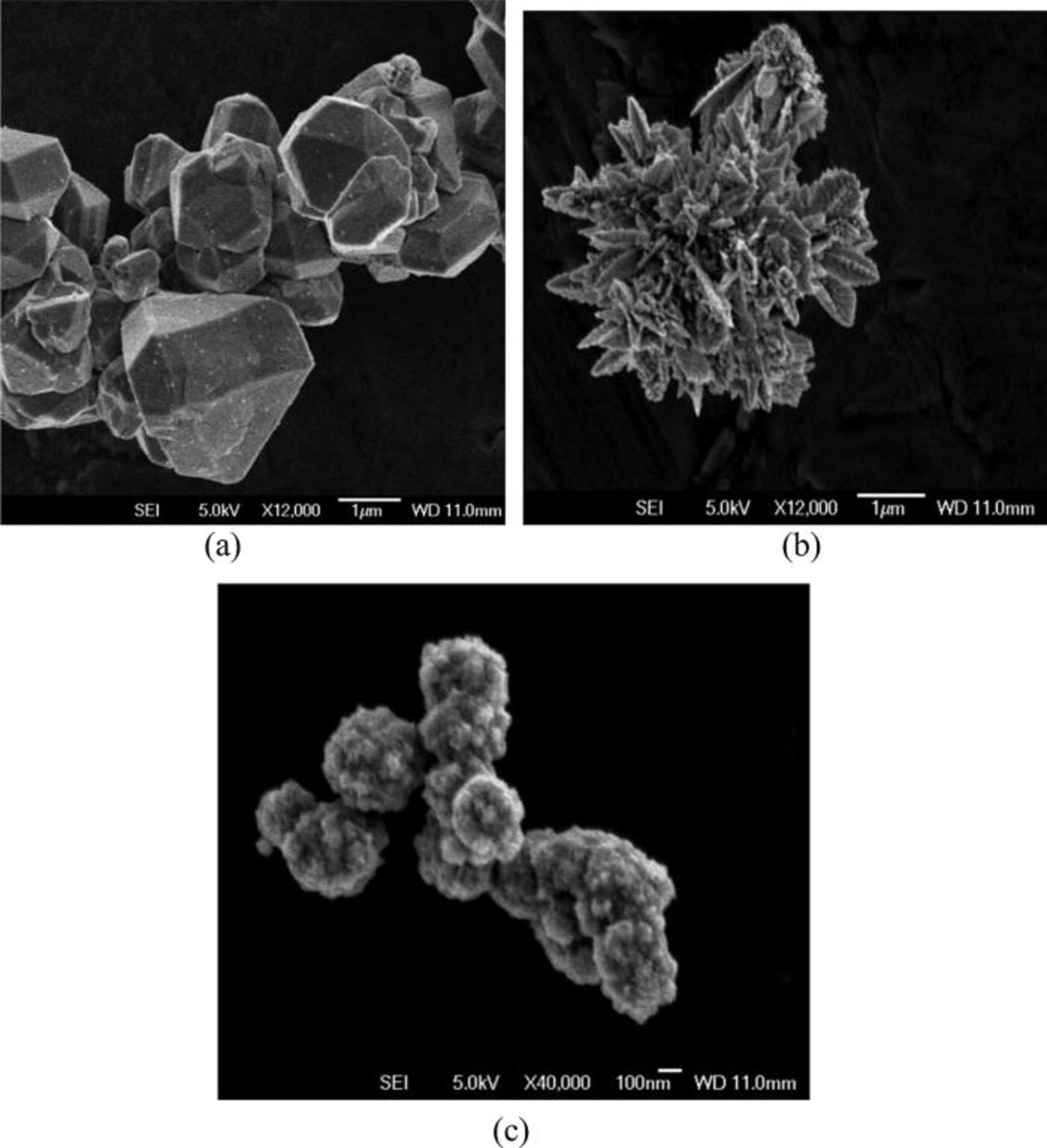
The SEM micrographs of Cu, Au, and Pd powders are presented in Fig. 5. As shown in Fig. 5a, copper represents agglomerated geometric crystals with a wide range in size between 500 nm and more than 2–3 μm. Gold powder produced with ascorbic acid (Fig. 5b) represents agglomerate composed of 3D dendrites, smaller than 500 nm. This result is in a way different from the results published before,8 where the gold particles were found to be star-like. The differences in the shape of gold particles are attributed to various conditions including different solutions used in experiments. The palladium powder presented in Fig. 5c consists of agglomerates of Pd particles. It seems that the individual Pd particles are spheres with a rough surface morphology. The size of the individual particles is estimated at about 300–600 nm. This result is different than previously reported images of Pd particles9 obtained with a reduction of Pd(II) ions with hypophosphite. While the previous paper9 shows smooth spherical Pd particles, in the present work, rough spherical surfaces were obtained. The differences are attributable to various reducing agents used for the deposition of Pd.
