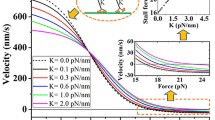
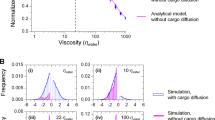
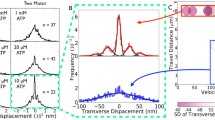
Abstract
Active, motor-based cargo transport is important for many cellular functions and cellular development. However, the cell interior is complex and crowded and could have many weak, non-specific interactions with the cargo being transported. To understand how cargo-environment interactions will affect single motor cargo transport and multi-motor cargo transport, we use an artificial quantum dot cargo bound with few (~ 1) to many (~ 5–10) motors allowed to move in a dense microtubule network. We find that kinesin-driven quantum dot cargo is slower than single kinesin-1 motors. Excitingly, there is some recovery of the speed when multiple motors are attached to the cargo. To determine the possible mechanisms of both the slow down and recovery of speed, we have developed a computational model that explicitly incorporates multi-motor cargos interacting non-specifically with nearby microtubules, including, and predominantly with the microtubule on which the cargo is being transported. Our model has recovered the experimentally measured average cargo speed distribution for cargo-motor configurations with few and many motors, implying that numerous, weak, non-specific interactions can slow down cargo transport and multiple motors can reduce these interactions thereby increasing velocity.
Graphic abstract








Similar content being viewed by others
Data availability
Data sets generated during the current study are available from the corresponding authors on reasonable request.
References
J.L. Ross, M.Y. Ali, D.M. Warshaw, Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 20, 41–47 (2008)
R.D. Vale, The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003)
N. Hirokawa, Y. Noda, Y. Tanaka, S. Niwa, Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682–696 (2009)
N. Hirokawa, R. Takemura, Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 6, 201–214 (2005)
E. Chevalier-Larsen, E.L. Holzbaur, Axonal transport and neurodegenerative disease. Biochimica et Biophysica Acta (BBA)-Mol. Basis Dis. 1762, 1094–1108 (2006)
S.T. Brady, G.A. Morfini, Regulation of motor proteins, axonal transport deficits and adult-onset neurodegenerative diseases. Neurobiol. Dis. 105, 273–282 (2017)
S. Rice, A.W. Lin, D. Safer, C.L. Hart, N. Naber, B.O. Carragher, S.M. Cain, E. Pechatnikova, E.M. Wilson-Kubalek, M. Whittaker, E. Pate, R. Cooke, E.W. Taylor, R.A. Milligan, R.D. Vale, A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999)
M. Kikkawa, Y. Okada, N. Hirokawa, 15 Å resolution model of the monomeric kinesin motor, KIF1A. Cell 100, 241–252 (2000)
S.A. Kuznetsov, V.I. Gelfand, Bovine brain kinesin is a microtubule-activated ATPase. Proc. Natl. Acad. Sci. 83, 8530–8534 (1986)
K. Svoboda, S.M. Block, Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994)
S.M. Block, L.S.B. Goldstein, B.J. Schnapp, Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990)
S.P. Gilbert, M.R. Webb, M. Brune, K.A. Johnson, Pathway of processive ATP hydrolysis by kinesin. Nature 373, 671–676 (1995)
W.O. Hancock, J. Howard, Kinesin’s processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. 96, 13147–13152 (1999)
W.H. Liang, Q. Li, K.M. Rifat Faysal, S.J. King, A. Gopinathan, J. Xu, Microtubule defects influence kinesin-based transport in vitro. Biophys. J. 110, 2229–2240 (2016)
M.W. Gramlich, L. Conway, W.H. Liang, J.A. Labastide, S.J. King, J. Xu, J.L. Ross, Single molecule investigation of kinesin-1 motility using engineered microtubule defects. Sci. Rep. 7(1), 44290 (2017)
