Abstract
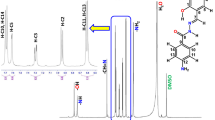
Derivatives of bis(2-bromoethyl) sulfide of the general formula (RSCH2CH2)2S, where R = Me, Et, Pr, i-Pr, Bu, i-Bu, t-Bu, C6H13, Cy, Ph, and Bn, were synthesized and characterized by IR and 1H and 13C NMR spectra. The synthesized compounds are potential electron-donating multidentate ligands that are promising for the preparation of coordination compounds and as components of catalytic systems for catalytic transformations of ethylene.


Similar content being viewed by others
REFERENCES
Baker, P.K., Clark, A.I., Coles, S.J., Hursthouse, M.B., and Richards, R.L., J. Organomet. Chem., 1996, vol. 518, p. 235. https://doi.org/10.1016/0022-328X(96)06164-5
Baker, P.K., Clark, A.I., Drew, M.G.B., Durrant, M.C., and Richards, R.L., Polyhedron, 1998, vol. 17, p. 1407. https://doi.org/10.1016/S0277-5387(97)00423-3
Baker, P.K., Clark, A.I., Coles, S.J., Drew, M.G.B., Durrant, M.C., Hursthouse, M.B., and Richards, R.L., J. Chem. Soc., Dalton Trans., 1998, p. 1281. https://doi.org/10.1039/a708648b
Connolly, J., Genge, A.R.J., Levason, W., Orchard, S.D., Popem, S.J.A., and Reid, G., J. Chem. Soc., Dalton Trans., 1999, p. 2343. https://doi.org/10.1039/A902820J
Cho, S.-Y. and Mochida, T., Inorg. Chem., 2020, vol. 59, p. 847. https://doi.org/10.1021/acs.inorgchem.9b03108
McGuinness, D.S., Wasserscheid, P., Keim, W., Morgan, D., Dixon, J.T., Bollmann, A., Maumela, H., Hess, F., and Englert, U., J. Am. Chem. Soc., 2003, vol. 125, p. 5272. https://doi.org/10.1021/ja034752f
Mohamadnia, Z., Ahmadi, E., Haghighi, M.N., and Salehi-Mobarakeh, H., Catal. Lett., 2011, vol. 141, p. 474. https://doi.org/10.1007/s10562-010-0492-z
Bespalova, N.B., Cheredilin, D.N., Kozlova, G.A., Dudin, A.V., and Afanas’ev, V.V., RU Patent no. 2470707 C1, 2011; Byull. Izobret., 2012, no. 36.
Bezborodov, V., Babenko, I., Rozentsveig, I., Korchevin, N., Levanova, E., Smirnov, V., Borodina, T., Saraev, V., and Vilms, A., Polyhedron, 2018, vol. 151, p. 287. https://doi.org/10.1016/j.poly.2018.05.053
Levanova, E.P., Vilms, A.I., Bezborodov, V.A., Babenko, I.A., Sosnovskaya, N.G., Istomina, N.V., Albanov, A.I., Russavskaya, N.V., and Rozentsveig, I.B., Russ. J. Gen. Chem., 2017, vol. 87, p. 396. https://doi.org/10.1134/S1070363217030069
Moulin, J.O., Evans, J., McGuinness, D.S., Reid, G., Rucklidge, A.J., Tooze, R.P., and Tromp, M., Dalton Trans., 2008, p. 1177. https://doi.org/10.1039/b716078j
Vshivtsev, V.Yu., Levanova, E.P., Grabel’nykh, V.A., Klyba, L.V., Zhanchipova, E.R., Sukhomazova, E.N., Tatarinova, A.A., Albanov, A.I., Russavskaya, N.V., and Korchevin, N.A., Russ. J. Org. Chem., 2008, vol. 44, p. 43. https://doi.org/10.1007/s11178-008-1005-z
Levanova, E.P., Vakhrina, V.S., Grabel’nykh, V.A., Rozentsveig, I.B., Russavskaya, N.V., Albanov, A.I., and Korchevin, N.A., Russ. Chem. Bull., Int. Ed., 2014, vol. 63, p. 1722. https://doi.org/10.1007/s11172-014-0659-7
Franke, S., Franz, P., and Warnke, W., Lehrbuch der Militärchemie, Berlin: Deutscher Militärverlag, 1967, vol. 1. Translated under the title Khimiya otravlyayushchikh veshchestv, Moscow: Khimiya, 1973, vol. 1, p. 281.
ACKNOWLEDGMENTS
This study was performed using the facilities of the joint analytical center at the Irkutsk State University (http://ckp-rf.ru/ckp/3264/) and Baikal joint analytical canter (Siberian Branch, Russian Academy of Sciences). The authors thank senior researcher M.V. Bykov (Research Institute of Petrochemical and Coal Chemical Synthesis, Irkutsk State University) for recording the IR spectra.
Funding
This work was supported by the Government Assignment for Scientific Research from the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-03-2020-176/3; project code in Parus 8: FZZE-2020-0022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 10, pp. 1482–1489 https://doi.org/10.31857/S0514749221100153.
Rights and permissions
About this article
Cite this article
Bezborodov, V.A., Babenko, I.A., Ratovskii, G.V. et al. Synthesis of Multidentate Chalcogen-Containing Ligands Based on Bis(2-bromoethyl) Sulfide. Russ J Org Chem 57, 1662–1667 (2021). https://doi.org/10.1134/S1070428021100158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021100158