Abstract
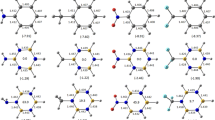
Quantum-chemical method DFT B3LYP/6-311G* was applied to stage by stage thermodynamic calculation of reactions of electrophilic iodination of benzene, naphthalene, phenanthrene, and anthracene with iodine and iodine monochloride, and comparison with chlorination reactions was performed. The main distinction of iodination process from chlorination was an enhanced reversibility owing to protodeiodination. The reversibility of iodination grows with the electron-donor properties of aromatic substrates. The calculations permit an assumption that the chlorination of anthracene and phenanthrene with iodine monochloride occurs most probably through stages of electrophilic iodination-dehydroiodination.
Similar content being viewed by others
References
Merkushev, E.B., Usp. Khim., 1984, vol. 53, p. 583.
Cacchi, S., Fabrizi, G., Parisi, L.M., Heterocycles, 2002, vol. 58, p. 667.
Negishi, E-J., J. Organometal. Chem., 1999, vol. 576, p. 179.
Merkushev, E.B., Synthesis, 1988, p. 923.
Cheprakov, A.V., Makhon’kov, D.I., and Beletskaya, I.P., Izv. Akad. Nauk SSSR, Ser. Khim., 1987, p. 11.
Skulski, L., Molecules, 2000, vol. 5, p. 1331.
Turner, D.E., O’Malley, R.F., Sardella, D.J., Barinelli, K.S., and Kaul, P. J. Org. Chem., 1994, vol. 59, p. 7335.
Chaikovskii, V.K. and Filimonov, V.D., Zh. Org. Khim., 2001, vol. 37, p. 1189.
Filimonov, V.D., Krasnokutskaya, E.A., Poleshchuk, O.Kh., and Lesina, Yu.A., Izv. Akad. Nauk, Ser. Khim., 2006, p. 1280.
Kekule, A., Lieb. Ann. 1866, vol. 137, p. 129.
Graham, W.S., Nichol, R.J., and Ubbelohde, A.R., J. Chem. Soc., 1955, p. 115.
Smith, W.B., J. Org. Chem., 1985, vol. 50, p. 3649.
Nicolet, B.H., J. Am. Chem. Soc., 1921, vol. 43, p. 2081.
Terent’ev, A.P., Belen’kii, L.I., and Yanovskaya, L.A., Zh. Obshch. Khim., 1954, vol. 24, p. 1265.
Meerwein, H., Hoffman, P., and Schill, F., J. Prakt. Chem., 1939, vol. 154, p. 266.
Suzuki, H. and Goto, R., Bull. Chem. Soc. Jpn., 1963, vol. 36, p. 389.
Mattern, D.L. and Chen, X., J. Org. Chem., 1991, vol. 56, p. 5903.
Filimonov, V.D., Krasnokutskaya, E.A., and Lesina, Yu.A., Zh. Org. Khim., 2003, vol. 39, p. 924.
Tomasi, J. and Perisco, M., Chem. Rev., 1994, vol. 94, p. 2027.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Gill, P.M.W., Johnson, B.G., Robb, M.A., Cheeseman, J.R., Keith, T., Petersson, G.A., Montgomery, J.A., Raghavachari, K., Al-Laham, M.A., Zakrzewski, V., Ortiz, J.V., Foresman, J.B., Closlowski, J., Stefanov, B.B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y., Chen, W., Wong, M.W., Andress, J.L., Replogle, E.S., Gomperts, R., Martin, R.L., Fox, D.J., Binkley, J.S., Defress, D.J., Baker, J., Stewart, J.P., Head-Gordon, M., Gonzales, C., and Pople, J.A., Gaussian 98, Revision A., Pittsburg: Gaussian Inc:, 1998.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648.
Lee, C., Yang, W., and Parr, R.G., Phys. Rev. B, 1988, vol. 37, p. 785.
Glukhovtsev, M.N., Pross, A., McGrath, M.P., and Radom, L., J. Chem. Phys., 1995, vol. 103, p. 1878.
Sakai, H., Maeda, Y., Ichiba, S., and Negita, H., J. Chem. Phys., 1980, vol. 72, p. 6192.
Su, J.T. and Zewail, A.H., J. Phys. Chem. A, 1998, vol. 102, p. 4082.
Tang, L-T., Wei, Y., Wang, Y., Hu, S.-W., Liu, X.-O., Chu, T.-W., and Wang, X.-Y., J. Mol. Struct. Theochem., 2004, vol. 686, p. 25.
Drepaul, I., Fagundez, V., Guiterrez, F., Lau, E.H., and Joens, J.A., J. Org. Chem., 1996, vol. 61, p. 3571.
Su, J.T. and Zewail, A.H., J. Phys. Chem. A, 1998, vol. 102, p. 4082.
Tustin, G.C. and Rule, M., J. Catalys., 1994, vol. 147, p. 186.
Duan, S., Turk, J., Spiegle, J., Corbin, J., Masnovi, J., and Baker, R.J., J. Org. Chem., 2000, vol. 65, p. 3005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.D. Filimonov, E.A. Krasnokytskaya, O.Kh. Poleshchuk, Yu.A. Lesina, 2008, published in Zhurnal Organicheskoi Khimii, 2008, Vol. 44, No. 5, pp. 691–696.
Rights and permissions
About this article
Cite this article
Filimonov, V.D., Krasnokytskaya, E.A., Poleshchuk, O.K. et al. Theoretical analysis of reactions of electrophilic iodination and chlorination of benzene and polycyclic arenes in density functional theory approximation. Russ J Org Chem 44, 681–687 (2008). https://doi.org/10.1134/S1070428008050072
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428008050072