J.L. Ross, H. Shuman, E.L.F. Holzbaur, Y.E. Goldman, Kinesin and dynein-dynactin at intersecting microtubules: motor density affects dynein function. Biophys. J. 94, 3115–3125 (2008)
L. Conway, D. Wood, E. Tüzel, J.L. Ross, Motor transport of self-assembled cargos in crowded environments. Proc. Natl. Acad. Sci. 109, 20814–20819 (2012)
P.C. Bressloff, J.M. Newby, Stochastic models of intracellular transport. Rev. Mod. Phys. 85, 135 (2013)
F. Jülicher, A. Ajdari, J. Prost, Modeling molecular motors. Rev. Mod. Phys. 69, 1269–1282 (1997)
R.D. Astumian, Thermodynamics and kinetics of a Brownian motor. Science 276, 917–922 (1997)
M.E. Fisher, A.B. Kolomeisky, Simple mechanochemistry describes the dynamics of kinesin molecules. Proc. Natl. Acad. Sci. 98, 7748–7753 (2001)
T. Guérin, J. Prost, P. Martin, J.-F. Joanny, Coordination and collective properties of molecular motors: theory. Curr. Opin. Cell Biol. 22, 14–20 (2010)
M. Badoual, F. Julicher, J. Prost, Bidirectional cooperative motion of molecular motors. Proc. Natl. Acad. Sci. 99, 6696–6701 (2002)
S. Klumpp, R. Lipowsky, Cooperative cargo transport by several molecular motors. Proc. Natl. Acad. Sci. 102, 17284–17289 (2005)
M.J.I. Müller, S. Klumpp, R. Lipowsky, Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc. Natl. Acad. Sci. U S A 105, 4609–4614 (2008)
D.K. Jamison, J.W. Driver, M.R. Diehl, Cooperative responses of multiple kinesins to variable and constant loads. J. Biol. Chem. 287, 3357–3365 (2012)
A. Kunwar, A. Mogilner, Robust transport by multiple motors with nonlinear force–velocity relations and stochastic load sharing. Phys. Biol. 7, 016012 (2010)
A. Vilfan, E. Frey, F. Schwabl, Force-velocity relations of a two-state crossbridge model for molecular motors. Europhys. Lett. 45, 283–289 (1999)
S. Leibler, D.A. Huse, Porters versus rowers: a unified stochastic model of motor proteins. J. Cell Biol. 121, 1357–1368 (1993)
B. Geislinger, R. Kawai, Brownian molecular motors driven by rotation-translation coupling. Phys. Rev. E 74, 011912 (2006)
F. Jülicher, J. Prost, Cooperative molecular motors. Phys. Rev. Lett. 75, 2618–2621 (1995)
P. Bressloff, J. Newby, Directed intermittent search for hidden targets. New J. Phys. 11, 023033 (2009)
A.V. Kuznetsov, A.A. Avramenko, D.G. Blinov, Numerical modeling of molecular-motor-assisted transport of adenoviral vectors in a spherical cell. Comput. Methods Biomech. Biomed. Engin. 11, 215–222 (2008)
D.A. Smith, R.M. Simmons, Models of motor-assisted transport of intracellular particles. Biophys. J. 80, 45–68 (2001)
C. Loverdo, O. Bénichou, M. Moreau, R. Voituriez, Enhanced reaction kinetics in biological cells. Nat. Phys. 4, 134–137 (2008)
S.M.A. Tabei, S. Burov, H.Y. Kim, A. Kuznetsov, T. Huynh, J. Jureller, L.H. Philipson, A.R. Dinner, N.F. Scherer, Intracellular transport of insulin granules is a subordinated random walk. Proc. Natl. Acad. Sci. 110, 4911–4916 (2013)
A. Kahana, G. Kenan, M. Feingold, M. Elbaum, R. Granek, Active transport on disordered microtubule networks: the generalized random velocity model. Phys. Rev. E 78, 051912 (2008)
A.E. Hafner, H. Rieger, Spatial organization of the cytoskeleton enhances cargo delivery to specific target areas on the plasma membrane of spherical cells. Phys. Biol. 13, 066003 (2016)
A.E. Hafner, H. Rieger, Spatial cytoskeleton organization supports targeted intracellular transport. Biophys. J. 114, 1420–1432 (2018)
