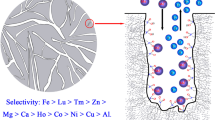
Abstract
SQS–6 as a cationic exchange resin was originally used for production of enriched nitrogen and separation of ultrapure N-14. Based on the literature, the application of such resin is very limited. Therefore, it is interesting to test its ability to extract and separate some of the rare earth ions from aqueous media like phosphoric acid solution. In this work, solid–liquid extraction of some rare earth metal ions namely; Ce(IV), Pr(III), Er(III) and Y(III) from 8.0 M phosphoric acid was studied using batch and column techniques with a strongly acidic cation exchange resin (SQS–6). Batch investigations data indicated that the amount of adsorbed metal ions studied decreased with increasing the phosphoric acid concentration. Thermodynamic results obtained from the study of the temperature effect on metal ions sorption showed that the process is endothermic and spontaneous associated with increasing the randomness of the system. The sorption isotherm was favorable and obeys Langmuir isotherm model based on a comparison between experimental and calculated sorption capacity. The experimental sorption capacity were found 5.2, 12.6, 8.8, and 3.8 mg/g for Ce(IV), Pr(III), Y(III), and Er(III), respectively. Column investigations were carried out in terms of the breakthrough curves of the metal ions under study. The breakthrough data obtained for the lanthanide metal ions proved that the experimental sorption capacity and the time required for 50% adsorbate breakthrough (τ) are close to that calculated from Thomas and Yoon–Nelson models. Possible separations of those metal ions were studied and assessed at different sorption conditions.











Similar content being viewed by others
Change history
19 December 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1070427222060143
REFERENCES
Habashi, F., J. Chem. Technol. Biotechnol., 1985, vol. 35, no. 1, pp. 5–14. https://doi.org/10.1002/jctb.5040350103
Habashi, F., Can. Metall. Q., 2013, vol. 52, pp. 224–233. https://doi.org/10.1179/1879139513Y.0000000081
Beker, P., Hignett, T.P., and Palgrave, D.A., Phosphates and Phosphoric Acid, Raw Materials, Technology and Economics of Wet Process, New York: Marcel Decker Inc., 1989.
Reddy, B.R., Kumar, B.N., and Radhika, S., Solvent Extr. Ion Exch., 2009, vol. 27, no. 5-6, pp. 695–711. https://doi.org/10.1080/07366290903270031
Reddy, B.R., and Kumar, B.N., Solvent Extr. Ion Exch., 2016, vol. 34, no. 3, pp. 226–240. https://doi.org/10.1080/07366299.2016.1169144
Radhika, S., Nagaraju, V., Kumar, B.N., Kantam, M.L., and Reddy, B.R., J. Rare Earths, 2012, vol. 30, no. 12, pp. 1270–1275. https://doi.org/10.1016/S1002-0721(12)60219-1
Kumar, B.N., Radhika, S., and Reddy, B.R., Chem. Eng. J., 2010, vol. 160, no. 1, pp. 38–144. https://doi.org/10.1016/j.cej.2010.03.021
Hérès, X., Blet, V., Natale, P.D., Ouaattou, A., Mazouz, H., Dhiba, D., and Cuer, F., Metals, 2018, vol. 8, no. 9, pp. 682. https://doi.org/10.3390/met8090682
Abu Elgoud, E.M., Ismail, Z.H., Ahmad, M.I., El-Nadi, Y.A., Abdelwahab, S.M., and Aly, H.F., Russ. J. Appl. Chem., 2019, vol. 92, no. 11, pp. 1581−1592. https://doi.org/10.1134/S1070427219110156
Marczenko, Z., Spectrophotometric Determination of Elements, New York: John Wiley, 1986.
Uddin, Md.T., Rukanuzzaman, Md., Khan, M.R., and Islam, Md.A., J. Environ. Manage, 2009, vol. 90, no. 11, pp. 3443–3450. https://doi.org/10.1016/j.jenvman.2009.05.030
Han, R.P., Zou, L.N., Zhao, X., Xu, Y.F., Xu, F., Li, Y.L., and Wang, Y., Chem. Eng. J., 2009, vol. 149, no. 1–3, pp. 123–131. https://doi.org/10.1016/j.cej.2008.10.015
El–Gammal, B. and Shady, S.A., Colloid Surf. A: Physicochem. Eng. Asp., 2006, vol. 287, no. 1–3, pp. 132–138 (2006). https://doi.org/10.1016/j.colsurfa.2006.02.068
Oguz, E. and Ersoy, M., Chem. Eng. J., 2010, vol. 164, no. 1, pp. 56–62. https://doi.org/10.1016/j.cej.2010.08.016
Xueyong, Z. and Xin, Z., Chem. Eng. Comm., 2014, vol. 201, no. 11, pp. 1459–1467. https://doi.org/10.1080/00986445.2013.818541
Rizkalla, E.N. and Choppin, G.R., J. Alloy. Compd., 1992, vol. 180, pp. 325–336. https://doi.org/10.1016/0925-8388(92)90398-S
Gupta, N.K. and Sengupta, A., Hydrometallurgy, 2017, vol. 171, pp. 8– https://doi.org/10.1016/j.hydromet.2017.03.016
Freundlich, H., Colloid and Capillary Chemistry, London: Methuen and Co. Ltd, 1926.
Langmuir, I., J. Am. Chem. Soc., 1918, vol. 40, no. 9, pp. 1361–1403. https://doi.org/10.1021/ja02242a004
Han, R.P., Wang, Y., Zhao, X., Wang, Y.F., Xie, F.L, Cheng, J.M., and Tang, M.S., Desalination, 2009, vol. 245, no. 1–3, pp. 284–297. https://doi.org/10.1016/j.desal.2008.07.013
Baral, S.S., Das, N., Ramulu, T.S., Sahoo, S.K., Das, S.N., and Chaudhury, G.R., J. Hazard. Mater., 2009, vol. 161, no. 2–3, pp. 1427–1435. https://doi.org/10.1016/j.jhazmat.2008.04.127
Salman, J.M., Njoku, V.O., and Hameed, B.H., Chem. Eng. J., 2011, vol. 174, no. 1, pp. 33–40 (2011). https://doi.org/10.1016/j.cej.2011.08.024
Thomas, H.C., J. Am. Chem. Soc., 1944, vol. 66, no. 10, pp. 1466–1664. https://doi.org/10.1021/ja01238a017
Liu, D. and Sun, D., Environ. Eng. Sci., 2012, vol. 29, no. 6, pp. 461–465. https://doi.org/10.1089/ees.2010.0435
Yoon, Y.N. and Nelson, J.H., Am. Ind. Hyg. Assoc. J., 1984, vol. 45, no. 8, pp. 509– 516. https://doi.org/10.1080/15298668491400197
ACKNOWLEDGMENTS
This research is funded by the Egyptian Atomic Energy Authority and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Elgoud, E.M.A., Ismail, Z.H., El-Nadi, Y.A. et al. Solid–Liquid Extraction of Rare Earth Elements Ce(IV), Pr(III), Er(III), and Y(III) from Concentrated Phosphoric Acid Solutions Using Strongly Acidic Cation Exchange Resin (SQS–6). Russ J Appl Chem 95, 602–615 (2022). https://doi.org/10.1134/S1070427222040176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222040176