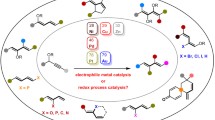
Abstract
The review presents analysis of the literature on the intramolecular cyclization of N-(2-acylaryl)amides, known as the Camps reaction, as well as close analogs of these compounds, N-(3-oxoalkenyl)- and N-(3-oxoalkyl)amides, which contain an amide and a carbonyl groups and react by the aldol–crotonic type. The potential, limitations, and regularities of these reactions leading to quinoline-2(1H)-ones, quinoline-4(1H)-ones, pyridin-2-ones, pyridin-4-ones, as well as their hydrogenated derivatives are highlighted.

























































Similar content being viewed by others
REFERENCES
Prajapati, S.M., Patel, K.D., Vekariya, R.H., Panchal, S.N., and Patel, H.D., RSC Adv., 2014, vol. 4, p. 24463. https://doi.org/10.1039/C4RA01814A
Heeb, S., Fletcher, M.P., Chhabra, R.S., Diggle, S.P., Williams, P., and Camara, M., FEMS Microbiol. Rev., 2011, vol. 35, p. 247. https://doi.org/10.1111/j.1574-6976.2010.00247
Horta, P., Secrieru, A., Coninckx, A., and Cristiano, M.L.S., Targets Heterocycl. Syst., 2018, vol. 11, p. 260. https://doi.org/10.17374/targets.2019.22.260
Beteck, R.M., Smit, F.J., Haynes, R.K., and N’Da, D.D., Malar. J., 2014, vol. 13, p. 339. https://doi.org/10.1186/1475-2875-13-339
Charushin, V.A., Nosova, E.V., Lyapunova, G.N., and Chupakhin, O.N., Ftorkhinoliny: sintez i primenenie (Fluoroquinolones: Synthesis and Application), Moscow: FIZMATLIT, 2013.
Manske, R.H., Chem. Rev., 1942, vol. 30, p. 113. https://doi.org/10.1021/cr60095a006
Jones, G., Comprehensive Heterocyclic Chemistry, Katritzky, A.R. and Rees, C.W., Eds. New York: Pergamon. 1997, vol. 2, p. 316.
Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., and Taylor, R.J.K., Comprehensive Heterocyclic Chemistry III, Amsterdam: Elsevier, 2008, vol. 7, p. 217.
Sharma, R., Kour, P., and Kumar, A., J. Chem. Sci., 2018, vol. 130, p. 73. https://doi.org/10.1007/s12039-018-1466-8
Kouznetsov, V.V., Méndez, L.Y.V., and Gómez, C.M.M., Curr. Org. Chem., 2005, vol. 9, p. 141. https://doi.org/10.2174/1385272053369196
Camps, R., Ber. Dtsch. Chem. Ges., 1899, vol. 32, p. 3228. https://doi.org/10.1002/cber.18990320389
Camps, R., Arch. Pharm., 1899, vol. 237, p. 659. https://doi.org/10.1002/ardp.18992370902
Camps, R., Arch. Pharm., 1901, vol. 239, p. 591. https://doi.org/10.1002/ardp.19012390805
Camps, R., Arch. Pharm., 1902, vol. 240, p. 135. https://doi.org/10.1002/ardp.19022400204
Vatsuro, K.V. and Mishchenko, G.V., Imennye reaktsii v organicheskoi khimii (Name Reactions in Organic Chemistry), Moscow: Kimiya, 1976.
Pflum, D.A., Name Reactions in Heterocyclic Chemistry, Li, J.J. and Corey, E.J., Eds. Hoboken: Wiley and Sons, 2005, p. 386.
Li, J.J., Name Reactions. A Collection of Detailed Mechanisms and Synthetic Applications, 4th Edn. Berlin: Springer-Verlag, 2009, p. 92. https://doi.org/10.1007/978-3-642-01053-84
Elderfield, R.C., Todd, W.H., and Gerber, S., Heterocyclic Compounds, Elderfield, R.C., Ed. New York: Wiley and Sons, 1957, p. 576.
