Abstract
abstract
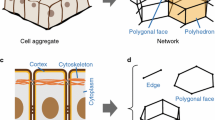
Vertex models describe biological tissues as tilings of polygons. In standard vertex models, the tissue dynamics result from a balance between isotropic stresses, which are associated with the bulk of the cells, and tensions associated with cell–cell interfaces. However, in this framework it is less obvious how to describe anisotropic stresses arising from the bulk of cells. In epithelia, such bulk anisotropic stresses could arise for instance through medial myosin fluctuations. Two recent publications—Tlili et al. (Proc Natl Acad Sci USA 116(51):25430–25439, 2019) and Comelles et al. (eLife 10:e57730, 2021)—have proposed different schemes to implement bulk anisotropic stresses in vertex models. Here we show that while both schemes transform in the same way under affine deformations, they lead to significantly different tissue dynamics. Our results are consistent with the interpretation that the Tilli et al. scheme describes bulk stresses that are uniform within each cell, while the Comelles et al. scheme corresponds to non-uniform bulk stresses. Finally, we wondered whether a standard vertex model can be fully expressed in terms of bulk cellular stresses alone. We find that, in general, neither scheme can mimic the vertex forces created by cell–cell interface tensions
Graphic abstract




Similar content being viewed by others
References
B. Ladoux, R.M. Mège, Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 18(12), 743–757 (2017)
S. Tlili, C. Gay, F. Graner, P. Marcq, F. Molino, P. Saramito, Colloquium: mechanical formalisms for tissue dynamics. Eur. Phys. J. E. 38, 1–31 (2015)
S. Tlili, J. Yin, J.-F. Rupprecht, M.A. Mendieta-Serrano, G. Weissbart, N. Verma, X. Teng, Y. Toyama, J. Prost, T.E. Saunders, Shaping the zebrafish myotome by intertissue friction and active stress. Proc. Natl. Acad. Sci. USA 116(51), 25430–25439 (2019)
J. Comelles, S.S. Soumya, L. Lu, E. LeMaout, S. Anvitha, G. Salbreux, F. Jülicher, M.M. Inamdar, D. Riveline, Epithelial colonies in vitro elongate through collective effects. eLife 10, e57730 (2021)
T. Nagai, H. Honda, A dynamic cell model for the formation of epithelial tissues. Philos. Magn. Part B 81(7), 699–719 (2001)
R. Farhadifar, J.C. Röper, B. Aigouy, S. Eaton, F. Jülicher, The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17(24), 2095–2104 (2007)
D.B. Staple, R. Farhadifar, J.C. Röper, B. Aigouy, S. Eaton, F. Jülicher, Mechanics and remodelling of cell packings in epithelia. Eur. Phys. J. E 33(2), 117–127 (2010)
A.G. Fletcher, J.M. Osborne, P.K. Maini, D.J. Gavaghan, Implementing vertex dynamics models of cell populations in biology within a consistent computational framework. Prog. Biophys. Mol. Biol. 113(2), 299–326 (2013)
D. Bi, J.H. Lopez, J.M. Schwarz, M.L. Manning, A density-independent rigidity transition in biological tissues. Nat. Phys. 11(12), 1074–1079 (2015)
S. Alt, P. Ganguly, G. Salbreux, Vertex models: from cell mechanics to tissue morphogenesis. Philos. Trans. R. Soc. B Biol. Sci. 372(1720), 20150520 (2017)
M. Rauzi, P. Lenne, T. Lecuit, Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468(7327), 1110–1114 (2010)
B. Dehapiot, R. Clément, H. Alégot, G. Gazsó-Gerhát, J.M. Philippe, T. Lecuit, Assembly of a persistent apical actin network by the formin Frl/Fmnl tunes epithelial cell deformability. Nat. Cell Biol. 22(7), 791–802 (2020)
R. Penrose, A generalized inverse for matrices. Math. Proc. Cambridge Philos. Soc. 51(3), 406–413 (1955)
G.K. Batchelor, The stress system in a suspension of force-free particles. J. Fluid Mech. 41(3), 545–570 (1970)
A.W.C. Lau, T.C. Lubensky, Fluctuating hydrodynamics and microrheology of a dilute suspension of swimming bacteria. Phys. Rev. E 80(1), 011917 (2009)
A. Nestor-Bergmann, G. Goddard, S. Woolner, O.E. Jensen, Relating cell shape and mechanical stress in a spatially disordered epithelium using a vertex-based model. Math. Med. Biol. 35, 1–27 (2018)
R. Farhadifar, J.-C. Röper, B. Aigouy, S. Eaton, F. Jülicher, The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17(24), 2095–2104 (2007)
S.Z. Lin, S. Ye, G.K. Xu, B. Li, X.Q. Feng, Dynamic migration modes of collective cells. Biophys. J. 115(9), 1826–1835 (2018)
