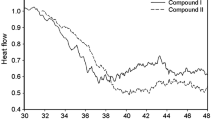
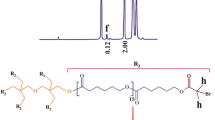
Abstract
A thermal-responsive Y-shaped amphiphilic block copolymer, namely poly(N-isopropylacrylamide-block-[poly(ε-caprolactone)]2 (PNIPAAm-b-PCL2), was synthesized through the combination of atom transfer radical polymerization (ATRP) and ring-opening polymerization (ROP) techniques, and its potential as smart anti-cancer drug delivery system was investigated preliminary. The fabricated polymers were characterized using FTIR and 1H NMR spectroscopy, and GPC analysis. The self-assembly behavior of the fabricated PNIPAAm-b-PCL2 copolymer under thermal stimuli was investigated using dynamic light scattering and UV–Vis spectroscopy. The lower critical solution temperature of the synthesized PNIPAAm-b-PCL2 was found to be 39–41°C. The doxorubicin hydrochloride loading and encapsulation capacities of the fabricated PNIPAAm-b-PCL2 were calculated to be 62 ± 3%, and 75 ± 4%, respectively; the drug release value was up to 46.5% during 40 h at 37°C and up to 88% at 40°C.








Similar content being viewed by others
REFERENCES
N.A.N. Hanafy, M. El-Kemary, and S. Leporatti, Cancers 10, 238 (2018).
B. Razavi, A. Abdollahi, H. Roghani-Mamaqani, and M. Salami-Kalajahi, Mater. Sci. Eng., C 109, 110524 (2020). https://doi.org/10.1016/j.msec.2019.110524
Q. Tian, C. Fei, H. Yin, and Y. Feng, Prog. Polym. Sci. 89, 108 (2019).
S. I. Y. Diouf, D. J. Williams, S. Seifert, A. Londoño-Calderon, M. T. Pettes, C. J. Sheehan, and M. A. Firestone, Polym. Chem. 10, 645 (2019).
A. Aied, W. Song, W. Wang, A. Baki, and A. Sigen, Bioprinting 9, 37 (2018).
Y. Zang, H. Zhu, and H. Xue, Electrochim. Acta 259, 676 (2018).
Y. Yang and A. Hashidzume, Macromol. Chem. Phys. 220, 1900317 (2019). https://doi.org/10.1002/macp.201900317
A. Ghamkhari, B. Massoumi, and M. Jaymand, Des. Monomers Polym. 20, 190 (2017).
Z. Ge, Y. Cai, J. Yin, Z. Zhu, J. Rao, and S. Liu, Langmuir 23, 1114 (2007).
Y. Cai and S. P. Armes, Macromolecules 38, 271 (2005).
A. Hirao, Y. Matsuo, and R. Goseki, J. Polym. Res. 26, article number 236 (2019).
X. Qiang, R. Chakroun, N. Janoszka, and A. H. Gröschel, Isr. J. Chem. 59, 945 (2019).
D. R. Perinelli, M. Cespi, G. Bonacucina, and G. F. Palmieri, J. Pharm. Invest. 49, 443 (2019).
M. Sahn, C. Weber, and U. S. Schubert, Polym. Rev. 59, 240 (2019).
M. Abbasian, L. Razavi, M. Jaymand, and S. G. Karaj-Abad, Sci. Iran. 26, 1447 (2019).
H. Sun, W. Choi, N. Zang, C. Battistella, M. P. Thompson, W. Cao, X. Zhou, C. Forman, and N. C. Gianneschi, Angew. Chem., Int. Ed. 58, 17359 (2019).
G. Xie, M. R. Martinez, M. Olszewski, S. S. Sheiko, and K. Matyjaszewski, Biomacromolecules 20, 27 (2019).
X. Wang, L. Shen, and Z. An, Prog. Polym. Sci. 83, 1 (2018).
M. Abbasian, M. Bakhshi, M. Jaymand, and S. G. Karaj-Abad, J. Elastomers Plast. 51, 473 (2019).
X. Tian, J. Ding, B. Zhang, F. Qiu, X. Zhuang, and Y. Chen, Polymers 10, 318 (2018).
P. Chmielarz, M. Fantin, S. Park, A. A. Isse, A. Gennaro, A. J. D. Magenau, A. Sobkowiak, and K. Matyjaszewski, Prog. Polym. Sci. 69, 47 (2017).