Fisyuk, A.S. and Bundel’, Yu.G., Chem. Heterocycl. Compd., 1999, vol. 35, p. 125. https://doi.org/10.1007/BF02251699
Konovalov, A.I., Antipin, I.S., Burilov, V.A., Madzhidov, T.I., Kurbangalieva, A.R., Nemtarev, A.V., Solovieva, S.E., Stoikov, I.I., Mamedov, V.A., Zakharova, L.Ya., Gavrilova, E.L., Sinyashin, O.G., Balova, I.A., Vasilyev, A.V., Zenkevich, I.G., Krasavin, M.Yu., Kuznetsov, M.A., Molchanov, A.P., Novikov, M.S., Nikolaev, V.A., Rodina, L.L., Khlebnikov, A.F., Beletskaya, I.P., Vatsadze, S.Z., Gromov, S.P., Zyk, N.V., Lebedev, A.T., Lemenovskii, D.A., Petrosyan, V.S., Nenaidenko, V.G., Negrebetskii, V.V., Baukov, Yu.I., Shmigol’, T.A., Korlyukov, A.A., Tikhomirov, A.S., Shchektikhin, A.E., Traven’, V.F., Voskresenskii, L.G., Zubkov, F.I., Golubchikov, O.A., Semeikin, A.S., Berezin, D.B., Stuzhin, P.A., Filimonov, V.D., Krasnokutskaya, E.A., Fedorov, A.Yu., Nyuchev, A.V., Orlov, V.Yu., Begunov, R.S., Rusakov, A.I., Kolobov, A.V., Kofanov, E.R., Fedotova, O.V., Egorova, A.Yu., Charushin, V.N., Chupakhin, O.N., Klimochkin, Yu.N., Osyanin, V.A., Reznikov, A.N., Fisyuk, A.S., Sagitullina, G.P., Aksenov, A.V., Aksenov, N.A., Grachev, M.K., Maslennikova, V.I., Koroteev, M.P., Brel’, A.K., Lisina, S.V., Medvedeva, S.M., Shikhaliev, Kh.S., Suboch, G.A., Tovbis, M.S., Mironovich, L.M., Ivanov, S.M., Kurbatov, S.V., Kletskii, M.E., Burov, O.N., Kobrakov, K.I., and Kuznetsov, D.N., Russ. J. Org. Chem., 2018, vol. 54, p. 157. https://doi.org/10.1134/S107042801802001X
Lechel, T. and Reissig, H.-U., Targets in Heterocyclic Systems – Chemistry and Properties, Attanasi, O.A., Merino, P., and Spinelli, D., Eds., Rome: Italian Society of Chemistry, 2016, vol. 20, p. 1. https://doi.org/10.17374/targets.2017.20.1
Bischler, A. and Howell, F.J., Ber. Dtsch. Chem. Ges., 1893, vol. 26, p. 1384. https://doi.org/10.1002/cber.18930260239
Bieshler, A. and Lang, Y., Ber. Dtsch. Chem. Ges., 1895, vol. 28, p. 279. https://doi.org/10.1002/cber.18950280169
Hewawasam, P., Fan, W., Ding, M., Flint, K., Cook, D., Goggins, G.D., Myers, R.A., Gribkoff, V.K., Boissard, C.G., Dworetzky, S.I., Starrett, J.E., and Lodge, N.J., J. Med. Chem., 2003, vol. 46, p. 2819. https://doi.org/10.1021/jm030005h
Wang, J.J., Discordia, R.P., Crispino, G.A., Li, J., Grosso, J.A., Polniaszek, R., and Truc, V.C., Tetrahedron Lett., 2003, vol. 44, p. 4271. https://doi.org/10.1016/S0040-4039(03)00889-X
Hameršak, Z., Litvić, M., Šepac, D., Lesac, A., Raza, Z., and Šunjić, V., Synthesis, 2002, vol. 15, p. 2174. https://doi.org/10.1055/s-2002-34835
Patel, M., McHugh, R.J., Cordova, B.C., Klabe, R.M., Bacheler, L.T., Erickson-Viitanen, S., and Rodgers, J.D., Bioorg. Med. Chem. Lett. 2001, vol. 11, p. 1943. https://doi.org/10.1016/S0960-894x(01)00331-6
Mitchell, L.H. and Samas, B., Synth. Commun., 2005, vol. 35, p. 955. https://doi.org/10.1081/Scc-200051700
Brouillette, Y., Martinez, J., and Lisowski, V., J. Org. Chem., 2009, vol. 74, p. 4975. https://doi.org/10.1021/jo900627a