M. Merkel, R. Etournay, M. Popović, G. Salbreux, S. Eaton, F. Jülicher, Triangles bridge the scales: quantifying cellular contributions to tissue deformation. Phys. Rev. E 95(3), 032401 (2017)
D. Bi, X. Yang, M.C. Marchetti, M.L. Manning, Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X 6(2), 021011 (2016)
F. Giavazzi, M. Paoluzzi, M. Macchi, D. Bi, G. Scita, L. Manning, R. Cerbino, M.C. Marchetti, Flocking transitions in confluent tissues. Soft Matter 14, 3471–7 (2018)
M. Paoluzzi, L. Angelani, G. Gosti, M.C. Marchetti, I. Pagonabarraga, G. Ruocco. Alignment interactions drive structural transitions in biological tissues. arXiv:2107.00523, pp. 1–15 (2021)
T.B. Saw, A. Doostmohammadi, V. Nier, L. Kocgozlu, S. Thampi, Y. Toyama, P. Marcq, C.T. Lim, J.M. Yeomans, B. Ladoux, Topological defects in epithelia govern cell death and extrusion. Nature 544(7649), 212–216 (2017)
K. Kawaguchi, R. Kageyama, M. Sano, Topological defects control collective dynamics in neural progenitor cell cultures. Nature 545(7654), 327–331 (2017)
G. Duclos, C. Blanch-Mercader, V. Yashunsky, G. Salbreux, J.F. Joanny, J. Prost, P. Silberzan, Spontaneous shear flow in confined cellular nematics. Nat. Phys. 14(7), 728–732 (2018)
P. Maiuri, J.-F. Rupprecht, S. Wieser, V. Ruprecht, O. Bénichou, N. Carpi, M. Coppey, S. De Beco, N. Gov, C.-P. Heisenberg, C.L. Crespo, F. Lautenschlaeger, M. LeBerre, A.-M. Lennon-Dumenil, M. Raab, H.-R. Thiam, M. Piel, M. Sixt, R. Voituriez, Actin flows mediate a universal coupling between cell speed and cell persistence. Cell 161(2), 374–386 (2015)
Acknowledgements
We thank M.M. Inamdar, F. Jülicher, G. Salbreux, D. Riveline, and S. Tlili for useful comments. This project was funded by grants from the Investissements d’Avenir French Government program managed by the French National Research Agency (ANR-16-CONV- 0001 and ANR-20-CE30-0023 COVFEFE) and from Excellence Initiative of Aix-Marseille University - A*MIDEX.
Author information
Authors and Affiliations
Contributions
All authors contributed to deriving the analytical results, writing the paper, and conceptualizing the research; S-ZL also carried out the simulations.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (mov 1085 KB)
Supplementary file 2 (mov 1642 KB)
Supplementary file 3 (mov 1321 KB)
Appendices
Appendix A: Cell and tissue aspect ratio change
In Figs. 2 and 3, we have quantified the relative cell aspect ratio change as \(\varepsilon _{\mathrm {cell}} = \mathrm{AR}_\mathrm{final} / \mathrm{AR}_\mathrm{initial} - 1\), where \(\mathrm AR_{initial}\) (\(\mathrm AR_{final}\)) is the aspect ratio of the cell in the initial (final) state. \(\mathrm AR\) is defined as \(\mathrm{AR} = \sqrt{{I_1}/{I_2}}\), where \(I_1\) and \(I_2\) (\(I_1 > I_2\)) are the two eigenvalues of the matrix \(\varvec{M}\) defined in Eq. (11).
Similarly, for free boundary conditions, we analogously define the relative tissue aspect ratio change \(\varepsilon _{\mathrm {tissue}}\). In this case however, the tensor \(\varvec{M}\) is defined via Eq. (11) using all margin vertices of the tissue.
Appendix B: Consistency checks
1.1 Appendix B.1: Zero net force and torque across each cell
Zero net force We check that the net force on all vertices of a cell satisfies the relation
in both schemes. Indeed, substituting Tlili et al. forces Eq. (4) into Eq. (B.1), we find that
Likewise, substitution of Comelles et al. definitions Eq. (13) into Eq. (B.1) leads to
Zero net torque We also check that the overall torque acting on a given cell satisfies the relation
in both schemes. Substituting Tlili et al. scheme definition Eq. (4) into Eq. (B.4), and adopting Einstein notation with Greek dimension indices, we obtain:
To further simplify this expression, we note that:
This relation can be verified by testing each dimension index combination for the pair \((\beta ,\mu )\). Using Eq. (B.6) in Eq. (B.5), we find that
For symmetric bulk stress tensors \(\varvec{\sigma }^{(\mathrm {b})}\), we thus obtain \(\varvec{T}=\varvec{0}\).
Substitution of Comelles et al. force definition Eq. (13) into Eq. (B.4), adopting index notation, yields
Hence, again, for symmetric \(\varvec{\sigma }^{(\mathrm {b})}\), the overall torque is zero: \(\varvec{T}=\varvec{0}\).
