Abstract
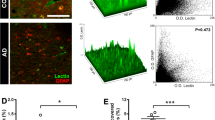
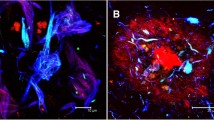
New RAGE- and CD147-mediated mechanisms of damage to the hippocampus of mice due to the accumulation of amyloid β (Aβ), the development of local inflammation, metabolic disorders and damage to the blood–brain barrier in two experimental models of Alzheimer’s disease were studied in vivo. The new effects of Aβ in the hippocampal tissue in chronic Alzheimer’s type neurodegeneration, which characterize neuroplasticity disorders, were studied, as were angiogenesis, the structural and functional integrity of the blood–brain barrier, and the development of local neuroinflammation in conjunction with the features of the expression of RAGE and CD147 proteins. Early neurodegenerative changes in the hippocampus associated with the accumulation of Aβ are associated with the intensification of neoangiogenesis and the formation of aberrant intercellular contacts in the endothelial layer of cerebral microvessels in individual hippocampal subregions and the development of local neuroinflammation. As neurodegeneration progresses, neoangiogenesis in the hippocampus is suppressed.

Similar content being viewed by others
REFERENCES
Agrawal, S.M., Williamson, J., Sharma, R., Kebir, H., Patel, K., Prat, A., and Yong, V.W., Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis, Brain, 2013, vol. 6, p. 1760.
Andrade, S., Ramalho, M.J., Pereira, M.D.C., and Loureiro, J.A., Resveratrol brain delivery for neurological disorders prevention and treatment, Front. Pharmacol., 2018, vol. 9, p. 1261.
Armstrong, R.A., What causes Alzheimer’s disease?, Folia Neuropathol., 2013, vol. 3, p. 169.
Arvanitakis, Z., Capuano, A.W., Leurgans, S.E., Bennett, D.A., and Schneider, J.A., Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study, Lancet Neurol., 2016, vol. 9, p. 934.
Bao, W., Min, D., Twigg, S.M., Shackel, N.A., Warner, F.J., Yue, D.K., and McLennan, S.V., Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications, Am. J. Physiol.—Cell Physiol., 2010, vol. 5, p. C1212.
Biron, K.E., Dickstein, D.L., Gopaul, R., and Jefferies, W.A., Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease, PLoS One, 2011, vol. 8, e23789.
Carare, R.O., Hawkes, C.A., Jeffrey, M., Kalaria, R.N., and Weller, R.O., Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy, Neuropathol. Appl. Neurobiol., 2013, vol. 6, p. 593.
Chen, F., Ghosh, A., Hu, M., Long, Y., Sun, H., Kong, L., Hong, H., and Tang, S., RAGE-NF-κB-PPARγ signaling is involved in AGEs-induced upregulation of amyloid-β influx transport in an in vitro BBB model, Neurotox. Res., 2018, vol. 2, p. 284.
Deane, R.J., Is RAGE still a therapeutic target for Alzheimer’s disease?, Future Med. Chem., 2012, vol. 7, p. 915.
Encinas, J.M. and Enikolopov, G., Identifying and quantitating neural stem and progenitor cells in the adult brain, Methods Cell Biol., 2008, vol. 85, p. 243.
Epelbaum, S., Youssef, I., Lacor, P.N., Chaurand, P., Duplus, E., Brugg, B., Duyckaerts, C., and Delatour, B., Acute amnestic encephalopathy in amyloid-β oligomer–injected mice is due to their widespread diffusion in vivo, Neurobiol. Aging, 2015, vol. 6, p. 2043.
Erickson, M.A. and Banks, W.A., Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease, J. Cereb. Blood Flow Metab., 2013, vol. 10, p. 1500.
Festoff, B.W., Sajja, R.K., van Dreden, P., and Cucullo, L., HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease, J. Neuroinflam., 2016, vol. 1, p. 194.
Fujita, K., Motoki, K., Tagawa, K., Chen, X., Hama, H., Nakajima, K., Homma, H., Tamura, T., Watanabe, H., Katsuno, M., Matsumi, C., Kajikawa, M., Saito, T., Saido, T., Sobue, G., Miyawaki, A., and Okazawa, H., HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease, Sci. Rep., 2016, vol. 6, p. 31895. https://doi.org/10.1038/srep31895
Graham, W.V., Bonito-Oliva, A., and Sakmar, T.P., Update on Alzheimer’s disease therapy and prevention strategies, Ann. Rev. Med., 2017, vol. 1, p. 413.
