Abstract
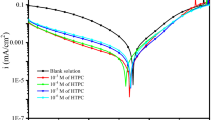
The effect of an heterocycle triazole, namely (1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methanol (MTM) on the corrosion of mild steel in hydrochloric acid solution has been investigated using electrochemical methods for a wide temperature range, FT-IR spectroscopy and SEM techniques. MTM was found to inhibit the corrosion of steel by adsorbing to a great extent, even at high temperatures. Computational results point to the phenyl ring as the main structural part which is responsible of the adsorption by electron-accepting to the mild steel surface, while the triazol ring is responsible for the electron-donating. Molecular dynamics simulations (MD), reduced density gradient (RDG), radial distribution function (RDF) provides further insights into the interpretation of inhibition mechanism in a more realistic condition, confirming that the MTM can effectively protect mild steel against corrosion by constraining the diffusion of the particles present on the steel surface.
















Similar content being viewed by others
REFERENCES
Umoren, S.A. and Obot, I.B., Surf. Rev. Lett., 2008, vol. 15, p. 277.
Benabdellah, M., Yahyi, A., Dafali, A., Aouniti, A., Hammouti, B., and Ettouhami, A., Arabian J. Chem., 2011, vol. 4, p. 243.
Negm, N.A., Migahed, M.A., Farag, R.K., Fadda, A.A., Awad, M.K., and Shaban, M.M., J. Mol. Liq., 2018, vol. 262, p. 363.
Quraishi, M.A. and Sharma, H.K., Mater. Chem. Phys., 2003, vol. 78, p. 18.
Tamilselvi, S. and Rajeswari, S., Methods Mater., 2003, vol. 50, p. 223.
Wang, L., Corros. Sci., 2006, vol. 48, p. 608.
Sanaei, Z., Bahlakeh, G., and Ramezanzadeh, B., J. Alloys Compd., 2017, vol. 728, p. 1289.
Nahlé, A.H., Harvey, T.J., and Walsh, F.C., J. Alloys Compd., 2018, vol. 765, p. 812.
Cruz, J., Pandiyan, T., and Garcia-Ochoa, E., J. Electroanal. Chem., 2005, vol. 583, p. 816.
Bashir, S., Sharma, V., Lgaz, H., Chung, I.M., Singh, A., and Kumar, A., J. Mol. Liq., 2018, vol. 263, p. 454.
Krishnaveni, K., Sampath, K., Ravichandran, J., and Chin, C., J. Chem. Eng., 2015, vol. 23, p. 1916.
Idouhli, R., Oukhrib, A., Koumya, Y., Abouelfida, A., Benyaich, A., and Benharref, A., Corros. Rev., 2018, vol. 36, p. 373.
Laamari, M.R., Benzakour, J., Berrekhis, F., Abouelfida, A., Derja, A., and Villemin, D., Arabian J. Chem., 2011, vol. 4, p. 271.
Eldesoky, A.M. and Nozha, S.G., J. Chem. Eng., 2017, vol. 25, p. 1256.
Materials Studio, Revision 6.0, San Diego, CA: Accelrys, 2013.
Sun, H., J. Phys. Chem. B, 1998, vol. 102, p. 7338.
Hosseini, S.M.A. and Azimi, A., Corros. Sci., 2009, vol. 51, p. 728.
Lorenz, W.J. and Mansfeld, F., Corros. Sci, 1981, vol. 21, pp. 647–672.
Yadav, M., Behera, D., Kumar, S., and Sinha, R.R., Ind. Eng. Chem. Res., 2013, vol. 52, p. 6318.
Pournazari, S., Moayed, M.H., and Rahimizadeh, M., Corros. Sci., 2013, vol. 71, p. 20.
Ita, B.I. and Offiong, O.E., Mater. Chem. Phys., 2001, vol. 70, p. 330.
El Mehdi, B., Mernari, B., Traisnel, M., Bentiss, F., and Lagrenee, M., Mater. Chem. Phys., 2003, vol. 77, p. 489.
Ghailane, T., Balkhmima, R.A., Ghailane, R., Souizi, A., Touir, R., EbnTouhami, M., Marakchi, K., and Komiha, N., Corros. Sci., 2013, vol. 76, p. 317.
Bagherzadeh, M. and Jaberinia, F., J. Alloys Compd., 2018, vol. 750, p. 677.
Solmaz, R., Kardaş, G., Çulha, M., Yazıcı, B., and Erbil, M., Electrochim. Acta., 2008, vol. 53, p. 5941.
Ma, Q., Qi, S., He, X., Tang, Y., and Lu, G., Corros. Sci., 2017, vol. 129, p. 91.
Chaouiki, A., Lgaz, H., Chung, I.M., Ali, I.H., Gaonkar, S.L., Bhat, K.S., Salghi, R., Oudda, H., and Khan, M.I., J. Mol Liq., 2018, vol. 266, p. 603.
Ouici, H.B., Benali, O., Harek, Y., Larabi, L., and Hammouti, B., Res. Chem. Intermed., 2013, vol. 39, p. 2777.
Yan, Y., Wang, X., Zhang, Y., Wang, P., Cao, X., and Zhang, J., Corros. Sci., 2013, vol. 73, p. 123.
Kumar, R., Chahal, S., Kumar, S., Lata, S., Lgaz, H., Salghi, R., and Jodeh, S., J. Mol. Liq., 2017, vol. 243, p. 439.
Contreras-García, J., Boto, R.A., Izquierdo-Ruiz, F., Reva, I., Woller, T., and Alonso, M., Theor. Chem. Acc., 2016, vol. 135, p. 242.
Roohi, H., Ghauri, K., and Salehi, R., J. Mol. Liq., 2017, vol. 243, p. 22.
ACKNOWLEDGMENTS
The authors are grateful to the Center of Analyses and Characterization (CAC) of University Caddy Ayyad, Morocco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Aziz Boutouil, Hrimla, M., Laamari, M.R. et al. Understanding the Corrosion Inhibition Mechanism of Mild Steel in Hydrochloric Acid by a Triazole Derivative: A Combined Experimental and Theoretical Approach. Prot Met Phys Chem Surf 55, 973–985 (2019). https://doi.org/10.1134/S2070205119050046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119050046