1.2 Appendix B.2: Self-consistency: the input bulk stress equals the output Batchelor stress
Here we show that in both schemes, Batchelor stress Eq. (15) is identical to the input bulk cell-stress \(\varvec{\sigma }^{(\mathrm {b})}\), i.e. that: \(\varvec{\sigma }_B\left[ \varvec{F}_{i}^{\left( T,C \right) } \right] =\varvec{\sigma }^{(\mathrm {b})}\).
Substituting Tlili et al. force expression Eq. (4) into Eq. (15) we immediately obtain:
where in the second step, we have applied Eq. (B.6).
Similarly, substitution of Comelles et al. force definition Eq. (13) into Eq. (15) leads to
with the matrix \(\varvec{M}\) defined in Eq. (11).
Appendix C: Capacity of both schemes to reproduce standard vertex model forces
1.1 Appendix C.1: Identity of forces for regular polygonal cells
Here we consider a regular polygonal cell composed of N vertices \(\{ \varvec{r}_i \}_{i = 1, \dots , N}\) with \({{\varvec{r}}_{i}}=r{{\varvec{e}}_{i}}\), with \(r >0\), \({{\varvec{e}}_{i}}=\left( \cos {{\theta }_{i}},\sin {{\theta }_{i}} \right) \), and the vertex angles \({\theta }_{i}=2\left( i-1 \right) \pi /N\). Area and perimeter of such a cell are:
To simplify standard vertex model forces Eq. (35), we use the relations
and obtain:
Differences between the forces according to both schemes and the standard vertex model forces for irregular cell shapes. Vertex positions correspond to a randomly perturbed hexagonal cell according to Eq. (C.17). a The Tlili et al. forces \(\varvec{F}_i^{(\mathrm {T})}\) (green arrows), the Comelles et al. forces \(\varvec{F}_i^{(\mathrm {C})}\) (blue arrows), and the standard vertex model forces \(\varvec{F}^{(\mathrm {svm})}_i\) (red arrows) at each cell vertex for an example configuration. b Dependence of the relative force deviations \(\zeta _\mathrm{T}\) and \(\zeta _\mathrm{C}\) defined in Eq. (C.18) on the amplitude \(\eta \) of the deviation of the cell shape from a regular hexagonal shape. Vertex model parameters are provided in Table 1
The Tlili et al. vertex forces in Eq. (38) simplify since we consider regular hexagons and thus \(\varvec{S} = \varvec{I} / 2\). Inserting Eqs. (C.11), (C.12) and (C.13) in Eq. (36), we recover indeed an expression identical to the one in Eq. (C.15).
To compute the Comelles et al. forces, we fist evaluate each component of the \(\varvec{M}\) tensor defined in Eq. (11). We find for \(N\ge 3\):
while
which leads to \(\varvec{M}=N{{r}^{2}}/2 \ \varvec{I}\). Therefore, the vertex forces given by the Comelles et al. scheme are
Injecting stress expression Eq. (36) into Eq. (C.16), we find that the Comelles et al. expression is identical to Eq. (C.15); thus \(\varvec{F}_i^\mathrm{(T)} = \varvec{F}_i^\mathrm{(C)} = \varvec{F}^{(\mathrm {svm})}_i\) for regular polygonal cells.
1.2 Appendix C.2: Numerical comparison of forces for irregular hexagons
We numerically compare the Tlili et al. and Comelles et al. forces with the standard vertex model forces for non-regular hexagonal cells. We define the corners of the hexagonal cell as \(\varvec{r}_i=(x_i,y_i)\) with \(i=1,2,\cdots ,6\) and:
Here, \(\vartheta _{ix}\) and \(\vartheta _{iy}\) are independent, zero-mean, unit-variance Gaussian random variables, and \(\eta \) is a parameter tuning the deviation of the cell shape from that of a regular hexagon.
Discrepancies between the two schemes and the standard vertex model forces are quantified in terms of
where \(X \in \lbrace T,C\rbrace \), indicating Tlili and Comelles et al. schemes, respectively. The average \(\langle \cdot \rangle \) is over all cell vertices and over 1, 000 realizations for the set of the Gaussian random variables \(\vartheta _{ix}\) and \(\vartheta _{iy}\).
We find that the deviations of both Tlili et al. and Comelles et al. forces from the standard vertex model forces increase with increasing cell shape deviation from a regular hexagonal shape, see Fig. 4b. However, the Tlili et al. forces deviated less from the standard vertex model forces than the Comelles et al. forces did.
Rights and permissions
About this article
Cite this article
Lin, SZ., Merkel, M. & Rupprecht, JF. Implementation of cellular bulk stresses in vertex models of biological tissues. Eur. Phys. J. E 45, 4 (2022). https://doi.org/10.1140/epje/s10189-021-00154-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-021-00154-2