Figure 5. SEM micrographs of (a) Cu, (b) Au, and (c) Pd powders produced from [Cu(NH3)4]2+, AuCl3, and [Pd(NH3)2]2+ solutions, respectively, using the ascorbic acid as a reducing agent.
Importantly, the morphologies of electrolessly produced powders illustrated in this paper are quite different and depend on the nature of the reducing agent used in the experiments. This can clearly be seen with a comparison of the SEM results presented in Figs. 2, 3, 4 and 5.
As suggested above for the case of cobalt, the difference in the shapes of powders is attributed to the various chemical composition of the powder itself. On the other hand, the composition of powder is determined by the nature of the reducing agent, e.g., hydrazine or hypophosphite. Furthermore, by using the same reducing agent but different metallic ions, e.g., Co2+ of Ni2+, different shapes of powders can be obtained, as illustrated in Figs. 3a and 3d. This suggests that not only reducing agent but also the nature of the metal, i.e., Ni or Co, would influence the surface morphology of the deposited powder. In addition, as demonstrated in Fig. 4 for the example of silver using the same reducing agent (ascorbic acid), pH may have a significant influence on the shape of electroless deposited powders.
It is obvious that, in order to find a more sophisticated correlation between the shape of the particles and the nature of the reducing agent, further investigations are required. Although the electroless deposition is frequently used in practice, the fundamental aspects governing this process are not yet established. In the following section, the mechanisms of electroless deposition are discussed.
Discussion of mechanistic aspects of the electroless deposition
An increased concentration of the reducing agent, pH, and an elevated temperature significantly contribute to the hydrolysis of the metal ions causing the formation of hydroxyl complexes, hydroxides, and even oxides by this way.1, 2, 6, 7 The electroless deposition of a metal M from a homogenous liquid solution, using a reducing agent Rn−, is described with the following reaction
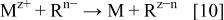
In the electroless deposition, the hydrolysis phenomena, as has already been published before,1, 2, 6, 7 play a very significant role in the reduction of metallic ions from aqueous solutions. Due to an increase in pH, the following reaction may occur
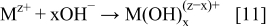
The hydrolyzed metallic species ( ) are further reduced in the presence of an appropriate reducing agent (Rn−) to metal according to the reaction
) are further reduced in the presence of an appropriate reducing agent (Rn−) to metal according to the reaction
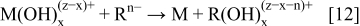
These hydroxides and/or oxides, formed into the bulk solution due to hydrolysis, serve as sites, which are further reduced to the metallic state with an appropriate reducing agent. Because various sizes of particles can be produced, it is obvious that the further growth of the metallic powder is autocatalytic. Furthermore, because the formation of hydrolyzed metallic species is significantly determined by pH, it seems that the degree of hydrolysis can significantly influence the shape of powder particles.
To prove the concept that the formation of hydroxides or oxides in the bulk solution leads to their further reduction to the metallic state, several experiments were performed in the following way. Oxide powders, i.e., Ag2O, Cu2O, and CuO, were used as starting materials. Slurries of the mentioned oxides were simply prepared by a dispersion of solid powders in distilled water. The dispersions were vigorously stirred with a magnetic stirrer, and an adequate amount of ascorbic acid, used as the reducing agent, was added. The mixing was continued until metallic powders (e.g., Ag and Cu) were obtained for approximately 30 min, which was visually observed.