Grammas, P., Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease, J. Neuroinflam., 2011, vol. 8, p. 26. https://doi.org/10.1186/1742-2094-8-26
van de Haar, H.J., Jansen, J.F.A., van Osch, M.J.P., van Buchem, M.A., Muller, M., Wong, S.M., Hofman, P.A.M., Burgmans, S., Verhey, F.R.J., and Backes, W.H., Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging, Neurobiol. Aging, 2016, vol. 45, p. 190.
Iturria-Medina, Y., Sotero, R.C., Toussaint, P.J., Mateos-Pérez, J.M., and Evans, A.C., Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis, Nat. Commun., 2016, vol. 21, p. 11934. https://doi.org/10.1038/ncomms11934
Kanyenda, L.J., Verdile, G., Martins, R., Meloni, B.P., Chieng, J., Mastaglia, F., Laws, S.M., Anderton, R.S., and Boulos, S., Is cholesterol and amyloid-β stress induced CD147 expression a protective response? Evidence that extracellular cyclophilin a mediated neuroprotection is reliant on CD147, J. Alzheimer’s Dis., 2014, vol. 3, p. 545.
Keable, A., Fenna, K., Yuen, H.M., Johnston, D.A., Smyth, N.R., Smith, C., Rustam, Al-Shahi, Salman, Samarasekera, N., James, N.A.R., Attems, J., Kalaria, R.N., Wellera, R.O., and Carare, R.O., Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy, Biochim. Biophys. Acta, 2016, vol. 5, p. 1037.
Kierdorf, K. and Fritz, G., RAGE regulation and signaling in inflammation and beyond, J. Leukocyte Biol., 2013, vol. 1, p. 55.
Kim, I.D., Lee, H., Kim, S.W., Lee, H.K., Choi, J., Han, P.L., and Lee, J.K., Alarmin HMGB1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model, Cell Death Dis., 2018, vol. 4, p. 426.
Liu, X., Hou, D., Lin, F., Luo, J., Xie, J., Wang, Y., and Tian, Y., The role of neurovascular unit damage in the occurrence and development of Alzheimer’s disease, Rev. Neurosci., 2018. https://doi.org/10.1515/revneuro-2018-0056
Magaki, S., Tang, Z., Tung, S., Williams, C.K., Lo, D., Yong, W.H., Khanlou, N., and Vinters, H.V., The effects of cerebral amyloid angiopathy on integrity of the blood-brain barrier, Neurobiol. Aging, 2018, vol. 70, p. 70.
Meneghini, V., Bortolotto, V., Francese, M.T., Dellarole, A., Carraro, L., Terzieva, S., and Grilli, M., High-mobility group box-1 protein and β-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-kb axis: relevance for Alzheimer’s disease, J. Neurosci., 2013, vol. 14, p. 6047.
Miedel, C.J., Patton, J.M., Miedel, A.N., Miedel, E.S., and Levenson, J.M., Assessment of spontaneous alternation, novel object recognition and limb clasping in transgenic mouse models of amyloid-β and tau neuropathology, J. Vis. Exp., 2017, vol. 123. https://doi.org/10.3791/55523
Miller, M.C., Tavares, R., Johanson, C.E., Hovanesian, V., Donahue, J.E., Gonzalez, L., Silverberg, G.D., and Stopa, E.G., Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease, Brain Res., 2008, vol. 1230, p. 273.
Muramatsu, T., Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners, J. Biochem., 2016, vol. 5, p. 481.
Paudel, Y.N., Shaikh, M.F., Chakraborti, A., Kumari, Y., Aledo-Serrano, Á., Aleksovska, K., Alvim, M.K.M., and Othman, I., HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction, Front. Neurosci., 2018, vol. 12, p. 628.
Ramasamy, R., Shekhtman, A., and Schmidt, A.M., The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic disease, Expert Opin. Ther. Targets, 2016, vol. 4, p. 431.
Reich, D., Gallucci, G., Tong, M., and de la Monte, S.M., Therapeutic advantages of dual targeting of PPAR-δ and PPAR-γ in an experimental model of sporadic Alzheimer’s disease, J. Parkinson’s Dis. Alzheimer’s Dis., 2018, vol. 1, p. 1.
Saito, S. and Ihara, M., Interaction between cerebrovascular disease and Alzheimer pathology, Curr. Opin. Psychiatry, 2016, vol. 2, p. 168.