Rehwald, M., Bellmann, P., Jeschke, T., and Gewald, K., J. Prakt. Chem., 2000, vol. 342, p. 371. https://doi.org/10.1002/(Sici)1521-3897(200004)342:4<371::Aid-Prac371>3.0.Co;2-M
Yanagisawa, H., Nakao, H., and Ando, A., Chem. Pharm. Bull., 1973, vol. 21, p. 1080. https://doi.org/10.1248/cpb.21.1080
Manley, P.J. and Bilodeau, M.T., Org. Lett., 2004, vol. 6, p. 2433. https://doi.org/10.1021/ol049165t
Ban, H., Muraoka, M., Morisita, K., and Ohashi, N., Heterocycles, 2005, vol. 65, p. 2763. https://doi.org/10.3987/Com-05-10511
Amer, A.M., Monatsh. Chem., 2001, vol. 132, p. 859. https://doi.org/10.1007/s007060170075
Gorelik, M.V., Titova, S.P., and Gordievskaya, E.V., Russ. Chem. Bull., 2006, vol. 55, p. 1487. https://doi.org/10.1007/s11172-006-0444-3
Ibrahim, H.M., Behbehani, H., Makhseed, S., and Elnagdi, M.H., Molecules, 2011, vol. 16, p. 3723. https://doi.org/10.3390/molecules16053723
Peters, J.U., Capuano, T., Weber, S., Kritter, S., and Sagesser, M., Tetrahedron Lett., 2008, vol. 49, p. 4029. https://doi.org/10.1016/j.tetlet.2008.04.088
El Mariah, F., J. Chem. Res., 2009, p. 593. https://doi.org/10.3184/030823409x12508790019612
Mochalov, S.S., Chasanov, M.I., Fedotov, A.N., and Zefirov, N.S., Chem. Heterocycl. Compd., 2011, vol. 47, p. 1105. https://doi.org/10.1007/s10593-011-0881-2
Park, K.K. and Lee, J.J., Tetrahedron, 2004, vol. 60, p. 2993. https://doi.org/10.1016/j.tet.2004.02.001
Mochalov, S.S., Fedotov, A.N., Trofimova, E.V., and Zefirov, N.S., Russ. J. Org. Chem., 2016, vol. 52, p. 956. https://doi.org/10.1134/S107042801607006x
Park, K.K. and Jung, J.Y., Heterocycles, 2005, vol. 65, p. 2095. https://doi.org/10.3987/COM-05-10462
Jones, C.P., Anderson, K.W., and Buchwald, S.L., J. Org. Chem., 2007, vol. 72, p. 7968. https://doi.org/10.1021/jo701384n
Wӧhnlich, E., Arch. Pharm., 1913, vol. 251, p. 526. https://doi.org/10.1002/ardp.19132510606
Kutsumura, N., Numata, K., and Saito, T., Tetrahedron Lett., 2016, vol. 57, p. 5581. https://doi.org/10.1016/j.tetlet.2016.10.070
Eidamshaus, C., Triemer, T., and Reissig, H.U., Synthesis, 2011, p. 3261. https://doi.org/10.1055/s-0030-1260198
Witkop, B., Patrick, J.B., and Rosenblum, M., J. Am. Chem. Soc., 1951, vol. 73, p. 2641. https://doi.org/10.1021/ja01150a065
Hayward, R.J. and Methcohn, O., J. Chem. Soc. Perkin Trans. 1, 1975, p. 212. https://doi.org/10.1039/p19750000212
Afsah, E.M., Fadda, A.A., Bondock, S., and Hammouda, M.M., Z. Naturforsch. B, 2015, vol. 70, p. 385. https://doi.org/10.1515/znb-2014-0271
Willemsens, B., Vervest, I., Ormerod, D., Aelterman, W., Fannes, C., Mertens, N., Marko, I.E., and Lemaire, S., Org. Process Res. Dev., 2006, vol. 10, p. 1275. https://doi.org/10.1021/op060099f
Liu, S., Scotti, J.S., and Kozmin, S.A., J. Org. Chem. 2013, vol. 78, p. 8645. https://doi.org/10.1021/jo401262v
Demerson, C.A. and Humber, L.G., Can. J. Chem., 1979, vol. 57, p. 3296. https://doi.org/10.1139/v79-538
Lemaire, S., Willemsens, B., and Marko, I.E., Synlett, 2007, p. 709. https://doi.org/10.1055/s-2007-970769