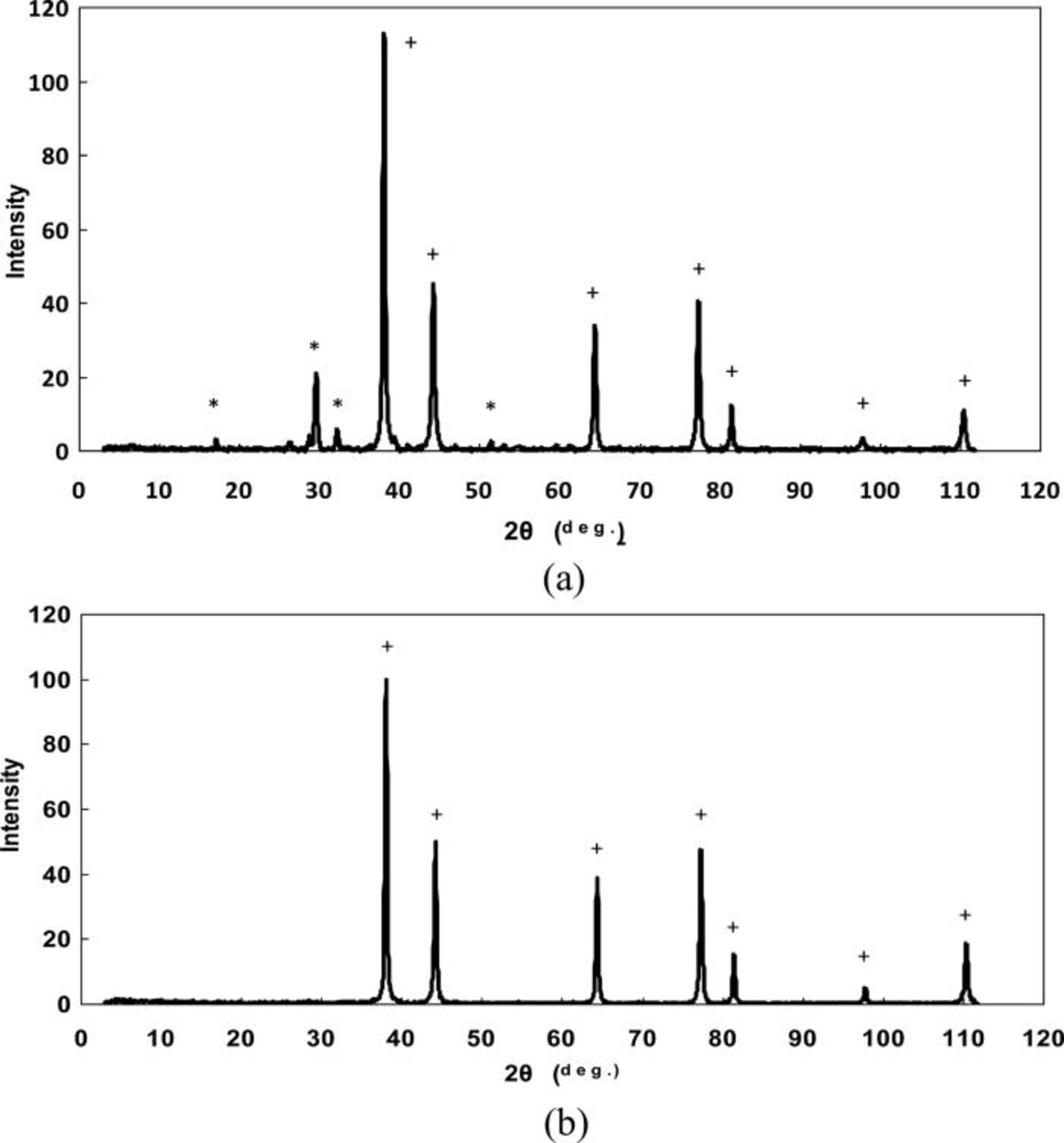
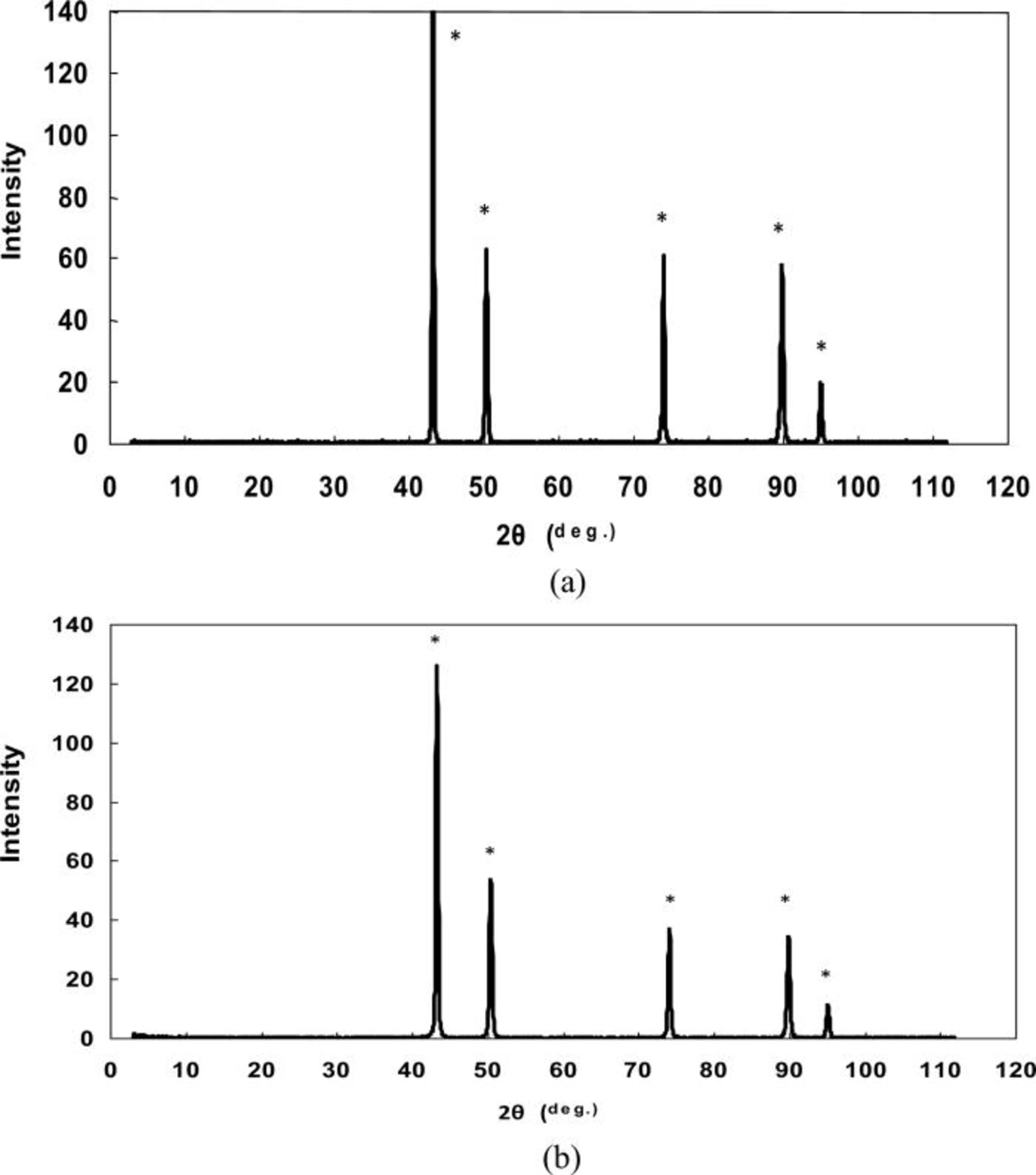
XRD patterns of silver powders produced from the slurry of Ag2O in water and using the ascorbic acid as a reducing agent are presented in Figs. 6a and 6b. Both patterns (a) and (b) in Fig. 6 clearly show Ag peaks, suggesting that Ag2O is reduced to elemental silver. The reduction of Ag2O to the elemental silver using the ascorbic acid can be presented as
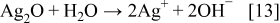
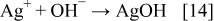
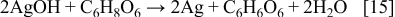
However, while the pattern (b) shows Ag peaks only, in the pattern (a) (Fig. 6), peaks related to silver oxalate (Ag2C2O4) and silver (II) oxide were identified. This result can be explained as follows.
Figure 6. XRD patterns of (a) coarse and (b) fine silver powders produced from a suspension of Ag2O and ascorbic acid as a reducing agent (+, Ag, *, Ag2C2O4).