Salmina, A.B., Pozhilenkova, E.A., Morgun, A.V., Kuvacheva, N.V., Shuvaev, A.N., Lopatina, O.L., Boitsova, E.B., and Taranushenko, T.E., Glial dysfunction and blood-brain barrier impairment in the developing brain, Adv. Neuroimmune Biol., 2016, vol. 2, p. 69.
Seizer, P., Gawaz, M., and May, A.E., Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases, Cardiovasc. Res., 2014, vol. 1, p. 17.
Sipos, E., Kurunczi, A., Kasza, A., Horváth, J., Felszeghy, K., Laroche, S., Toldi, J., Párducz, A., Penke, B., and Penke, Z., Beta-amyloid pathology in the entorhinal cortex of rats induces memory deficits: implications for Alzheimer’s disease, Neuroscience, 2007, vol. 1, p. 28.
Steinberg, M., Hess, K., Corcoran, C., Mielke, M.M., Norton, M., Breitner, J., Green, R., Leoutsakos, J., Welsh-Bohmer, K., Lyketsos, C., and Tschanz, J., Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s disease: the cache county study, Int. J. Geriatric Psychiatry, 2014, vol. 2, p. 153.
Ungern-Sternberg, S.N.I., von, Zernecke, A., and Seizer, P., Extracellular matrix metalloproteinase inducer EMMPRIN (CD147) in cardiovascular disease, Int. J. Mol. Sci., 2018, vol. 19. pii: E507. https://doi.org/10.3390/ijms19020507
Uspenskaya, Yu.A., Komleva, Yu.K., Gorina, Ya.V., Pozhilenkova, E.A., Belova, O.A., and Salmina, A.B., Multifunctionality of CD147 and new opportunities for diagnosis and therapy, Sib. Med. Obozr., 2018, vol. 4, p. 22.
Vetrivel, K.S., Zhang, X., Meckler, X., Cheng, H., Lee, S., Gong, P., Lopes, K.O., Chen, Y., Iwata, N., Yin, K.J., Lee, J.M., Parent, A.T., Saido, T.C., Li, Y.M., Sisodia, S.S., and Thinakaran, G., Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta, J. Biol. Chem., 2008, vol. 28, p. 19489.
Weber, D.J., Allette, Y.M., Wilkes, D.S., and White, F.A., The HMGB1-RAGE inflammatory pathway: implications for brain injury-induced pulmonary dysfunction, Antioxid. Red. Signaling, 2015, vol. 17, p. 1316.
Wei, M., Li, H., Shang, Y., Zhou, Z., and Zhang, J., Increased CD147 (EMMPRIN) expression in the rat brain following traumatic brain injury, Brain Res., 2014, vol. 1585, p. 150.
Yamamoto, Y., Liang, M., Munesue, S., Deguchi, K., Harashima, A., Furuhara, K., Yuhi, T., Zhong, J., Akther, S., Goto, H., Eguchi, Y., Kitao, Y., Hori, O., Shiraishi, Y., Ozaki, N., et al., Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice, Commun. Biol., 2019, vol. 1, p. 76.
Zinchuk, V., Zinchuk, O., and Okada, T., Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: Pushing pixels to explore biological phenomena, Acta Histochem. Cytochem., 2007, vol. 40, p. 101.
Funding
This work was carried out with state financial support of the Presidential Program for Leading Scientific Schools of the Russian Federation, project no. NSh-6240.2018.7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare they have no conflict of interest.
Statement on the welfare of animals. Animal experiments were carried out in accordance with generally accepted ethical international standards in compliance with the principles of humaneness set out in the European Community Directive (2010/63/EC) and the requirements of order of the Russian Ministry of Health no. 267 dated June 19, 2003, “On the Approval of the Guidelines of Laboratory Practice in the Russian Federation.”
Additional information
Abbreviations: Aβ—beta-amyloid β, AD—Alzheimer’s disease, BBB—blood–brain barrier, NVU—neurovascular unit, RAGE—protein glycation end product receptor.
Rights and permissions
About this article
Cite this article
Morgun, A.V., Osipova, E.D., Boitsova, E.B. et al. Vascular Component of Neuroinflammation in Experimental Alzheimer’s Disease in Mice. Cell Tiss. Biol. 14, 256–262 (2020). https://doi.org/10.1134/S1990519X20040057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X20040057