Barret, R., Ortillon, S., Mulamba, M., Laronze, J.Y., Trentesaux, C., and Levy, J., J. Heterocycl. Chem., 2000, vol. 37, p. 241. https://doi.org/10.1002/jhet.5570370204
Desmaële, D., Tetrahedron Lett., 1996, vol. 37, p. 1233. https://doi.org/10.1016/0040-4039(96)00007-X
Szamosvari, D. and Bottcher, T., Angew. Chem., Int. Ed., 2017, vol. 56, p. 7271. https://doi.org/10.1002/anie.201702944
Abe, H., Kawada, M., Inoue, H., Ohba, S., Masuda, T., Hayashi, C., Igarashi, M., Nomoto, A., Watanabe, T., and Shibasaki, M., Tetrahedron, 2013, vol. 69, p. 7608. https://doi.org/10.1016/j.tet.2013.05.033
Abe, H., Kawada, M., Igarashi, M., Ohba, S., Hayashi, C., Sakashita, C., Watanabe, T., and Shibasaki, M., J. Antibiot., 2018, vol. 71, p. 86. https://doi.org/10.1038/ja.2017.123
Fackler, P., Huber, S.M., and Bach, T., J. Am. Chem. Soc., 2012, vol. 134, p. 12869. https://doi.org/10.1021/ja305890c
Mochalov, S.S., Fedotov, A.N., Trofimova, E.V., and Zefirov, N.S., Chem. Heterocycl. Compd., 2014, vol. 49, p. 1469. https://doi.org/10.1007/s10593-014-1398-2
Clémence, F., Lemartret, O., and Collard, J., J. Heterocycl. Chem., 1984, vol. 21, p. 1345. https://doi.org/10.1002/jhet.5570210520
Szamosvari, D., Sylvester, K., Schmid, P., Lu, K.Y., Derbyshire, E.R., and Bottcher, T., Chem. Commun., 2019, vol. 55, p. 7009. https://doi.org/10.1039/c9cc01689a
Hadjeri, M., Mariotte, A.M., and Boumendjel, A., Chem. Pharm. Bull., 2001, vol. 49, p. 1352. https://doi.org/10.1248/cpb.49.1352
Kumar, S., Verma, N., Zubair, S., Faisal, S.M., Kazmi, S., Chakraborty, S., Owais, M., and Ahmed, N., J. Heterocycl. Chem., 2017, vol. 54, p. 2242. https://doi.org/10.1002/jhet.2812
Gao, H. and Kawabata, J., Bioorg. Med. Chem., 2005, vol. 13, p. 1661. https://doi.org/10.1016/j.bmc.2004.12.010
Nilsson, J., Nielsen, E.O., Liljefors, T., Nielsen, M., and Sterner, O., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 5713. https://doi.org/10.1016/j.bmcl.2008.09.092
Manfroni, G., Gatto, B., Tabarrini, O., Sabatini, S., Cecchetti, V., Giaretta, G., Parolin, C., Del Vecchio, C., Calistri, A., Palumbo, M., and Fravolini, A., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 714. https://doi.org/10.1016/j.bmcl.2008.12.034
Bera, S.S., Sk, M.R., and Maji, M.S., Chem. Eur. J., 2019, vol. 25, p. 1806. https://doi.org/10.1002/chem.201805376
Jiang, C., Yang, L., Wu, W.T., Guo, Q.L., and You, Q.D., Bioorg. Med. Chem., 2011, vol. 19, p. 5612. https://doi.org/10.1016/j.bmc.2011.07.029
Takami, H., Kishibayashi, N., Ishii, A., and Kumazawa, T., Bioorg. Med. Chem., 1998, vol. 6, p. 2441. https://doi.org/10.1016/S0968-0896(98)80018-7
Popova, N.N., Kulikov, S.V., and Piotrovskii, L.B., Russ. J. Gen. Chem., 2004, vol. 74, p. 1467. https://doi.org/10.1007/s11176-005-0037-0
Chang, Y.H., Hsu, M.H., Wang, S.H., Huang, L.J., Qian, K., Morris-Natschke, S.L., Hamel, E., Kuo, S.C., and Lee, K.H., J. Med. Chem., 2009, vol. 52, p. 4883. https://doi.org/10.1021/jm900456w