D. Ando, N. Korabel, K.C. Huang, A. Gopinathan, Cytoskeletal network morphology regulates intracellular transport dynamics. Biophys. J. 109, 1574–1582 (2015)
M. Scholz, S. Burov, K.L. Weirich, B.J. Scholz, S.M.A. Tabei, M.L. Gardel, A.R. Dinner, Cycling state that can lead to glassy dynamics in intracellular transport. Phys. Rev. X 6, 011037 (2016)
I. Goychuk, V.O. Kharchenko, R. Metzler, How molecular motors work in the crowded environment of living cells: coexistence and efficiency of normal and anomalous transport. PLoS ONE 9(3), e91700 (2014)
K. Sozański, F. Ruhnow, A. Wiśniewska, M. Tabaka, S. Diez, R. Hołyst, Small crowders slow down kinesin-1 stepping by hindering motor domain diffusion. Phys. Rev. Lett. 115, 218102 (2015)
G. Knoops, C. Vanderzande, Motion of kinesin in a viscoelastic medium. Phys. Rev. E 97, 052408 (2018)
J.P. Bergman, M.J. Bovyn, F.F. Doval, A. Sharma, M.V. Gudheti, S.P. Gross, J.F. Allard, M.D. Vershinin, Cargo navigation across 3D microtubule intersections. Proc. Natl. Acad. Sci. U.S.A. 115, 537–542 (2018)
C. Kural, Kinesin and dynein move a peroxisome in vivo: A Tug-of-War or coordinated movement? Science 308, 1469–1472 (2005)
M.C. De Rossi, D.E. Wetzler, L. Benseñor, M.E. De Rossi, M. Sued, D. Rodríguez, V. Levi, Mechanical coupling of microtubule-dependent motor teams during peroxisome transport in Drosophila S2 cells. Biochimica et Biophysica Acta BBA General Subj. 1861, 3178–3189 (2017)
C. Kural, A.S. Serpinskaya, Y.-H. Chou, R.D. Goldman, V.I. Gelfand, P.R. Selvin, Tracking melanosomes inside a cell to study molecular motors and their interaction. Proc. Natl. Acad. Sci. 104, 5378–5382 (2007)
V. Levi, A.S. Serpinskaya, E. Gratton, V. Gelfand, Organelle transport along microtubules in Xenopus melanophores: evidence for cooperation between multiple motors. Biophys. J. 90, 318–327 (2006)
D.B. Hill, M.J. Plaza, K. Bonin, G. Holzwarth, Fast vesicle transport in PC12 neurites: velocities and forces. Eur. Biophys. J. 33, 623–632 (2004)
H.T. Vu, S. Chakrabarti, M. Hinczewski, D. Thirumalai, Discrete step sizes of molecular motors lead to bimodal non-Gaussian velocity distributions under force. Phys. Rev. Lett. 117, 078101 (2016)
L. Scharrel, R. Ma, R. Schneider, F. Jülicher, S. Diez, Multimotor transport in a system of active and inactive kinesin-1 motors. Biophys. J. 107, 365–372 (2014)
J. Xu, Z. Shu, S.J. King, S.P. Gross, Tuning multiple motor travel via single motor velocity: velocity control of travel distance. Traffic 13, 1198–1205 (2012)
A.K. Efremov, A. Radhakrishnan, D.S. Tsao, C.S. Bookwalter, K.M. Trybus, M.R. Diehl, Delineating cooperative responses of processive motors in living cells. Proc. Natl. Acad. Sci. 111, E334–E343 (2014)
E.L. Holzbaur, Y.E. Goldman, Coordination of molecular motors: from in vitro assays to intracellular dynamics. Curr. Opin. Cell Biol. 22, 4–13 (2010)
J. Xu, S.J. King, M. Lapierre-Landry, B. Nemec, Interplay between velocity and travel distance of kinesin-based transport in the presence of Tau. Biophys. J. 105, L23–L25 (2013)
D.W. Pierce, R.D. Vale, Single-molecule fluorescence detection of green fluorescence protein and application to single-protein dynamics. Methods Cell Biol. 1999, 49–74 (1998)
J. Peloquin, Y. Komarova, G. Borisy, Conjugation of fluorophores to tubulin. Nat. Methods 2, 299–303 (2005)
L. Conway, J.L. Ross, Measuring transport of motor cargos. Fluoresc. Methods Mol. Motors 235–52 (2014)
A.M. Stein, D.A. Vader, L.M. Jawerth, D.A. Weitz, L.M. Sander, An algorithm for extracting the network geometry of three-dimensional collagen gels. J. Microsc. 232, 463–475 (2008)
