Abstract
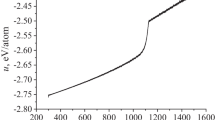
Melting of fcc Lennard-Jones (LJ) nanoparticles is studied by heating up models from low temperature toward liquid phase using molecular dynamics (MD) simulation. Atomic mechanism of melting is analyzed via temperature dependence of potential energy, heat capacity, analysis of the spatio-temporal arrangements of liquidlike atoms occurred during the heating process. Moreover, radial distribution function (RDF), mean-squared displacement (MSD) of atoms and radial density profile are also used for deeper analyzing melting. Surface melting is under much attention. We also analyze the evolution of structure of nanoparticles upon heating via the global order parameter Q 6 and Honeycutt-Andersen (HA) analysis. We find previously unreported information as follows. At temperature far below a melting point, a quasi-liquid layer containing both liquidlike and solidlike atoms occurs in the surface shell of nanoparticles unlike that thought in the past. Further heating leads to the formation of a purely liquid layer at the surface and homogeneous occurrence/growth of liquidlike atoms throughout the interior of nanoparticles. Melting proceeds further via two different mechanisms: homogeneous one in the interior and propagation of liquid front from the surface into the core leading to fast collapse of crystalline matrix.
Similar content being viewed by others
References
Q.S. Mei, K. Lu, Prog. Mater. Sci. 52, 1175 (2007)
K. Lu, Z.H. Jin, Curr. Opin. Solid State Mater. Sci. 5, 39 (2001)
J.G. Dash, Contemp. Phys. 30, 89 (1989)
H. Sakai, Surf. Sci. 351, 285 (1996)
K.F. Peters, Y.-W. Chung, J.B. Cohen, Appl. Phys. Lett. 71, 2391 (1997)
K.F. Peters, J.B. Cohen, Y.-W. Chung, Phys. Rev. B 57, 13430 (1998)
M. Polcik, L. Wilde, J. Haase, Phys. Rev. Lett. 78, 491 (1997)
P.Z. Pawlow, Z. Phys. Chem. 65, 545 (1909)
P.A. Buffat, J.P. Borel, Phys. Rev. A 13, 2287 (1976)
H. Reiss, I.B. Wilson, J. Colloid Sci. 3, 551 (1948)
C.R.M. Wronski, J. Appl. Phys. 18, 1731 (1967)
K.J. Hanszen, Z. Phys. 157, 523 (1960)
P.R. Couchman, W.A. Jesser, Nature (London) 269, 481 (1977)
V.P. Skripov, V.P. Koverda, V.N. Skokov, Phys. Stat. Sol. A 66, 109 (1981)
R.R. Vanfleet, J.M. Mochel, Surf. Sci. 341, 40 (1995)
V.V. Hoang, J. Phys. Chem. C 116, 14728 (2012)
J.D. Honeycutt, H.C. Andersen, J. Phys. Chem. 91, 4950 (1987)
Z.H. Jin, P. Gumbsch, K. Lu, E. Ma, Phys. Rev. Lett. 87, 055703 (2001)
E.G. Noya, P.K. Doye, J. Chem. Phys. 124, 104503 (2006)
P.A. Frantsuzov, V.A. Mandelshtam, Phys. Rev. E 72, 037102 (2005)
V.A. Mandelshtam, P.A. Frantsuzov, F. Calvo, J. Phys. Chem. A 110, 5326 (2006)
V.M. Samsonov, S.S. Kharechkin, S.L. Gafner, L.V. Redel, Y.Y. Gafner, Cryst. Rep. 54, 526 (2009)
J. Deckman, V.A. Mandelshtam, Phys. Rev. E 79, 022101 (2009)
K. Nishio, J. Koga, T. Yamaguchi, F. Yonezawa, J. Phys. Soc. Jpn 73, 627 (2004)
T. Bachels, H.-J. Guntherodt, Phys. Rev. Lett. 85, 1250 (2000)
Y. Qi, T. Cagin, W.L. Johnson, W.A. Goddard III, J. Chem. Phys. 115, 385 (2001)
S. Alavi, D.L. Thompson, J. Phys. Chem. A 110, 1518 (2006)
F.A. Lindemann, Z. Phys. 11, 609 (1910)
V.V. Hoang, Philos. Mag. 91, 3443 (2011)
F.H. Stillinger, Science 267, 1935 (1995)
A. Pavlovska, D. Dobrev, E. Bauer, Surf. Sci. 286, 176 (1993)
J.C. Heyraud, J.J. Metois, J.M. Bermond, J. Cryst. Growth 98, 355 (1989)
S.J. Peppiatt, J.R. Sambles, Proc. R. Soc. Lond. A 345, 387 (1975)
G.L. Allen, R.A. Bayles, W.W. Gile, W.A. Jesser, Thin Solid Film 144, 297 (1986)
R. Garrigos, P. Cheyssac, R. Kofman, Z. Phys. D 12, 497 (1989)
Y. Oshima, K. Takayanagi, Z. Phys. D 27, 287 (1993)
F. Ercolessi, W. Andreoni, E. Tosatti, Phys. Rev. Lett. 66, 911 (1991)
D.S. Ivanov, L.V. Zhigilei, Phys. Rev. Lett. 98, 195701 (2007)
D.S. Ivanov, L.V. Zhigilei, Phys. Rev. B 68, 064114 (2003)
Z. Lin, L.V. Zhigilei, Phys. Rev. B 73, 184113 (2006)
Y.G. Chushak, L.S. Bartell, J. Phys. Chem. B 105, 11605 (2001)
X. Li, J. Huang, J. Solid State Chem. 176, 234 (2003)
C.-L. Kuo, P. Clancy, J. Phys. Chem. B 109, 13743 (2005)
M.H. Ghatee, K. Shekoohi, Fluid Phase Equilib. 327, 14 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Sang, L., Van Hoang, V. & Thuy Hang, N. Molecular dynamics simulation of melting of fcc Lennard-Jones nanoparticles. Eur. Phys. J. D 67, 64 (2013). https://doi.org/10.1140/epjd/e2013-30584-9
Received:
Revised:
Published:
DOI: https://doi.org/10.1140/epjd/e2013-30584-9