Huang, J., Chen, Y., King, A.O., Dilmeghani, M., Larsen, R.D., and Faul, M.M., Org. Lett., 2008, vol. 10, p. 2609. https://doi.org/10.1021/ol800837z
Sui, Z.H., Nguyen, V.N., Altom, J., Fernandez, J., Hilliard, J.J., Bernstein, J.I., Barrett, J.F., and Ohemeng, K.A., Eur. J. Med. Chem., 1999, vol. 34, p. 381. https://doi.org/10.1016/S0223-5234(99)80087-7
Beney, C., Hadjeri, M., Mariotte, A.M., and Boumendjel, A., Tetrahedron Lett., 2000, vol. 41, p. 7037. https://doi.org/10.1016/S0040-4039(00)01226-0
Kobayashi, K., Nishikawa, K., and Nogi, T., Heterocycles, 2016, vol. 92, p. 2225. https://doi.org/10.3987/Com-16-13566
Jensen, S. and Torssell, K.B.G., Acta Chem. Scand., 1995, vol. 49, p. 53. https://doi.org/10.3891/acta.chem.scand.49-0053
Kapti, T., Dengiz, C., and Balci, M., Synthesis, 2017, vol. 49, p. 1898. https://doi.org/10.1055/s-0036-1588119
Kim, G. and Keum, G., Heterocycles, 1997, vol. 45, p. 1979. https://doi.org/10.3987/COM-96-7676
Kim, S.H., Jeong, J.U., Choi, D.O., and Lee, K.J., Bull. Korean Chem. Soc., 1993, vol. 14, p. 11. https://doi.org/10.1002/chin.199346179
Alkhathlan, H.Z. and Al-Farhan, K.A. Heterocycles, 1998, vol. 48, p. 641. https://doi.org/10.3987/COM-97-7949
Alkhathlan, H.Z., Al-Jaradah, M.A., Al-Farhan, K.A., and Mousa, A.A., Phosphorus, Sulfur Silicon Relat. Elem., 2004, vol. 179, p. 373. https://doi.org/10.1080/10426500490262513
Khalikov, I.G., Galin, F.Z., Sakhautdinov, I.M., and Tukhvatullin, O.R., Baskir. Khim. Zh., 2007, vol. 14, p. 22.
Peifer, C., Urich, R., Schattel, V., Abadleh, M., Rottig, M., Kohlbacher, O., and Laufer, S., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 1431. https://doi.org/10.1016/j.bmcl.2007.12.073
Krayushkin, M.M., Lichitskii, B.V., Pashchenko, D.V., Antonov, I.A., Nabatov, B.V., and Dudinov, A.A., Russ. J. Org. Chem. 2007, vol. 43, p. 1357. https://doi.org/10.1134/S1070428007090163
Mendez, M.V., Heredia, D.A., Larghi, E.L., Bracca, A.B.J., and Kaufman, T.S., RSC Adv., 2017, vol. 7, p. 28298. https://doi.org/10.1039/c7ra05349e
Doleans-Jordheim, A., Veron, J.B., Fendrich, O., Bergeron, E., Montagut-Romans, A., Wong, Y.S., Furdui, B., Freney, J., Dumontet, C., and Boumendjel, A., ChemMedChem, 2013, vol. 8, p. 652. https://doi.org/10.1002/cmdc.201200551
Zheng, J., Li, Z., Wu, W.Q., and Jiang, H.F., Org. Lett., 2016, vol. 18, p. 6232. https://doi.org/10.1021/acs.orglett.6b02710
Yan, J., Pang, Y.Q., Chen, J., Sheng, J.F., Hu, J.H., Huang, L., and Li, X.S., RSC Adv., 2015, vol. 5, p. 98527. https://doi.org/10.1039/c5ra19270f
Peifer, C., Kinkel, K., Abadleh, M., Schollmeyer, D., and Laufer, S., J. Med. Chem., 2007, vol. 50, p. 1213. https://doi.org/10.1021/jm061097o
Balasubramaniyan, V. and Argade, N.P., Synth. Commun., 1989, vol. 19, p. 3103. https://doi.org/10.1080/00397918908052708
Huang, L.J., Hsieh, M.C., Teng, C.M., Lee, K.H., and Kuo, S.C., Bioorg. Med. Chem., 1998, vol. 6, p. 1657. https://doi.org/10.1016/S0968-0896(98)00141-2
Hellal, M. and Cuny, G.D., J. Org. Chem., 2010, vol. 75, p. 3465. https://doi.org/10.1021/jo1003339