Ascorbic acid, C6H8O6, can be oxidized into the dehydroascorbic acid C6H6O6, as presented with the following reaction10–12
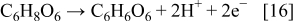
The reduction of metallic ions with ascorbic acid should very carefully be carried out, because its consumption in side reactions is also possible. For example, in the oxidation reaction with the dissolved oxygen, which maybe present in the working solution, ascorbic acid is converted into dehydroascorbic acid13
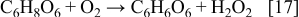
In addition, H2O2 formed during the oxidation, as presented by Reaction 17, reacts with ascorbic acid forming, again, dehydroascorbic acid10
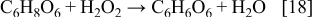
In this way, a significant amount of the ascorbic acid may be consumed during the electroless deposition on side reactions. Furthermore, the complications may arise due to a rapid hydrolysis of dehydroascorbic acid. In fact, it is well established that the hydrolysis of dehydroascorbic acid leads to a formation of the oxalic acid (H2C2O4) and threonic (C4H8O5) acids,10, 14 as shown with the following reaction
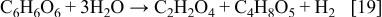
The presence of oxalic acid can lead to the precipitation of silver oxalate according to the reaction
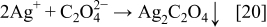
Silver oxalate is a white precipitate15 and, as such, is identified in the pattern (a) of Fig. 6. Traces of AgO (Fig. 6a) can be attributed to the oxidation Ag2O. As shown above with Reaction 17, in a side reaction, H2O2 can be formed. The oxidation of Ag(I) to Ag(II) with hydrogen peroxide is described in the literature.15 Consequently, the formation of AgO, which is identified in the XRD pattern of the final product (Fig. 6b), is described as
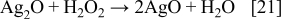
The observations in the present work strongly confirm that the concentration of the ascorbic acid, if used as a reducing agent in the electroless deposition of silver, should very carefully be controlled. It seems that due to consumption of the ascorbic acid on the side reactions, the amount used in the electroless deposition of the corresponding metallic powder should be larger than that required by the stoichiometry.
XRD patterns of the powders produced by the reduction of the aqueous slurries of Cu2O and CuO with the ascorbic acid are presented in Fig. 7. It is very clear from the patterns in Fig. 7 that only Cu peaks were identified. No other impurities were found.
Figure 7. XRD patterns of copper powders produced from suspensions of (a) Cu2O or (b) CuO and ascorbic acid as a reducing agent (*, Cu).
The reduction of Cu2O or CuO to the metallic state is explained with the following reactions
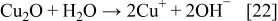
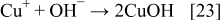
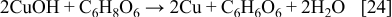
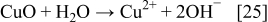
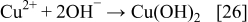
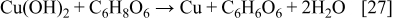
The SEM images of silver and copper powders produced by the reduction of the respective oxides with ascorbic acid are presented in Fig. 8. In both cases, i.e., when the starting material was Cu2O or CuO, similar shapes of Cu particles were obtained. The results presented in Figs. 8a and 8b show that the Cu particles obtained via reduction of Cu2O or CuO with ascorbic acid are geometric crystals with sizes from 500 nm to about 2 μm. Silver powder (Figs. 8c and 8d), produced via reduction of Ag2O with ascorbic acid as a reducing agent represents agglomerates of nanoparticles. The individual size of these nanoparticles is about 100 nm or less.
Figure 8. Copper and silver powders produced by the reduction of (a) Cu2O, (b) CuO, and (c and d) Ag2O with ascorbic acid.
Conclusions
By using the electroless deposition, metallic powders can be obtained via a few different approaches. Galvanic deposition of metallic powders is achieved as a consequence of the porosity of more positive metal or due to simultaneous hydrogen evolution reaction. From homogenous solutions, many metallic powders can be produced when the instability of the solution takes place. This is seen when the concentration of the reducing agent is too high or at elevated temperatures. Formation of metallic powders (e.g., Cu, Ni, Co, Ag, and Pd) using an appropriate reducing agent from homogenous aqueous solutions was demonstrated in the present work. The observations suggest that the hydrolysis of metallic ions is crucial in the deposition of metallic powders via the electroless deposition from homogenous aqueous solutions. It was shown that if starting sources of metallic ions are oxides (e.g., Ag2O, CuO, and Cu2O), using an appropriate reducing agent (ascorbic acid in the present work), metallic powders (Ag or Cu) can successfully be produced.
ACKNOWLEDGMENTS
We are thankful to Dr. H. Wan from the University of Alberta for the experimental help.