Abstract
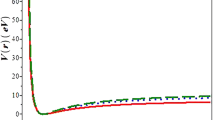
In the first order of perturbation theory, the total energy of a diatomic molecule in the ground state is calculated taking into account the Pauli principle and plasma oscillations of atomic electrons. The Fourier component of the potential energy of the interaction of an atom with another atom has the form of a polynomial of the fourth degree from the atomic-form factor. The numerical calculation is performed for the atomic-form factor in the approximation of wave functions, which approximate the solution of the Hartree–Fock equation for an isolated atom. It is shown that taking into account plasma oscillations of atomic electrons leads to a self-consistent system of equations, the numerical solution of which makes it possible to determine the elastic constant, that is, the value of the second derivative at the minimum potential energy of the molecule. The total energy for nitrogen and fluorine molecules is calculated.



Similar content being viewed by others
REFERENCES
V. P. Koshcheev and Yu. N. Shtanov, Tech. Phys. Lett. 44, 566 (2018). https://doi.org/10.1134/S1063785018070088
V. P. Koshcheev and Yu. N. Shtanov, J. Surf. Invest.: X‑ray, Synchrotron Neutron Tech. 14, 841 (2020). https://doi.org/10.1134/S102745102004028X
P. A. M. Dirac, The Principles of Quantum Mechanics (Clarendon, Oxford, 1958).
V. A. Fock, Fundamentals of Quantum Mechanics (LKI, Moscow, 2007; Mir, Moscow, 1978).
E. Clementi and C. Roetti, At. Data Nucl. Data Tables 14, 177 (1974). https://doi.org/10.1016/S0092-640X(74)80016-1
V. P. Koshcheev, Y. N. Shtanov, and D. A. Morgun, Bull. Russ. Acad. Sci.: Phys. 82, 179 (2018). https://doi.org/10.3103/S1062873818020156
H. Bethe, in Handbuch der Physik (Springer, Berlin, 1933), Vol. 24/1.
E. M. Livshitz and L. P. Pitaevskii, Physical Kinetics (Pergamon, Oxford, 1981; Fizmatlit, Moscow, 2007).
Yu. N. Shtanov and V. P. Koshcheev, State Registration Certificate of Computer Program No. 2020617054 (2020).
Yu. N. Shtanov, V. P. Koshcheev, and D. A. Morgun, JINRLIB Program Library. http://wwwinfo.jinr.ru/programs/jinrlib/tropics/index.html. Accessed January 10, 2022.
L. Seunghoon, Z. Huanchen, S. Sandeep, C. J. Umrigar, and G. Kin-Lic Chan, J. Chem. Theory Comput. 17, 3414 (2021). https://doi.org/10.1021/acs.jctc.1c00205
A. A. Radtsig and B. M. Smirnov, Reference Data on Atoms, Molecules, and Ions (Atomizdat, Moscow, 1980; Springer, Berlin, 1985).
Funding
This study was carried out with the financial support of the Russian Foundation for Basic Research as part of research project no. 20-07-00236 a.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Koshcheev, V.P., Shtanov, Y.N. A New Approach to the Calculation of the Total Energy of a Diatomic Molecule in the First Order of Perturbation Theory. Tech. Phys. Lett. 48, 123–127 (2022). https://doi.org/10.1134/S1063785022040083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063785022040083