Hellal, M. and Cuny, G.D., Org. Lett., 2010, vol. 12, p. 4628. https://doi.org/10.1021/ol101890t
González-Vera, J.A., Fueyo-Gonzalez, F., Alkorta, I., Peyressatre, M., Morris, M.C., and Herranz, R., Chem. Commun., 2016, vol. 52, p. 9652. https://doi.org/10.1039/c6cc04566a
Tawada, H., Natsugari, H., Ishikawa, E., Sugiyama, Y., Ikeda, H., and Meguro, K., Chem. Pharm. Bull., 1995, vol. 43, p. 616. https://doi.org/10.1248/cpb.43.616
Wang, L., Xie, S., Ma, L.J., Chen, Y., and Lu, W., Bioorg. Med. Chem., 2015, vol. 23, p. 1950. https://doi.org/10.1016/j.bmc.2015.03.031
Barile, E., De, S.K., Feng, Y.M., Chen, V., Yang, L., Ronai, Z., and Pellecchia, M., Chem. Biol. Drug. Des., 2013, vol. 82, p. 520. https://doi.org/10.1111/cbdd.12177
Robl, J.A., Synthesis, 1991, vol. 1, p. 56. https://doi.org/10.1055/s-1991-26379
Yu, S.B., Huang, Q.Q., Luo, Y., and Lu, W., J. Org. Chem., 2012, vol. 77, p. 713. https://doi.org/10.1021/jo201974f
Yadav, J.S., Reddy, E.J., Madhavi, G., and Ramalingam, T., Heterocycl. Commun., 2000, vol. 6, p. 403. https://doi.org/10.1515/HC.2000.6.5.403
Hewawasam, P., Fan, W.H., Cook, D.A., Newberry, K.S., Boissard, C.G., Gribkoff, V.K., Starrett, J., and Lodge, N.J., Bioorg. Med. Chem. Lett., 2004, vol. 14, p. 4479. https://doi.org/10.1016/j.bmcl.2004.06.051
Boy, K.M., Guernon, J.M., Sit, S.Y., Xie, K., Hewawasam, P., Boissard, C.G., Dworetzky, S.I., Natale, J., Gribkoff, V.K., Lodge, N., and Starrett, J.E., Bioorg. Med. Chem. Lett., 2004, vol. 14, p. 5089. https://doi.org/10.1016/j.bmcl.2004.07.080
Ribeiro, N., Tabaka, H., Peluso, J., Fetzer, L., Nebigil, C., Dumont, S., Muller, C.D., and Desaubry, L., Bioorg. Med. Chem. Lett., 2007, vol. 17, p. 5523. https://doi.org/10.1016/j.bmcl.2007.08.036
Prezent, M.A. and Dorokhov, V.A., Russ. Chem. Bull., 2003, vol. 52, p. 2454. https://doi.org/10.1023/B:Rucb.0000012369.78621.36
Hashim, S.R. and Reddy, P.T., Indian J. Chem. B, 2001, vol. 40, p. 357.
Ruzi, R.H.G.L., Ma, J.Y., Yuan, X.A., Wang, W.L., Wang, S.S., Zhang, M.L., Dai, J., Xie, J., and Zhu, C.J., Chem. Eur. J., 2019, vol. 25, p. 12724. https://doi.org/10.1002/chem.201903816
Li, X.Y., Huo, X., Li, J.P., She, X.G., and Pan, X.F., Chin. J. Chem., 2009, vol. 27, p. 1379. https://doi.org/10.1002/cjoc.200990230
Rehwald, M., Gewald, K., Lankau, H.J., and Unverferth, K., Heterocycles, 1997, vol. 45, p. 483. https://doi.org/10.3987/COM-96-7700
Cheng, P., Zhang, Q., Ma, Y.B., Jiang, Z.Y., Zhang, X.M., Zhang, F.X., and Chen, J.J., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 3787. https://doi.org/10.1016/j.bmcl.2008.05.065
Esteve, M.E. and Gaozza, C.H., J. Heterocycl. Chem., 1981, vol. 18, p. 1061. https://doi.org/10.1002/jhet.5570180542
Petersen, J.B. and Lakowitz, K.H., Acta Chem. Scand., 1969, vol. 23, p. 971. https://doi.org/10.3891/acta.chem.scand.23-0971
Sokolova, M.S., Lavrikova, T.I., and Gornostaev, L.M., Russ. J. Org. Chem., 2007, vol. 43, p. 625. https://doi.org/10.1134/S1070428007040252