Y. Zhou, K. Wang, and D. Hu, Sci. Rep. 9, (2019). https://doi.org/10.1038/s41598-019-46366-7
J. C. Theriot, B. G. McCarthy, C. H. Lim, and G. M. Miyake, Macromol. Rapid Commun. 38, (2017). https://doi.org/10.1002/marc.201700040
P. Krys and K. Matyjaszewski, Eur. Polym. J. 89, 482 (2017).
K. Matyjaszewski, Adv. Mater. 30, (2018). https://doi.org/10.1002/adma.201706441
P. J. Yunker, K. Chen, M. D. Gratale, M. A. Lohr, T. Still, and A. G. Yodh, Report. Prog. Phys.77, 056601 (2014).
J. Ramos, A. Imaz, and J. Forcada, Polym. Chem. 3, 852 (2012).
H. Wei, S. X. Cheng, X. Z. Zhang, and R. X. Zhuo, Prog. Polym. Sci. 34, 893 (2009).
K. Nagase, M. Yamato, H. Kanazawa, and T. Okano, Biomaterials 153, 27 (2018).
Z. Zhang, S. Wang, G. I. N. Waterhouse, Q. Zhang, and L. Li, J. Appl. Polym. Sci. 137, (2020). https://doi.org/10.1002/app.48391
L. J. Luo, D. D. Nguyen, and J. Y. Lai, J. Controlled Release 317, 246 (2020).
J. Li, X. Fan, L. Yang, F. Wang, J. Zhang, and Z. Wang, Int. J. Polym. Mater. Polym. Biomater. 67, 371 (2018).
A. Ehterami, M. Salehi, S. Farzamfar, A. Vaez, H. Samadian, H. Sahrapeyma, M. Mirzaii, S. Ghorbani, and A. Goodarzi, Int. J. Biol. Macromol. 117, 601 (2018).
A. R. Pohlmann, F. N. Fonseca, K. Paese, C. B. Detoni, K. Coradini, R. C. Beck, and S. S. Guterres, Exp. Opin. Drug Delivery 10, 623 (2013).
T. K. Dash and V. B. Konkimalla, J. Controlled Release 158, 15 (2012).
S. Farzamfar, M. Naseri-Nosar, H. Samadian, S. Mahakizadeh, R. Tajerian, M. Rahmati, A. Vaez, and M. Salehi, J. Bioact. Compat. Polym. 33, 282 (2018).
O. Ozkan and H. Turkoglu Sasmazel, J. Biomater. Appl. 32, 1300 (2018).
S. Deng, J. Ma, Y. Guo, F. Chen, and Q. Fu, Compos. Sci. Technol. 157, 168 (2018).
A. Morro, F. Catalina, J. L. Pablos, T. Corrales, I. Marin, and C. Abrusci, Eur. Polym. J. 94, 405 (2017).
J. Yang, Q. Zhou, K. Shen, N. Song, and L. Ni, RSC Adv. 8, 3705 (2018).
B. S. Lele and J. C. Leroux, Polymer 43, 5595 (2002).
M. Aghajanzadeh, M. Zamani, K. Rostamizadeh, A. Sharafi, and H. Danafar, J. Macromol. Sci., Part A: Pure Appl. Chem. 55, 559 (2018).
D. Neugebauer, A. Mielańczyk, R. Bielas, J. Odrobińska, M. Kupczak, and K. Niesyto, Pharmaceutics 11, 337 (2019).
J. M. Ren, T. G. McKenzie, Q. Fu, E. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer, and G. G. Qiao, Chem. Rev. 116, 6743 (2016).
K. Wang, M. C. Hawley, and S. J. DeAthos, Ind. Eng. Chem. Res. 42, 2913 (2003).
Y. Wang, J. Yan, N. Wen, H. Xiong, S. Cai, Q. He, Y. Hu, D. Peng, Z. Liu, and Y. Liu, Biomaterials 230, 119619 (2020).
A. Zhang, K. Jung, A. Li, J. Liu, and C. Boyer, Prog. Polym. Sci. 99, 101164 (2019).
T. Alejo, L. Uson, and M. Arruebo, J. Controlled Release 314, 162 (2019).
Funding
The authors gratefully acknowledge the partial financial support from Payame Noor University, Tehran, Iran, and Nano Drug Delivery Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant no. 3009887).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Farnaz Fazlalizadeh, Massoumi, B., Banaei, A. et al. A Thermal-Responsive Y-Shaped Miktoarm Amphiphilic Block Copolymer Composed of Poly(ε-caprolactone) and Poly(N-isopropylacrylamide) as a Nano-micellar Carrier for Anti-cancer Drugs. Polym. Sci. Ser. B 62, 540–549 (2020). https://doi.org/10.1134/S1560090420050061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090420050061