A. Kunwar, M. Vershinin, J. Xu, S.P. Gross, Stepping, strain gating, and an unexpected force velocity curve for multiple-motor-based transport. Curr. Biol. 18, 1173–1183 (2008)
N. Sarpangala, A. Gopinathan, Cargo surface fluidity can reduce inter-motor mechanical interference, promote load-sharing and enhance processivity in teams of molecular motors. PLoS Comput. Biol. 18, 1–32 (2022)
J.O. Wilson, D.A. Quint, A. Gopinathan et al., Cargo diffusion shortens single-kinesin runs at low viscous drag. Sci. Rep. 9, 4104 (2019)
C. Leduc, O. Campas, K.B. Zeldovich, A. Roux, P. Jolimaitre, L. Bourel-Bonnet et al., Cooperative extraction of membrane nanotubes by molecular motors. Proc. Natl. Acad. Sci. U.S.A. 101(49), 17096–17101 (2004)
J.W. Driver, D.K. Jamison, K. Uppulury, A.R. Rogers, A.B. Kolomeisky, M.R. Diehl, Productive cooperation among processive motors depends inversely on their mechanochemical efficiency. Biophys. J. 101(2), 386–395 (2011)
S. Sahu, L. Herbst, R. Quinn, J.L. Ross, Crowder and surface effects on self-organization of microtubules. Phys. Rev. E 103, 062408 (2021)
M. Xu, J.L. Ross, L. Valdez, A. Sen, Direct single molecule imaging of enhanced enzyme diffusion. Phys. Rev. Lett. 123, 128101 (2019)
Acknowledgements
The work presented here was partially supported by National Science Foundation grant PRFB# 1611801 to JAL and NSF INSPIRE Award #1344203 to JLR. JLR was also partially supported by a grant from the Mathers Foundation. RKC was supported by a grant from the Mathers Foundation and Moore Foundation grant # 4308.1.AG and DQ acknowledge support from the National Science Foundation (NSF-DMS-1616926 to AG) and NSF-CREST: Center for Cellular and Biomolecular Machines at UC Merced (NSF-HRD-1547848 and NSF-HRD-2112675 to AG). AG also acknowledges support from the NSF Center for Engineering Mechanobiology grant CMMI-1548571. AG would also like to acknowledge the hospitality of the Aspen Center for Physics, which is supported by National Science Foundation grant PHY-1607611, where some of this work was done.
Author information
Authors and Affiliations
Contributions
JAL conceived and designed the work, performed data acquisition, analysis, and interpretation of data, drafted and edited the manuscript, and is accountable for the work. DAQ conceived and designed the work, performed simulations and theoretical calculations, analyzed and interpreted data, drafted and edited the manuscript, and is accountable for the work. JAL and DAQ contributed equally to the work. RKC helped with data acquisition, analysis, and interpretation of data. BM helped with data analysis and interpretation of data. AG conceived and designed the simulation work, analyzed and interpreted data, drafted and edited the manuscript, and is accountable for the work. JLR conceived and designed the experimental work, analyzed and interpreted data, drafted and edited the manuscript, and is accountable for the work.
Corresponding authors
Ethics declarations
Ethical approval
This study does not report experiments on live vertebrates and/or higher invertebrates. No animals were killed as part of this study.
Additional information
This work is dedicated to Fyl's love of solving a good experimental mystery.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labastide, J.A., Quint, D.A., Cullen, R.K. et al. Non-specific cargo–filament interactions slow down motor-driven transport. Eur. Phys. J. E 46, 134 (2023). https://doi.org/10.1140/epje/s10189-023-00394-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-023-00394-4