Kumar, S., Luxami, V., and Kumar, A., Org. Lett., 2008, vol. 10, p. 5549. https://doi.org/10.1021/ol802352j
Qian, W.Y., Wang, H., and Allen, J., Angew Chem., Int. Ed., 2013, vol. 52, p. 10992. https://doi.org/10.1002/anie.201305970
He, B., Dai, J., Zherebetskyy, D., Chen, T.L., Zhang, B.A., Teat, S.J., Zhang, Q.C., Wang, L.W., and Liu, Y., Chem. Sci., 2015, vol. 6, p. 3180. https://doi.org/10.1039/c5sc00304k
Kumar, A., Vanita, V., Walia, A., and Kumar, S., Sens. Actuators B Chem., 2013, vol. 177, p. 904. https://doi.org/10.1016/j.snb.2012.11.093
Kumar, A. and Kumar, S., Tetrahedron Lett., 2012, vol. 53, p. 2030. https://doi.org/10.1016/j.tetlet.2012.01.134
Lv, D.J., Cui, J., Wang, Y.F., Zhu, G.H., Zhang, M.J., and Li, X.J., RSC Adv., 2017, vol. 7, p. 33494. https://doi.org/10.1039/c7ra04024e
Elizbarashvili, E.N., Lagvilava, I.V., and Samsoniya, S.A., Chem. Heterocycl. Compd., 2005, vol. 41, p. 1868. https://doi.org/10.1021/ja305890c
He, B., Pun, A.B., Klivansky, L.M., McGough, A.M., Ye, Y.F., Zhu, J.F., Guo, J.H., Teat, S.J., and Liu, Y., Chem Mater. 2014, vol. 26, p. 3920. https://doi.org/10.1021/cm5018272
Gewald, K., Rehwald, M., Muller, H., and Bellmann, P., Liebigs Ann., 1995, vol. 1995, p. 787. https://doi.org/10.1002/jlac.1995199505115
Goncharov, D.S., Kostuchenko, A.S., and Fisyuk, A.S., Chem. Heterocycl. Compd., 2009, vol. 45, p. 793. https://doi.org/10.1007/s10593-009-0358-8
Fisyuk, A.S., Kulakov, I.V., Goncharov, D.S., Nikitina, O.S., Bogza, Y.P., and Shatsauskas, A.L., Chem. Heterocycl. Compd., 2014, vol. 50, p. 217. https://doi.org/10.1007/s10593-014-1464-9
Kulakov, I.V., Matsukevich, M.V., Shulgau, Z.T., Sergazy, Sh., Seilkhanov, T.M., Puzari, A., and Fisyuk, A.S., Chem. Heterocycl. Compd., 2015, vol. 51, p. 991. https://doi.org/10.1007/s10593-016-1809-7
Kulakov, I.V., Palamarchuk, I.V., Shulgau, Z.T., Seilkhanov, T.M., Gatilov, Y.V., and Fisyuk, A.S., J. Mol. Struct., 2018, vol. 1166, p. 262. https://doi.org/10.1016/j.molstruc.2018.04.036
Shatsauskas, A.L., Abramov, A.A., Chernenko, S.A., Kostyuchenko, A.S., and Fisyuk, A.S., Synthesis, 2020, vol. 52, p. 227. https://doi.org/10.1055/s-0039-1690231
Kulakov, I.V., Shatsauskas, A.L., Matsukevich, M.V., Palamarchuk, I.V., Seilkhanov, T.M., Gatilov, Y.V., and Fisyuk, A.S., Synthesis, 2017, vol. 49, p. 3700. https://doi.org/10.1055/s-0036-1590470
Kulakov, I.V., Matsukevich, M.V., Levin, M.L., Palamarchuk, I.V., Seilkhanov, T.M., and Fisyuk, A.S., Synlett, 2018, vol. 29, p. 1741. https://doi.org/10.1055/s-0037-1610445
Shatsauskas, A.L., Saibulina, E.R., Gatilov, Y.V., Kostyuchenko, A.S., and Fisyuk, A.S., Chem. Heterocycl. Compd., 2019, vol. 55, p. 1080. https://doi.org/10.1007/s10593-019-02581-8
Fisyuk, A.S., Bogza, Y.P., Poendaev, N.V., and Goncharov, D.S., Chem. Heterocycl. Compd., 2010, vol. 46, p. 844. https://doi.org/10.1007/s10593-010-0592-0
Goncharov, D.S., Kulakov, I.V., and Fisyuk, A.S., Chem. Heterocycl. Compd., 2017, vol. 53, p. 1335. https://doi.org/10.1007/s10593-018-2215-0
Goncharov, D.S., Garkushenko, A.K., Savelieva, A.P., and Fisyuk, A.S., Arkivoc, 2015, vol. 5, p. 176. https://doi.org/10.3998/ark.5550190.p009.126
Hommes, P., Berlin, S., and Reissig, H.U., Synthesis, 2013, vol. 45, p. 3288. https://doi.org/10.1055/s-0033-1338548
Unger, L., Accorsi, M., Eidamshaus, C., Reich, D., Zimmer, R., and Reissig, H.U., Synthesis, 2018, vol. 50, p. 4071. https://doi.org/10.1055/s-0037-1609576
Eidamshaus, C. and Reissig, H.-U., Eur. J. Org. Chem., 2011, p. 6056. https://doi.org/10.1002/ejoc.201100681
Hommes, P., Jungk, P., and Reissig, H.-U., Synlett, 2011, p. 2311. https://doi.org/10.1055/s-0030-1260304
Hommes, P., Fischer, C., Lindner, C., Zipse, H., and Reissig, H.-U., Angew. Chem., Int. Ed., 2014, vol. 53, p. 7647. https://doi.org/10.1002/anie.201403403
Dash, J. and Reissig, H.-U., Chem. Eur. J., 2009, vol. 15, p. 6811. https://doi.org/10.1002/chem.200900939
Fyssiuk, A.S., Vorontsova, M.A., and Sagitullin, R.S., Mendeleev Commun., 1993, vol. 3, p. 249. https://doi.org/10.1070/MC1993v003n06ABEH000315
Fisyuk, A.S., Vorontsova, M.A., and Ivanov, S.A., Chem. Heterocycl. Compd., 1994, vol. 30, p. 709. https://doi.org/10.1007/BF01166313
Fisyuk, A.S. and Vorontsova, M.A., Chem. Heterocycl. Compd., 1998, vol. 34, p. 195. https://doi.org/10.1007/BF02315183
Fisyuk, A.S. and Poendaev, N.V., Molecules, 2002, vol. 7, p. 119. https://doi.org/10.3390/70200119
Fissyuk, A.S., Vorontsova, M.A., and Temnikov, D.V., Tetrahedron Lett., 1996, vol. 37, p. 5203. https://doi.org/10.1016/0040-4039(96)01051-9
Fisyuk, A.S., Berdovich, L.V., Temnlkov, D.V., and Knyaz’kova, L.N., Chem. Heterocycl. Compd., 1997, vol. 33, p. 805. https://doi.org/10.1007/BF02253030
Fisyuk, A.S. and Vorontsova, M.A., Chem. Heterocycl. Compd., 1998, vol. 34, p. 65. https://doi.org/10.1007/BF02290614
Fisyuk, A.S. and Poendaev, N.V., Molecules, 2002, vol. 7, p. 124. https://doi.org/10.3390/70200124
Chen, Q.-B., Xin, X.-L., Aisa, H.A., Phytochem. Lett., 2017, vol. 19, p. 168. https://doi.org/10.1016/j.phytol.2016.12.024
Fisyuk, A.S. and Poendaev, N.V., Chem. Heterocycl. Compd., 2003, vol. 39, p. 895. https://doi.org/10.1023/A:1026146421293
Fisyuk, A.S., Poendaev, N.V., and Bundel’, Yu.G., Mendeleev Commun., 1998, vol. 8, p. 12. https://doi.org/10.1070/MC1998v008n01ABEH000877
Fisyuk, A.S., Poendaev, N.V., and Bundel’, Yu.G., Chem. Heterocycl. Compd., 1998, vol. 34, p. 258. https://doi.org/10.1007/BF02315198
Fisyuk, A.S. and Poendaev, N.V., Chem. Heterocycl. Compd., 2003, vol. 39, p. 891. https://doi.org/10.1023/A:1026194204454
Funding
The review of the winners of the Ekspansiya Competition of the Russian Foundation for Basic Research 2019 (project no. 19-13-50277).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fisyuk, A.S., Kostyuchenko, A.S. & Goncharov, D.S. Camps Reaction and Related Cyclizations. Russ J Org Chem 56, 1863–1892 (2020). https://doi.org/10.1134/S1070428020110019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020110019